Abstract
Asthma and chronic rhinosinusitis are heterogeneous airway diseases of the lower and upper airways, respectively. Molecular and cellular studies indicate that these diseases can be categorized into unique endotypes, which have therapeutic implications. One such endotype is aspirin-exacerbated respiratory disease (AERD), which encompasses the triad of asthma, aspirin (or nonsteroidal anti-inflammatory drug) hypersensitivity, and nasal polyposis. AERD has unique pathophysiological features that distinguish it from aspirin-tolerant asthma and other forms of chronic rhinosinusitis. This review details molecular and cellular features of AERD and highlights current and future therapies that are based on these insights.
Introduction
The constellation of findings of asthma, aspirin (and nonselective nonsteroidal anti-inflammatory drug [NSAID]) hypersensitivity, and nasal polyposis encompasses the phenomena known as aspirin-exacerbated airway disease (AERD). Described by Samter and BeersCitation1 in 1968, and formally called Samter’s triad, the defining feature of this disease is the worsening of respiratory symptoms following ingestion of cyclooxygenase-1 (COX-1) inhibitors such as aspirin and nonselective NSAIDs. Despite being a relatively minor fraction of all asthma and sinusitis cases, aspirin hypersensitivity is associated with increased severity of both upper and lower airway diseases. A recent meta-analysis indicates that 7% of asthmatics are aspirin-sensitive but that the prevalence doubles in severe asthmatics.Citation2 Recent insights into AERD pathophysiology suggest that it represents a unique phenotype of airway disease. Unlike allergic asthma, this disease tends to develop in adulthood, occurs in patients without an atopic history, and displays a slightly higher prevalence in females.Citation3–Citation6 Hallmark features include eosinophilia, expression of Th2 cytokines, and elevated levels of cysteinyl leukotrienes (CysLTs).Citation7–Citation9 Recently, a prominent role for interferon (IFN)-γ in the maturation of eosinophil progenitors in AERD has been proposed.Citation10 Based on these characteristics, it is not surprising that steroids and leukotriene pathway modifiers are the mainstays of therapy. Perhaps paradoxically, aspirin desensitization is a keystone therapy for patients who can tolerate the procedure. This review highlights some recent advances into the cellular and molecular mechanisms involved in AERD.
Role of eicosanoids
Perhaps most central to the underlying pathophysiology of AERD is the dysregulation of pro- and anti-inflammatory lipid mediators. For example, it is well established that proinflammatory CysLTs are markedly upregulated in AERD, whereas the prostanoid prostaglandin E2 (PGE2) is constitutively decreased.Citation9,Citation11–Citation13 The fundamental factors that contribute to this underlying dysregulation remain a source of active investigation, although work discussed later suggest a role for both Th1 and Th2 cytokines.
The enzymes COX and 5-lipoxygenase (5-LO) act as critical switches for regulating downstream production of prostanoids and leukotrienes (), respectively, from a common pool of arachidonic acid precursors. Expression and regulation of these and other enzymes involved in eicosanoid metabolism are thought to be important in AERD pathogenesis.
Figure 1 IL-4 and IFN-γ favor leukotriene pathway.
