Abstract
Upper and lower airways are considered a unified morphological and functional unit, and the connection existing between them has been observed for many years, both in health and in disease. There is strong epidemiologic, pathophysiologic, and clinical evidence supporting an integrated view of rhinitis and asthma: united airway disease in the present review. The term “united airway disease” is opportune, because rhinitis and asthma are chronic inflammatory diseases of the upper and lower airways, which can be induced by allergic or nonallergic reproducible mechanisms, and present several phenotypes. Management of rhinitis and asthma must be jointly carried out, leading to better control of both diseases, and the lessons of the Allergic Rhinitis and Its Impact on Asthma initiative cannot be forgotten.
Introduction
Upper and lower airways are considered a unified morphological and functional unit, and the connection existing between them has been observed for many years, both in health and in disease.Citation1,Citation2 More than 2,000 years ago, Claudius Galenus studied the upper airway and paranasal sinuses as integral parts of the respiratory tract, and he assumed that rhinitis and asthma were caused by secretions dripping from the brain into the nose and lung.Citation3 More recently, the concept of united airway disease (UAD) was suggested.Citation4–Citation6
The nose is situated at the entrance of the airway and protects the lower airway from the harmful effects of the inspired air by acting as efficient air-conditioning. The nose warms, filters, and humidifies the inspired air so that clean air that is fully saturated with water vapor at a temperature of 37°C is delivered to the lungs. During nose breathing, the majority of particles with an aerodynamic equivalent diameter (AED) >15 μm are deposited in the upper respiratory tract. Particles with AEDs >2.5 μm are primarily deposited in the trachea and bronchi, whereas those with lower AEDs penetrate into the gas-exchange region of the lungs.Citation7 The nasal and bronchial mucosa present similarities, and one of the most important concepts regarding nose–lung interactions is the functional complementarity, which assigns the protector role of the nose to the lungs.Citation4 However, the functions of the upper airway and their interactions with the lower airway are much broader than merely air-conditioning.
The Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines published in 2001Citation1 achieved some goals: 1) development of a guideline proposing a standardized management plan for allergic rhinitis (AR), 2) establishment of the ARIA concept, 3) spreading of the guideline to general and specialist physicians, and 4) establishment of a multiprofessional forum to study rhinitis and asthma. There is strong epidemiologic, pathophysiologic, and clinical evidence supporting an integrated view of rhinitis and asthma: UAD in the present review. We can also consider UAD an airway-hypersensitivity syndrome, because rhinitis and asthma are chronic inflammatory diseases of the upper and lower airways, which are induced and reproduced by allergic or nonallergic hypersensitivity reactions, and present several phenotypes ().
Table 1 Airway hypersensitivity syndrome phenotypes
United airway disease: epidemiologic evidence
AR is the most common of all atopic diseases, and although it can develop at any age, most patients report the onset of symptoms before 30 years of age, making it the most common chronic disorder in children.Citation6 AR can be considered a major public health problem, due to its prevalence and impact on patients’ quality of life, work/school performance, and productivity economic burden.Citation4,Citation6 It is characterized by the classic symptoms of nasal itching, sneezing, rhinorrhea, and nasal obstruction. In addition, AR is associated with a variety of comorbidities, such as atopic dermatitis, sleep-disordered breathing, conjunctivitis, rhinosinusitis, otitis media, asthma, and emotional problems.Citation6,Citation8 At the same time, AR is a disease that is underdiagnosed and overlooked by patients and physicians.Citation9,Citation10
AR is considered a risk factor for developing asthma.Citation4,Citation6 Asthma is a heterogeneous disease characterized by chronic airway inflammation and hyperresponsiveness (AHR) to direct or indirect stimuli, which can persist even when symptoms are absent or lung function is normal but may normalize with treatment.Citation11 Asthma is defined by the history of episodic respiratory symptoms, such as wheeze, shortness of breath, chest tightness, and cough, and is associated with variable expiratory airflow limitation.Citation11
Allergic asthma is the most prevalent disease phenotype, which often begins in childhood and is associated with a personal and/or family history of allergic diseases, such as eczema and AR.Citation11 In the same way of the allergic phenotype, patients with non-AR (NAR) are at increased risk of developing nonallergic asthma. NAR presents later in life than AR and is not a single disorder but is composed of a heterogeneous group of diseases.Citation12
According to the International Study on Asthma and Allergy in Childhood, the prevalence of AR in Europe was found to be ∼25% and in Brazil ∼15%–20%. The prevalence of asthma worldwide was observed to be ∼20% (Global Initiative for Asthma) and 10%–20% in Brazil (Global Initiative for Asthma, ARIA). Countries with a very high prevalence of rhinitis had asthma prevalence ranging from 10% to 25%.Citation4,Citation11,Citation13
