Abstract
Tracheobronchial amyloidosis is a rare disease characterized by amyloid deposits on the tracheal and bronchial tissue. Patients with tracheobronchial amyloidosis are asymptomatic or exhibit symptoms, such as chronic wheezing, dyspnea, and cough, that are common manifestations of other disorders, including asthma. A bronchoscopic tissue biopsy using Congo red staining is the key standard for diagnosing tracheobronchial amyloidosis. Treatment strategies vary depending on the degree of airway obstruction. If the obstruction is significant and the patient is symptomatic, repeated bronchoscopic treatment, including local resection, laser therapy, stent placement, and radiation therapy, is considered a safer and better option. It is often misdiagnosed as asthma, but cases of tracheobronchial amyloidosis accompanied with asthma have not been reported. We report a case of intermittent wheezing, cough for 33 years, and shortness of breath on exertion for 7 years, which had aggravated in the previous 22 days. A pulmonary examination revealed diffuse wheezing. Pulmonary function testing revealed an obstructive ventilation dysfunction. Computerized tomography (CT) imaging revealed circumferential and irregular thickening of the tracheobronchial wall tissue with calcification and atelectasis of the right middle and lower lobe of the lung. Bronchoscopy revealed diffuse thickening of the mucosa of the trachea and bilateral main bronchi, with multiple nodular protuberances and relatively narrow lumens. The bronchial biopsies revealed massive amyloid deposits under the bronchial mucosa. The deposits exhibited a green birefringence under crossed polarized light after Congo red positive staining. The patient received standard treatment for asthma, and remains in good general condition without wheezing. It is not difficult to distinguish tracheobronchial amyloidosis through chest CT examination for patients with wheezing as long as this disease was considered. It was interesting that we present a rarer case of patient with tracheobronchial amyloidosis accompanied with asthma which both can cause symptoms such as wheezing.
Introduction
Amyloidosis is a rare disease characterized by abnormal extracellular deposition of autologous proteins, or amyloids, in various organs, causing functional damage. The disease is categorized as systemic or localized in one organ with little or no spread.Citation1 About 50% of amyloidosis cases are estimated to be localized in the respiratory system.Citation2,Citation3 Respiratory amyloidosis mostly occurs in three forms: tracheobronchial, nodular parenchymal, and diffuse parenchymal or alveolar septal amyloidosis.Citation4 Tracheobronchial amyloidosis (TA) is a form of localized amyloidosis. It is a rare entity, with only a few cases or series of case reports or described in the literature.Citation5–7 At present, there is no exact data on gender or geographic differences in the prevalence of TA. Patients with TA are asymptomatic or exhibit symptoms, such as chronic wheezing, dyspnea, and cough, that are common manifestations of other disorders, including asthma.Citation8 Of note, there are 6 case reports in PubMed describing TA misdiagnosed as asthma but not accompanied with asthma.Citation9–14 For patients with wheezing, it is easy to diagnosis TA, as long as we consider the diagnosis of TA and remember to do a CT examination, but it is difficult to diagnose bronchial asthma for a patient with TA, requiring sufficient diagnosis evidences. Herein, we present a case of a patient with TA and asthma.
Case Report
Chief Complaints and History of Present Illness
A 67-year-old woman from Beijing, China, was admitted to the hospital with a history of intermittent wheezing for 33 years and shortness of breath on exertion for 7 years. She developed intermittent wheezing accompanied by coughs 33 years ago after getting a cold and the symptoms subsided after antibiotic treatment (details unknown). The symptoms occurred once every 1 to 2 years. There was shortness of breath that occurred after walking 500 meters 7 years ago. She experienced these symptoms 22 days prior to hospitalization after catching a cold and was accompanied with a fever reaching 38.2°C. She was admitted to emergency department and treated for asthma with infection using intravenous methylprednisolone (40 mg/d) and moxifloxacin infusions, and her symptoms slightly improved. She never experienced hemoptysis, night sweats, skin rash, joint swelling and pain since the onset.
History of Past Illnesses and Personal History
She was diagnosed with allergic rhinitis 7 years prior and highlighted no history of tuberculosis and no history of smoking, alcohol, or drug use.
Physical Examination
She had no obvious abnormalities in other physical examinations except for diffuse wheezing during expiration and inspiration.
Laboratory Examinations Before Admission
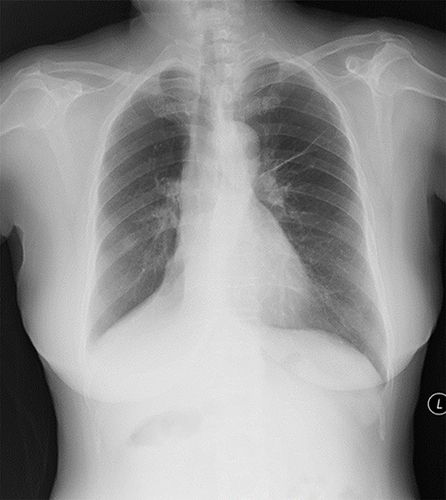
Blood routine tests revealed a white blood cell count of 11.7 × 109/L, 82.5% neutrophil percentage, and 0.0% eosinophil percentage. Chest radiograph revealed atelectasis of the right middle and lower lobes, and a fiber chord could be seen in the left lung ().