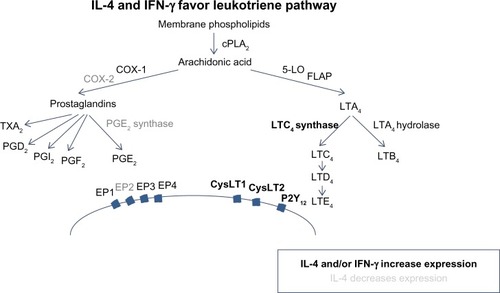
COX-1 and COX-2 are prostaglandin synthases that convert arachidonic acid into prostaglandin H2, which acts as a gateway to the synthesis of a number of prostanoids including other prostaglandins, thromboxanes, and prostacyclines (). It has been demonstrated that expression of COX-2, but not COX-1, is markedly reduced in AERD.Citation12,Citation14,Citation15 Given that microsomal prostaglandin E2 synthase (mPGES-1), one of the downstream enzymes that generates PGE2, is functionally coupled to COX-2, it is not surprising that PGE2 is decreased in AERD.Citation16 This prostaglandin has pleiotropic effects mediated by four different G-protein-coupled receptors (EP1–EP4); however, acting via the EP2 receptor, it exerts anti-inflammatory effects relevant to AERD by impairing eosinophil activation and mast cell degranulation.Citation17–Citation19 The importance of this pathway has been further demonstrated as PGE2 inhalation into the lung protects against aspirin-induced bronchoconstriction.Citation20,Citation21
In contrast to the impaired prostaglandin production, products of the 5-lipoxygenase pathway are markedly increased in AERD (). This reflects increased expression of 5-lipoxygenase itself as well as the downstream enzyme leukotriene C4 synthase (LTC4S). This has been demonstrated in both lung and nasal polyps, largely localizing to mast cell and eosinophil populations.Citation11,Citation12,Citation22 Initially described as the slow-reacting substance of anaphylaxis, studies quickly discerned three distinct lipid mediators that contributed to bronchoconstriction observed in experimental models.Citation23 LTC4 is the first product of this pathway but is readily converted to LTD4, which itself is rapidly metabolized to the more stable LTE4. Levels of LTE4 are elevated in the urine and respiratory secretions of AERD subjects and are further increased upon aspirin provocation.Citation24,Citation25 Signaling is mediated, at least in part, through the G-protein-coupled receptors CysLT1 and CysLT2. Of these, CysLT1 is the high-affinity receptor preferentially binding LTD4, whereas CysLT2 recognizes both LTC4 and LTD4 with equal affinity, albeit at a 10-fold lower affinity than the CysLT1 receptor.Citation26 Despite being able to mediate bronchoconstriction and other proinflammatory activities, LTE4 does not bind avidly to either of these receptors.Citation27–Citation29 CysLT1, but not CysLT2, is upregulated in leukocytes from AERD patients as compared with aspirin-tolerant controls.Citation30,Citation31 A prominent role for CysLT1 in this disease is further supported by its expression on airway smooth muscle and the capacity of CysLT1 receptor antagonists to ameliorate aspirin-induced bronchoconstriction.Citation32–Citation34 The importance of LTE4 has proved challenging, in part, owing to the elusive search for its cognate receptor. Recent reports suggest that LTE4 signals through P2Y12 or GPR99, although additional studies are needed to clarify their role in AERD.Citation35–Citation37
In summary, AERD reflects a state of dysregulated eicosanoid metabolism, with a pathway programmed in favor of enhanced CysLT expression and signaling, against a backdrop of impaired PGE2 expression. Collectively, this action will lead to airway constriction and stimulation of eosinophil and mast cell degranulation, all key factors in AERD pathogenesis. Aspirin or nonselective NSAIDs further contribute to this dysregulation via COX-1 blockade and forcing arachidonic acid into the leukotriene synthesis pathway.
Cells and cytokines
One of the defining features of AERD is the presence of nasal polyps with pronounced eosinophilic infiltrate.Citation7,Citation8,Citation12,Citation22 Additional cellular players include mast cells, macrophages, and T-cells.Citation38,Citation39 Consistent with the eosinophilic predominance is the marked upregulation of eosinophilic cationic protein (ECP), which is a marker of eosinophilic degranulation. A recent study has shown a greater increase in ECP levels, more than eosinophil numbers would predict, suggesting an increased activation state of eosinophils in AERD.Citation40 This is especially interesting as eosinophilia is also pronounced in subjects with certain forms of aspirin-tolerant polyposis. In the same study, the authors reported that chemokines including CCL11 (eotaxin-1) and CCL24 (eotaxin-2), in addition to other mediators involved in proliferation and recruitment (such as interleukin [IL]-5), are not increased in AERD compared with relevant acetyl-salicylic-acid-tolerant polyp subjects.Citation40 Notably though, these mediators are increased in AERD compared with polyp-free sinus disease.