The management of asthma should include assessment of asthma control, future risks, and any comorbidity that could contribute to symptom burden and poor quality of life. The main associated comorbidities are rhinitis, rhinosinusitis, gastroesophageal reflux, obesity, obstructive sleep apnea, depression, and anxiety.Citation11 We evaluated the prevalence of comorbidities in patients with severe asthma and observed that rhinitis and gastroesophageal reflux disease were the most common, rhinitis being observed in 91% and gastroesophageal reflux disease in 71% of the asthmatic patients.Citation14
Interactions between the lower and the upper airways are well known and have been extensively studied since 1990. Over 80% of asthmatics have rhinitis, and 10%–40% of patients with rhinitis have asthma, suggesting the concept of “one airway, one disease”.Citation4 Rhinitis symptoms have been reported in 98.9% of allergic asthmatics and in 78.4% of nonallergic asthmatics. Furthermore, ∼30% of patients with only AR who do not have asthma present hyperresponsiveness to methacholine or histamine.Citation2,Citation4,Citation15 However, there are large differences in the magnitude of airway reactivity between patients with rhinitis and asthma. Patients with perennial rhinitis have greater bronchial reactivity than those with seasonal rhinitis, in whom the presence of hyperresponsiveness was observed especially during the pollen season.Citation4,Citation15,Citation16 AHR, which is a paramount feature of asthma, is a strong risk factor for the onset of asthma in patients presenting with AR.Citation6
Several studies suggest that AR and NAR are risk factors for new onset of asthma and persistence of asthma.Citation17,Citation18 In a cohort of 690 individuals with a follow-up of 23 years, it was observed that the incidence of asthma was 10.5% in subjects with rhinitis and 3.6% in those without rhinitis. Therefore, the development of asthma was tripled in rhinitis patients compared to those without rhinitis.Citation19 In the Tucson Epidemiologic Study of Obstructive Lung Diseases, the odds ratio for developing asthma was 2.59 (95% confidence interval 1.54–4.34) if rhinitis was present and 6.28 (95% confidence interval 4.01–9.82) in the presence of rhinitis plus sinusitis.Citation20 A European Survey confirmed the presence of perennial rhinitis as a major risk factor for asthma, with odds ratios of 11 for the atopic and 17 for the nonatopic phenotype.Citation21
Asthma and rhinitis share common risk factors and present common susceptibility to different agents, such as allergens (atopy) and infections.Citation4,Citation6 The presence of AHR and concomitant atopic manifestations in childhood increases the risk of developing asthma and should be recognized as a marker of prognostic significance, whereas the absence of these manifestations predicts a very low risk of future asthma.Citation22
United airway disease: pathophysiological evidence
The upper and lower respiratory tracts form a continuum, allowing the passage of air into and out of the lungs and sharing many anatomical and histological properties.Citation2 They share common structures, including the ciliary epithelium, basement membrane, lamina propria, glands, and goblet cells, forming the so-called united airway.Citation23 On the other hand, differences between the upper and lower airways do exist. Nasal mucosa, which is attached to bone, is enriched with vessels, whereas bronchial mucosa, which is attached to cartilage, is enriched with smooth-muscle cells.Citation24 Therefore, the major cause of airway obstruction, especially in the early phase of the allergic response, is different: upper airway obstruction is caused by vasodilation and edema, whereas lower airway obstruction arises from smooth-muscle constriction.Citation24
It is reasonable to think that because of anatomic reasons, the upper airway constitutes the first target for allergens and for physical and chemical environmental stimuli; therefore, they tend to be the first to be affected by the allergic airway disease, and if the intensity of this disease is low, the upper airway may be the only part of the respiratory tract that is affected. However, when the entire respiratory tract is involved, rhinosinusitis and asthma follow a parallel course.Citation25 Unfortunately, systematic research in this field has not been performed, and the evidence supporting these postulates is scarce.Citation25
UAD presents two main phenotypes: allergic (atopic or extrinsic) and nonallergic (nonatopic or intrinsic). With regard to asthma, most children and at least 50% of adults have the allergic phenotype, in which the disease is associated with allergic sensitization defined by the presence of serum-specific immunoglobulin (Ig)E antibodies and/or positive skin tests to the proteins of common inhaled allergens, such as house dust mites, animal dander, fungal spores, pollens, and cochroaches.Citation26 On the other hand, in nonallergic asthma, we do not observe IgE reactivity to allergens.Citation26 In the same way, there are two important phenotypes or rhinitis, allergic and nonallergic, both of them associated with increased prevalence of asthma.Citation27 We focus on the allergic pathophysiology of UAD.