Further Diagnostic Workup
Further diagnostic tests revealed total serum immunoglobulin (sIgE) of 444.0KU/L, sIgE against Artemisia argyi was 100.0KU/L. Pulmonary function testing revealed an obstructive ventilation dysfunction, forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) 55.73%, and FEV1% pred 61.5% (before bronchodilator-aided inhalation), and the airway reversibility test was negative. A chest CT scan revealed irregular high-density thickening of the trachea wall, both stem bronchi, lobar, and proximal segmental bronchi, leading to tracheobronchial luminal stenosis and atelectasis of the right middle and lower lobe (– and –). A bronchoscopy revealed diffuse, circumferential and irregular thickening of the mucosa of the trachea and bilateral main bronchi, with multiple nodular protuberances and relatively narrow lumens (). The bronchial biopsies () revealed massive amyloid deposits under the bronchial mucosa. Amyloid deposits are blue in cellulose staining, negative in Van Gieson method and were demonstrated by apple-green birefringence with polarized light with Congo red. There was no granuloma, multinucleated giant cells or other special lesions. Further tests for systemic disease and etiology, including electromyogram, serum immunofixation electrophoresis, blood and urine light chain, autoimmune examinations, T-spot, sputum culture, bone marrow puncture smear, and biopsy and flow cytology of bone marrow and so on were all negative.
Figure 2 CT scan of the chest (lung windows) showing lung windows before (A–C) and after treatment (D–F). (A–C) Lung windows showing irregular high-density thickening of the tracheal wall (A), both stem bronchi, lobar, and proximal segmental bronchi (B), leading to tracheobronchial luminal stenosis and atelectasis of the right middle and lower lobe (C). (D–F) Lung windows showing the relieved tracheobronchial wall thickening and luminal stenosis; there was bronchial inflation sign in atelectasis.
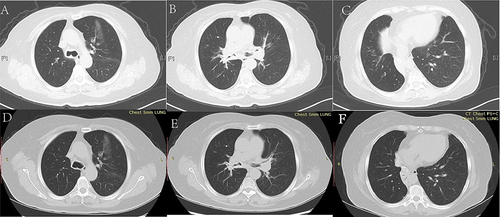
Figure 3 CT scan of the chest (soft tissue window) showing mediastinal windows before (A–C) and after treatment (D–F). (A–C) Soft tissue windows revealing the irregular thickening of tracheobronchial wall tissue accompanied by submucosal circumferential and granular calcification (A and B) and atelectatic lung tissue of the right middle and lower lobe with gathered bronchi outlined by the wall calcification (the bronchi are obstructed without air bronchogram sign) (C). (D–F) Soft tissue windows showing the relieved tracheobronchial wall thickening, luminal stenosis, and atelectasis of the right lower lobe; there was bronchial inflation sign in atelectasis.
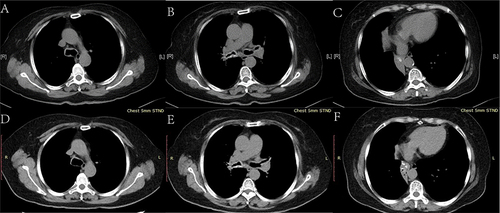
Figure 4 Tracheobronchial images showing the bronchoscopy findings. The mucosa of the trachea and bilateral main bronchi is diffusely and circumferentially thickened, with multiple nodular protuberances and relatively narrow lumens. (A) Trachea. (B) Tracheal carina. (C) Proximal left main bronchus. (D) Distal left main bronchus. (E) Right main bronchus. (F) Right upper lobe bronchus. (G) Middle segment bronchus of right lung.
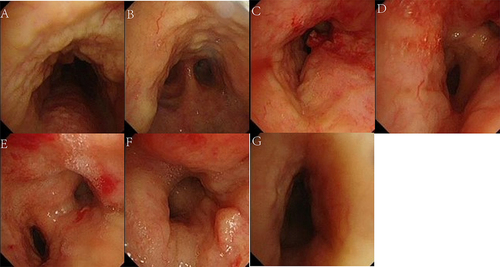
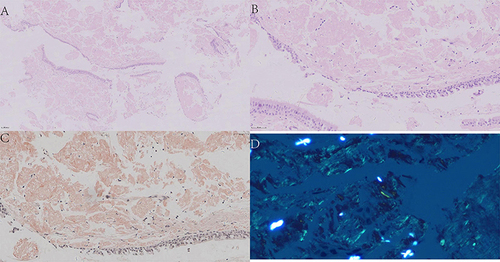
Figure 5 Pathological findings of bronchial biopsies: (A) (HE×100), (B) (HE×400): Low and high magnification views showing abundant amorphous eosinophilic material depositing under the epithelium of bronchial mucosa. (C) (Congo red staining ×400): Congo red stain highlight the dense accumulation of brick red amyloid material in bronchial mucosa; (D) (Congo red staining ×400): Congo red stain typically shows apple-green birefringence in polarized light.