AR and atopic asthma result from an IgE-mediated allergic reaction associated with airway inflammation of variable intensity.Citation4 Since the first class of Ig presented on the surface of B-cells is IgM, it is necessary that IgM is switched to IgE so that allergic inflammation can develop. Isotype switching to IgE requires antigen presentation and two other signals.Citation28 Signal one is provided by interleukin (IL)-4 and/or IL-13, acting through IL-4R and IL-13R via STAT6, which activate transcription to the IgE isotype. Signal two is provided mainly by ligation of CD40 on B-cells to CD40L on T-cells, which activates DNA-switch recombination.Citation28 The IgE-mediated immune response is initiated when the allergens are taken up by antigen-presenting cells via the cell-surface Ig receptor. Processed fragments are then presented in the context of major histocompatibility complex class II to T-helper (TH) cells, which recognize the allergen–major histocompatibility complex II composite and are activated. The allergen-specific TH2 cells produce IL-4 and IL-13, and express CD154, leading to IgE class switching.Citation26,Citation28 Although class switching is generally thought to occur in the germinal center of lymphoid tissues, it has also been reported to occur in the respiratory mucosa of patients with AR and atopic asthma and in the gastrointestinal tract in patients with food allergy.Citation28
Once IgE is produced by B-cells, the Ig will bind to the high-affinity receptor FcεR1 on mast cells and basophils.Citation28 In future, contacts with the polyvalent sensitizing allergen, these cells will be activated through FcεR1, initiating an immediate hypersensitivity reaction that is central in the pathogenesis of AR and allergic asthma.Citation28 The reaction has an immediate phase that is induced by the release of preformed and rapidly synthesized mediators from mast cells and basophils, resulting in erythema, edema, and itching in the skin, sneezing and rhinorrhea in the upper respiratory tract, and cough, bronchospasm, edema, and mucous secretion in the lower respiratory tract.Citation28 A late phase mediated by cytokines and chemokines and characterized by edema and leukocytic influx can occur 6–24 hours after the immediate phase. Eosinophils recruited mainly by IL-5 produced by TH2 cells stand out and are essential to maintain the chronic inflammatory process and tissue damage.Citation28
Eosinophil activation directly contributes to vasodilation, edema, mucous production, bronchoconstriction, and dysfunctional remodeling of the airway.Citation29 These processes are mainly induced by eosinophil-derived products, such as eosinophil peroxidase, which causes AHR and activates dendritic cells.Citation26 Murine studies have shown that eosinophils also contribute to airway-wall remodeling and subepithelial membrane thickening via the release of TGFβ.Citation26 Finally, similarly to neutrophils, upon activation, eosinophils undergo cytolysis and release mediators from the eosinophilic granules, such as eosinophil-derived neurotoxin, cationic proteins (eosinophil peroxidase), and major basic protein, which can damage structural cells of the airway.Citation26 It has been demonstrated that humans who died from asthma presented eosinophilic inflammation all over the respiratory tract, from nasal mucosa to lung tissue, showing that the airways really are unique, even in pathologic conditions.Citation30 Therefore, AR and asthma share immunopathological features, including a TH2-type immune response, thickness of the basement membrane, and goblet-cell hyperplasia.Citation24
In contrast to allergic UAD, the pathophysiology of which is well characterized, the etiology of and mechanisms involved in nonallergic UAD remain unclear. Some of the possibilities include allergy triggered by unknown antigens (fungi), persistent infection (caused by Chlamydia trachomatis, Mycoplasma spp., or viruses), and autoimmunity.
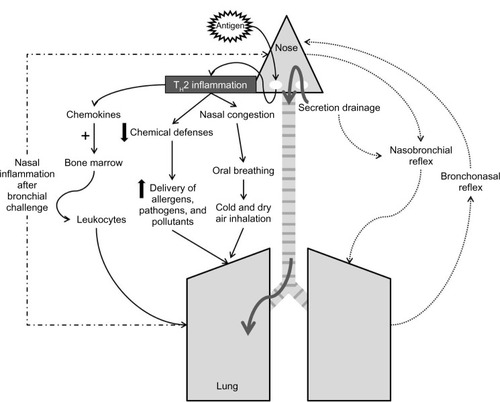
A central concept of UAD is the influence of the upper airway in the function of the lower airway, which is particularly evident and relevant in the allergic phenotype.Citation25 The pathological interactions between the upper and lower airways are summarized in and can be divided into:
air-conditioning
inflammation
neural reflexes.
Air-conditioning
Galen was the first to offer insights on the function of the nose as protector of the lower airway through its ability to clean, warm, and humidify inhaled air.Citation5 In addition, the nasal mucosa, with its abundant submucosal glands, takes part of the innate and adaptive immune defense by releasing antibacterial proteins, such as lysozyme and lactoferrin, chemical defenses, antioxidants, and secretory IgA, that can protect the lower airway from pathogens and allergens.Citation25 Patients with AR present partial or complete loss of function of the nose due to mucosal congestion, since nasal airways are bypassed during oral breathing.Citation25 In this situation, inhalation of cold and dry air may directly induce bronchoconstriction. Therefore, the lower airway would be quite “opened” to the entrance of allergens and pathogens, increasing the risk of asthma exacerbation.
Inflammation
Propagation of inflammation from the upper airway to lower airway may occur via postnasal drip and systemic circulation. The concept that inflammatory secretions from the upper airway of patients with rhinosinusitis or even with rhinitis are aspirated into the lower airway with adverse consequences has been viewed as one of the principal mechanisms for lower airway symptoms, especially after an upper respiratory infection.Citation25 It is quite possible that early morning coughing in individuals with rhinitis is associated with accumulation of secretions in the lower pharyngeal area stimulating irritant receptors.Citation25 It is questionable, however, whether these secretions can reach the intrathoracic lower airway in adequate quantities to alter their physiology and to generate exacerbations or chronically worsen lower airway function in patients with asthma.Citation25 The development of sinusitis in rabbits is associated with lower AHR, even after eliminating upper–lower airway communication with the use of an inflated endotracheal tube cuff.Citation31
On the other hand, there is good evidence that allergic inflammation developing in the respiratory mucosa may result in systemic inflammatory events.Citation25 Blood eosinophilia may be observed in patients with allergic asthma, and can be considered a biomarker of inflammation of the lower airway. There is less evidence that upper airway inflammation can lead to an increase in eosinophil blood count.Citation25,Citation32 Moreover, there is no experimental information indicating that nasal inflammation leads to systemic inflammatory signals that induce changes in lower airway physiology,Citation25 even though it seems reasonable to speculate that cytokines released in nasal mucosa could activate bone marrow with chemotaxis of white blood cells to both upper and lower airways.Citation25
The opposite direction for propagation of the inflammatory process, beginning in the lower airway and getting to the upper airway, has been postulated. A study showed that segmental bronchial allergen provocation in patients with nonasthmatic AR can induce nasal inflammation, nasal and bronchial symptoms, and reduction in pulmonary and nasal function.Citation33 However, there are recent data suggesting that this lung–nasal propagation of inflammation might not be relevant. In a very elegant murine model of allergic respiratory inflammation induced by ovalbumin, Balb/c mice were submitted to intratracheal challenge after sensitization by an intraperitoneal route. This provocation induced lung inflammation and AHR, but no signs of inflammation were found in the nose.Citation34
Neural reflexes
The existence of a nasobronchial reflex that originates from the sensory nerve endings in the nose, travels to the central nervous system through the trigeminal nerve, and follows an efferent pathway through the vagus nerve to produce airway smooth-muscle contraction has been under debate for years.Citation25 Despite being well documented in animal models, its existence and relevance in humans are still controversial.