Methylprednisolone (80mg per day) concurrently with inhaled corticosteroids and bronchodilators were administered. The symptoms gradually subsided. She was discharged and put on regular inhalation of budesonide formoterol (160/4.5 ug, 2 inhaled twice per day) and oral intake of montelukast.
Follow-up of the patient was done for 4 years. Three months after discharge, the patient had no symptoms, and the activity tolerance was not limited. A chest CT scan demonstrated the partially relieved tracheobronchial wall thickening and luminal stenosis, with bronchial inflation signs in atelectasis (– and –). Reexamination of the pulmonary function showed that obstructive ventilation dysfunction was slightly improved, with FEV1/FVC 59.0%, FEV1% pred 70%. However, she suffered cough, with wheezing sound during her allergy season and got significantly better after the allergy season when budesonide/formoterol was reduced to 1 inhaled BID. Currently, she is in good condition and is under regular therapy with ICS/LABA and montelukast.
Discussion
Pulmonary amyloidosis is a rare disease. Its exact incidence in China remains unclear, with about 6–10 cases occurring annually per 100,000 people in Western Europe and the United States.Citation1 TA is the most common form of pulmonary amyloidosis. Patients are often asymptomatic but can exhibit dyspnea, cough and recurrent pneumonia.Citation8,Citation15,Citation16 TA is strongly suspected if a CT scan presents high-attenuation thickening of the airway wall with long-segment narrowing of the lumen.Citation1 In contrast to other diffuse tracheal diseases, such as tracheobronchopathia, osteochondroplastica, and relapsing polychondritis, TA may involve the posterior tracheal and bronchial membranes. Definitive diagnosis of TA typically depends on bronchoscopy biopsy coupled with Congo red staining.Citation1,Citation8,Citation17
The present case was diagnosed as TA accompanied with asthma. There were typical characteristics of CT scan and pathological changes that supported a clear diagnosis of TA. Moreover, the patient had a history of wheezing and cough, exacerbated by allergies and cold, and responded well to steroids and β2 agonist nebulization, with the past history of allergic rhinitis. These clinical characteristics supported the diagnosis of asthma. According to the type of the fibrillar component in amyloid deposits, there are over two dozen subtypes of amyloidosis in the biochemical classification.Citation1 The fibrillar proteins amyloid light chain (AL) and serum amyloid A (AA) are most common types. AL fibrillar protein is derived from the abnormal breakdown of normal immunoglobulin light chains produced by a clonal population of plasma cells. Systemic infections and inflammatory disorders (eg, rheumatoid arthritis, chronic bronchitis, and so on) cause elevation of AA in subjects.Citation18 Whether long-term poorly controlled asthma which is a chronic airway inflammatory disease can cause the generation of AA and the occurrence of airway amyloidosis is not very clear at present. More clinical cases are needed to study the relationship between TA and asthma. Some articlesCitation9,Citation11,Citation19–21suggested the clinical manifestations of TA are similar to severe and poorly controlled asthma, which makes it difficult to differentiate and often requires combining bronchoscopy and chest CT scan for auxiliary diagnosis.
Treatment strategies vary depending on the degree of airway obstruction.Citation9,Citation20 Asymptomatic patients should be monitored without treatment, while more aggressive local and systemic therapy should be undertaken if the obstruction is significant and the patient is symptomatic.Citation9 To date, there is no established effective medical treatment for TA. Repeated bronchoscopic treatment, including local resection, laser therapy, stent placement, and radiation therapy, is considered a safer and better option.Citation1,Citation17,Citation21 The 5-year survival rate of patients with TA range between 30% and 50%.Citation1 The present patient’s symptoms were improved when subjected to active asthma treatment. Therefore, she was not treated but was closely monitored. If asthma is well controlled but the degree of airway obstruction remains severe or progresses significantly, leading to more severe dyspnea for the patient, local treatment (bronchoscopic treatment or radiation therapy) should be considered.
Conclusion
Tracheobronchial amyloidosis is a localized variant of amyloidosis and a rare disease that is relatively difficult to diagnose because of its non-specific clinical manifestation. It should be considered in the differential diagnosis of patients with wheezing or recurrent respiratory infections. The disease often requires a combination of bronchoscopy and chest CT scan for auxiliary diagnosis. Definitive diagnosis of tracheobronchial amyloidosis typically depends on bronchoscopy biopsy coupled with Congo red staining. Repeated bronchoscopic treatment was the main treatment for tracheobronchial amyloidosis.