Some studies performed in healthy individuals and asthmatics have demonstrated that lower airway resistance increased after nasal inhalation of cold and dry air.Citation35,Citation36 Another important study showed an increase in AHR after a nasal allergen provocation in asthmatics who had reported worsening of asthma symptoms following seasonal exacerbations of rhinitis.Citation37 The authors observed that none of the solutions delivered to the nose during allergen provocation could be detected in the lower airway, showing that the increase in AHR was not due to inadvertent inhalation of the allergen.Citation37 It is important to point out that the classic nasobronchial reflex is a component of the diving reflex.Citation38 Immersion of the head into cold water leads to immediate suppression of respiration (apnea), laryngospasm, and bronchoconstriction, in order to protect the lower airway from diving.Citation38 Nasal inhalation of dust, pollutants, and irritants can induce immediate bronchoconstriction with cessation of respiration in the expiratory phase, due to relaxation of inspiratory muscles.Citation38 Therefore, in individuals with allergic respiratory disease, this reflex could lead to an increase in asthma symptoms after nasal injury.
There is less evidence showing the occurrence of a bronchonasal reflex. It has been demonstrated that inhalation of ultrasonically nebulized distilled water increased nasal airway resistance in patients with AR, without the involvement of parasympathetic efferent reflexes, since patients did not present sneezing or rhinorrhea.Citation39 The clinical relevance of this bronchonasal reflex has yet to be demonstrated.
In conclusion, upper and lower airways seem to constitute a unique system, named “united airway”, that share similarities in terms of histology, physiology, and pathology. UAD is triggered by a TH2 immune response of the airway, leading to an extended inflammatory process that begins in nasal mucosa and ends in bronchioles and alveoli, particularly in symptomatic asthmatics.
United airway disease: clinical evidence
There is also clinical evidence supporting the concept of UAD. Studies have demonstrated that the presence of severe rhinitis is associated with an increased risk of asthmaCitation20 and in patients with asthma a less favorable evolution.Citation40–Citation42 It has also been shown that the treatment of rhinitis can be beneficial to the lower airway, reducing symptoms, emergency room visits, and hospitalizations, as well as the severity of bronchial hyperresponsiveness.Citation43–Citation47 In protocols of difficult-to-control asthma, rhinitis was included as one of the main comorbidities to be assessed and treated.Citation48
Therapy for UAD includes avoidance of relevant allergens and irritants, pharmacotherapy, and allergen-specific immunotherapy (SIT). Allergen avoidance has been suggested not only to prevent UAD onset and progression but also to reduce its burden, improving symptoms and quality of life. However, there is a lack of evidence supporting the effectiveness of environmental control.Citation4
The pharmacologic approach of AR includes antihistamines, oral leukotriene antagonists, and intranasal corticosteroids, the last being considered the most efficacious drug.Citation4 Agondi et al reported a decrease in asthma symptoms and AHR after intranasal corticosteroid treatment of rhinitis.Citation43 A recent meta-analysis confirmed the beneficial effect of intranasal steroids in AHR.Citation44 Oral and intranasal antihistamines, as well as leukotriene antagonists, are less effective than intranasal corticosteroids in improving the symptoms of AR.Citation49–Citation52 A protective effect of cetirizine against AHR measured 6 hours after nasal allergen challenge in patients with AR was shown.Citation53
Allergen-SIT is defined as a procedure to administer increasing amounts of specific allergens in patients diagnosed with IgE-mediated disease, in order to induce immune tolerance.Citation8,Citation54 Subcutaneous and sublingual IT can reduce symptoms of AR and need of reliever medication, as well as improve the control of comorbid conditions, such as asthma and conjunctivitis.Citation8,Citation55
Allergen-SIT is indicated in moderate/severe AR for which response to pharmacotherapy is inadequate. Other potential indications are adverse effects of medications, coexisting allergic asthma, bad adherence to therapy, and patient preference for IT instead of pharmacotherapy.Citation8,Citation56 Furthermore, SIT has been positioned as the only treatment that can modify the natural course of allergic diseases that includes prevention of new sensitizations and reduction of risk of developing asthma in subjects with AR, even after termination of treatment.Citation57–Citation61 The Preventive Allergy Treatment study was a randomized controlled trial that showed clinical benefits and a preventive effect on asthma development in children suffering from seasonal rhinoconjuctivitis undergoing subcutaneous IT with grass- and/or birch-allergen extracts for 3 years. This positive effect of SIT in preventing the progression from rhinitis to asthma was observed to persist in the same patients for 7 years after the termination of the treatment.Citation57–Citation59 Another study found that after 3 years of sublingual grass-pollen IT in children with AR, eight of 45 actively treated subjects and 18 of 44 controls developed asthma, with 3.8-fold more frequent development of asthma in the untreated patients.Citation62
Some studies have shown that IT was able to prevent new sensitizations in monosensitized individuals.Citation63 A research assessing the effects of subcutaneous IT in 147 house dust mite-monosensitized children over 5 years found similar results: 75.3% in the treated group and 46.7% in the control group had no new sensitizations.Citation64 A randomized controlled study involved 216 children with AR (with or without intermittent asthma) receiving drugs alone or drugs plus sublingual IT for 3 years showed new sensitizations in 34.8% of controls and in 3.1% of the IT group. Moreover, they demonstrated that this protective effect extended to AHR, which significantly decreased in the IT group.Citation65
Novel targeted therapeutic approaches using biological agents have been studied in the treatment of AR and allergic asthma, especially for the management of severe uncontrolled phenotypes. Among these, omalizumab, a humanized monoclonal antibody that binds circulating IgE and prevents its attachment to high-affinity IgE receptors, is available worldwide. Omalizumab improves both upper and lower airway diseases, reducing nasal and asthma symptoms, decreasing exacerbations, and improving quality of life.Citation66 Mepolizumab, a monoclonal antibody that blocks the binding of IL-5 to eosinophils, has also shown a beneficial effect on severe eosinophilic airway diseases, such as asthma and nasal polyposis in adults.Citation67–Citation69 Because these treatments have systemic effects, it is not possible to design a study to assess how much the improvement in asthma is, due to direct effects or indirect effects associated with rhinitis improvement.
The management of rhinitis may promote better adherence to therapy. It should consider severity and duration of the disease and patient preference, as well as the efficacy, availability, and cost of medications. Therefore, management of rhinitis and asthma must be jointly carried out, including environmental control, pharmacotherapy, and SIT.
Conclusion
The treatment of rhinitis is indispensable in patients with asthma, since it leads to better control of both diseases, and the lessons of the ARIA initiative cannot be forgotten. Further studies regarding UAD are needed to better understand the interactions between the upper and lower airways, but there is no doubt that rhinitis and asthma have to be studied and managed in an integrated manner.
Disclosure
The authors report no conflicts of interest in this work.
References
- BousquetJvan CauwenbergePKhaltaevNAllergic rhinitis and its impact on asthmaJ Allergy Clin Immunol20011085 SupplS147S33411707753
- CingiCMulukNBCobanogluBÇatliTDikiciONasobronchial interactionWorld J Clin Cases20153649950326090369
- YusufOMStreptococcus pyogenes upper respiratory infections and their effect on atopic conditionsPrim Care Respir J201221212612722596247
- BousquetJKhaltaevNCruzAAAllergic rhinitis and its impact on asthma (ARIA) 2008Allergy200863Suppl 86816018331513
- KalinerMMcFaddenFBronchial asthmaSamterSImmunological Diseases4th edBostonLittle Brown198810671118
- CiprandiGCaimmiDMiraglia Del GiudiceMLa RosaMSalpietroCMarsegliaGLRecent developments in united airways diseaseAllergy Asthma Immunol Res20124417117722754709
- EcclesCAnatomy and physiology of the nose and control of nasal airflowAdkinsonNFYungingerJWBusseWWMiddleton’s Allergy: Principles and Practice7th edPhiladelphiaElsevier2008701711
- SeidmanMDGurgelRKLinSYClinical practice guideline: allergic rhinitisOtolaryngol Head Neck Surg2015152Suppl 1S1S4325644617