Ethics Approval and Informed Consent
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study followed the ethical standards of the institutional and national research committee(s) and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient to publish this case report and accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank every member of our team.
Additional information
Funding
References
- Czeyda-Pommersheim F, Hwang M, Chen SS, et al. Amyloidosis: modern cross-sectional imaging. Radiographics. 2015;35(5):1381–1392. doi:10.1148/rg.2015140179
- Aylwin AC, Gishen P, Copley SJ. Imaging appearance of thoracic amyloidosis. J Thorac Imaging. 2005;20(1):41–46. doi:10.1097/01.rti.0000154074.29194.09
- Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med. 1996;124(4):407–413. doi:10.7326/0003-4819-124-4-199602150-00004
- Milani P, Basset M, Russo F, et al. The lung in amyloidosis. Eur Respir Rev. 2017;26(145):170046. doi:10.1183/16000617.0046-2017
- Mangla L, Vadala R, Kadli SK, et al. Tracheobronchial amyloidosis: an uncommon disease with a common presentation. Respirol Case Rep. 2020;8(7):e00630. doi:10.1002/rcr2.630
- Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc. 2000;75(11):1148–1152. doi:10.4065/75.11.1148
- Papla B, Dubiel-Bigaj M. Tracheobronchial amyloidosis. Pol J Pathol. 1998;49(1):27–34. PMID: 9640972.
- Crain MA, Lakhani DA, Balar AB, et al. Tracheobronchial amyloidosis: a case report and review of literature. Radiol Case Rep. 2021;16(9):2399–2403. doi:10.1016/j.radcr.2021.05.082
- Serraj M, Kamaoui I, Znati K, et al. Pseudotumoral tracheobronchial amyloidosis mimicking asthma: a case report. J Med Case Rep. 2012;6:40. doi:10.1186/1752-1947-6-40
- Tanrıverdi E, Özgül MA, Uzun O, et al. Tracheobronchial amyloidosis mimicking tracheal tumor. Case Rep Med. 2016;2016:1084063. doi:10.1155/2016/1084063
- Rekik WK, Ayadi H, Ayoub A. L’amylose trachéobronchique localisée: une cause rare de pseudo-asthme [Localized tracheobronchial amyloidosis: a rare cause of pseudo-asthma]. Rev Pneumol Clin. 2001;57(4):308–310. French.
- Kunal S, Dhawan S, Kumar A, et al. Middle lobe syndrome: an intriguing presentation of tracheobronchial amyloidosis. BMJ Case Rep. 2017;2017:bcr2017219480. doi:10.1136/bcr-2017-219480
- Sharma SK, Ahluwalia G, Ahluwalia A, et al. Tracheobronchial amyloidosis masquerading as bronchial asthma. Indian J Chest Dis Allied Sci. 2004;46(2):117–119.
- Rodríguez Vázquez JC, Pino Alfonso PP, Gassiot Nuño C, et al. Amiloidosis traqueobronquial primaria. Reporte de un caso [Primary tracheobronchial amyloidosis. Report of a case]. An Med Interna. 1998;15(6):319–320. Spanish, English Abstract.
- Urban BA, Fishman EK, Goldman SM, et al. CT evaluation of amyloidosis: spectrum of disease. Radiographics. 1993;13(6):1295–1308. doi:10.1148/radiographics.13.6.8290725
- de Almeida RR, Zanetti G, Pereira E, et al. Respiratory tract amyloidosis. state-of-the-art review with a focus on pulmonary involvement. Lung. 2015;193(6):875–883. doi:10.1007/s00408-015-9791-x
- Riehani A, Soubani AO. The spectrum of pulmonary amyloidosis. Respir Med. 2023;218:107407. doi:10.1016/j.rmed.2023.107407
- Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute phase reactant for clinical practice. Eur J Clin Invest. 1996;26(6):427–435. doi:10.1046/j.1365-2362.1996.159291.x
- Sreetharan SS, Prepageran N, Razak A, et al. Aerodigestive amyloidosis presenting as acute asthma. Med J Malaysia. 2003;58(2):290–293.
- Segura Méndez NH, Barragán Estrada Mde L, Paredes Delgado Mde L, et al. Asma o amiloidosis laríngea? Comunicación de un caso y revisión de la literatura [Asthma or laryngeal amyloidosis? A report of a case and literature review]. Rev Alerg Mex. 2006;53(1):30–33. Spanish.
- Kang HW, Oh HJ, Park HY, et al. Endobronchial amyloidosis mimicking bronchial asthma: a case report and review of the literature. Open Med. 2016;11(1):174–177.