- CeledonJCPalmerLJWeissSAsthma, rhinitis, and skin test reactivity to aeroallergens in families of asthmatic subjects in Anqing, ChinaAm J Respir Crit Care Med200116351108111211316644
- GagaMLambrouPPapageorgiouNEosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitisClin Exp Allergy200030566366910792358
- FitzGeraldJBatemanEBouletLGlobal strategy for asthma management and prevention2015 Available from: http://www.ginasthma.org/documents/4Accessed June 17, 2015
- LiebermanPPattanaikDNonallergic rhinitisCurr Allergy Asthma Rep201414643924715611
- VannaATYamadaEArrudaLKNaspitzCKSoléDInternational Study of Asthma and Allergies in Childhood: validation of the rhinitis symptom questionnaire and prevalence of rhinitis in schoolchildren in São Paulo, BrazilPediatr Allergy Immunol20011229510111338293
- BisaccioniCAunMVCajuelaEKalilJAgondiRCGiavina-BianchiPComorbidities in severe asthma: frequency of rhinitis, nasal polyposis, gastroesophageal reflux disease, vocal cord dysfunction and bronchiectasisClinics200964876977319690661
- CrapoROCasaburiRCoatesALGuidelines for methacholine and exercise challenge testing – 1999Am J Respir Crit Care Med2000161130932910619836
- MadoniniEBriatico-VangosaGPappacodaAMaccagniGCardaniASaporitiFSeasonal increase of bronchial reactivity in allergic rhinitisJ Allergy Clin Immunol19877923583633819219
- ShaabanRZureikMSoussanDRhinitis and onset of asthma: a longitudinal population-based studyLancet200837296431049105718805333
- RochatMKIlliSEgeMJAllergic rhinitis as a predictor for wheezing onset in school-aged childrenJ Allergy Clin Immunol201012661170117521051078
- SettipaneRJHagyGWSettipaneGALong-term risk-factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college studentsAllergy Proc199415121258005452
- GuerraSSherrillDLMartinezFDBarbeeRARhinitis as an independent risk factor for adult-onset asthmaJ Allergy Clin Immunol2002109341942511897985
- LeynaertBBousquetJNeukirchCPerennial rhinitis: an independent risk factor for asthma in nonatopic subjects – results from the European Community Respiratory Health SurveyJ Allergy Clin Immunol19991042 Pt 130130410452748
- PorsbjergCvon LinstowMLUlrikCSNepper-ChristensenSBackerVRisk factors for onset of asthma: a 12-year prospective follow-up studyChest2006129230931616478846
- FengCHMillerMDSimonRAThe united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitisAm J Rhinol Allergy201226318719022643942
- OkanoMKariyaSOhtaNImotoYFujiedaSNishizakiKAssociation and management of eosinophilic inflammation in upper and lower airwaysAllergol Int201564213113825838087
- TogiasAMechanisms of nose-lung interactionAllergy199954Suppl 579410510565484
- LambrechtBNHammadHThe immunology of asthmaNat Immunol2015161455625521684
- ChawesBLBønnelykkeKKreiner-MøllerEBisgaardHChildren with allergic and nonallergic rhinitis have a similar risk of asthmaJ Allergy Clin Immunol2010126356757320816191
- StoneKDPrussinCMetcalfeDDIgE, mast cells, basophils, and eosinophilsJ Allergy Clin Immunol20101252 Suppl 2S73S8020176269
- RosenbergHFDyerKDFosterPSEosinophils: changing perspectives in health and diseaseNat Rev Immunol201313192223154224
- de Magalhães SimõesSdos SantosMAda Silva OliveiraMInflammatory cell mapping of the respiratory tract in fatal asthmaClin Exp Allergy200535560261115898982
- BrugmanSMLarsenGLHensonPMHonorJIrvinCGIncreased lower airways responsiveness associated with sinusitis in a rabbit modelAm Rev Respir Dis199314723143208430954
- ChungKFAdcockIMClinical phenotypes of asthma should link up with disease mechanismsCurr Opin Allergy Clin Immunol2015151566225504141
- BraunstahlGJKleinjanAOverbeekSEPrinsJBHoogstedenHCFokkensWJSegmental bronchial provocation induces nasal inflammation in allergic rhinitis patientsAm J Respir Crit Care Med200016162051205710852787
- XieJXXiYZhangQAn intratracheal challenge murine model of asthma: can bronchial inflammation affect the nose?Allergy Asthma Immunol Res201571768225553266
- FontanariPBurnetHZattara-HartmannMCJammesYChanges in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individualsJ Appl Physiol (1985)1996814173917438904594
- FontanariPZattara-HartmannMCBurnetHJammesYNasal eupnoeic inhalation of cold, dry air increases airway resistance in asthmatic patientsEur Respir J19971010225022549387948
- CorrenJAdinoffADIrvinCGChanges in bronchial responsiveness following nasal provocation with allergenJ Allergy Clin Immunol19928926116181740589
- BaraniukJNMerckSJNasal reflexes: implications for exercise, breathing, and sexCurr Allergy Asthma Rep20088214715318417057
- GhersonGMoscatoGVidiISalvaterraACanduraFNonspecific nasal reactivity: a proposed method of studyEur J Respir Dis198669124283743685
- AntonicelliLMicucciCVoltoliniSRelationship between ARIA classification and drug treatment in allergic rhinitis and asthmaAllergy20076291064107017686109
- HalpernMTSchmierJKRichnerRGuoCTogiasAAllergic rhinitis: a potential cause of increased asthma medication use, costs, and morbidityJ Asthma200441111712615046386
- GreisnerWASettipaneRJ3rdSettipaneGAThe course of asthma parallels that of allergic rhinitis: a 23-year follow-up study of college studentsAllergy Asthma Proc200021637137511191104
- AgondiRCMachadoMLKalilJGiavina-BianchiPIntranasal corticosteroid administration reduces nonspecific bronchial hyperresponsiveness and improves asthma symptomsJ Asthma200845975475718972290
- LohiaSSchlosserRJSolerZMImpact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysisAllergy201368556957923590215
- Crystal-PetersJNeslusanCCrownWHTorresATreating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visitsJ Allergy Clin Immunol20021091576211799366
- StelmachRdo PatrocínioTNunesMRibeiroMCukierAEffect of treating allergic rhinitis with corticosteroids in patients with mild-to-moderate persistent asthmaChest200512853140314716304254
- TaramarcazPGibsonPGIntranasal corticosteroids for asthma control in people with coexisting asthma and rhinitisCochrane Database Syst Rev20034CD00357014583983
- Giavina-BianchiPAunMVBisaccioniCAgondiRKalilJDifficult-to-control asthma management through the use of a specific protocolClinics (Sao Paulo)201065990591821049219
- BenningerMFarrarJRBlaissMEvaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by classAnn Allergy Asthma Immunol20101041132920143641
- YamamotoHYonekuraSSakuraiDComparison of nasal steroid with antihistamine in prophylactic treatment against pollinosis using an environmental challenge chamberAllergy Asthma Proc201233539740323026181
- WilsonAMO’ByrnePMParameswaranKLeukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysisAm J Med2004116533834414984820
- NayakALangdonRBMontelukast in the treatment of allergic rhinitis: an evidence-based reviewDrugs200767688790117428106
- AubierMNeukirchCPeifferCMelacMEffect of cetirizine on bronchial hyperresponsiveness in patients with seasonal allergic rhinitis and asthmaAllergy2001561354211167350
- ZuberbierTBachertCBousquetPJGA2LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthmaAllergy201065121525153021039596
- LinSErekosimaNSuarez-CuervoCAllergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review Rockville (MD)Agency for Healthcare Research and Quality2013
- WallaceDVDykewiczMSBernsteinDIThe diagnosis and management of rhinitis: an updated practice parameterJ Allergy Clin Immunol20081222 SupplS1S8418662584
- NiggemannBJacobsenLDreborgSFive-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in childrenAllergy200661785585916792584
- MöllerCDreborgSFerdousiHAPollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-Study)J Allergy Clin Immunol2002109225125611842293
- JacobsenLNiggemannBDreborgSSpecific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT studyAllergy200762894394817620073
- PolosaRLi GottiFManganoGEffect of immunotherapy on asthma progression, BHR and sputum eosinophils in allergic rhinitisAllergy200459111224122815461606
- MorjariaJBCarusoMRosaliaERussoCPolosaRPreventing progression of allergic rhinitis to asthmaCurr Allergy Asthma Rep201414241224408536
- NovembreEGalliELandiFCoseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitisJ Allergy Clin Immunol2004114485185715480326
- Des RochesAParadisLMenardoJLBougesSDaurésJPBousquetJImmunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in childrenJ Allergy Clin Immunol19979944504539111487
- InalAAltintasDUYilmazMKarakocGBKendirliSGSertdemirYPrevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust miteJ Investig Allergol Clin Immunol20071728591
- MarognaMTomassettiDBernasconiAPreventive effects of sublingual immunotherapy in childhood: an open randomized controlled studyAnn Allergy Asthma Immunol2008101220621118727478
- GevaertPCalusLVan ZeleTOmalizumab is effective in allergic and nonallergic patients with nasal polyps and asthmaJ Allergy Clin Immunol20131311110e1116e123021878
- RobinsonDSMepolizumab for severe eosinophilic asthmaExp Rev Respir Med2013711317
- PavordIDKornSHowarthPMepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trialLancet2012380984265165922901886
- GevaertPVan BruaeneNCattaertTMepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposisJ Allergy Clin Immunol20111285989995e1e821958585