Most studies have shown a cytokine and chemokine milieu consistent with type 2 immunity; however, recent work from our group and others suggests an important role for IFN-γ as well.Citation10,Citation41 We have shown that IL-4 and IFN-γ mRNA, but not IL-5 or IL-13, are upregulated in AERD polyp tissue and that eosinophils are the primary source of these cytokines.Citation10 Interestingly, both IL-4 and IFN-γ increase LTC4S expression on mast cells and eosinophils, providing a mechanistic link for the upregulation of CysLTs seen in AERD.Citation42 For IFN-γ, the effect was not direct but was mediated by promoting the maturation of eosinophils as measured by the upregulation of CCR3 and Siglec-8.Citation6,Citation10 Additionally, IL-4 has also been shown to inhibit COX-2 and mPGES-1.Citation43 Taken together, this suggests that AERD may represent a “mixed” Th1/Th2 disease, where IFN-γ plays an important role in pathogenesis. The mixed nature of cytokine expression fits with the observation that AERD subjects are not atopic as in classical Th2 allergic diseases. The precise role of IFN-γ remains to be determined, because some other studies have not supported this finding, which may reflect the different techniques used to detect expression.Citation40,Citation44
Recent work suggests that IL-33 may be another important cytokine involved in AERD pathogenesis. This alarmin-like cytokine is upregulated in the airway epithelial layer and has been shown to be dependent on CysLT expression in mouse models. It is able to function as a mediator of mast cell activation.Citation45 This is intriguing, because as an innate cytokine, IL-33 represents another mechanism to explain eosinophilic and mast cell involvement in the absence of IgE-mediated atopy.
Additional factors that have recently been shown to be preferentially increased in AERD tissue include granulocyte–macrophage colony-stimulating factor (GM-CSF) and MCP-1, while tissue plasminogen activator (tPA) was decreased.Citation40 Although the pathophysiological relevance of these findings is not clear, prior investigations offer hints. GM-CSF has previously been shown to enhance eosinophil survival in tissues in the absence of IL-5, and GM-CSF-mediated eosinophilic activation has recently been demonstrated in another mucosal inflammatory disease, colitis.Citation46,Citation47 Reduced tPA levels suggest a possible role for the fibrinolytic pathway to contribute to fibrin deposition and thus the remodeling seen in AERD IFN-γ.Citation48 In a similar vein, a role for platelet-associated inflammation has been described in AERD.Citation49 Interestingly, platelet-associated leukocytes were increased in AERD and were shown to enhance LTC4S expression.Citation49
Genetic studies
Microarray and genome-wide association studies have been employed to identify genes relevant to AERD pathogenesis. Stankovic et alCitation50 reported periostin upregulation in chronic rhinosinusitis polyps, although the levels were notably similar in aspirin-tolerant and -sensitive subjects in that study. Follow-up work assessing periostin protein levels with ELISA, however, did show a significant increase in AERD versus aspirin-tolerant asthma and also showed correlation in periostin levels with severity of sinus disease.Citation51 This finding is consistent with recent work establishing periostin as a potential biomarker for Th2 inflammation, congruent with the fact that it is upregulated by IL-4 and IL-13 and is associated with tissue eosinophilia.Citation52 Expression profiling of peripheral blood mononuclear cells from AERD versus aspirin-tolerant asthmatics demonstrated ten genes that exhibited >8-fold change. Interestingly, traditional cytokine and chemokine genes related to type 2 immunity were not represented among these. The two genes that were validated with high sensitivity and specificity were CNKSR family member 3 (CNKSR3) and spectrin β nonerythrocytic 2 (SPTBN2), both of whose functions in AERD remain elusive.Citation53
An association to human leukocyte antigen (HLA) has been described in three separate genome-wide association studies of Korean subjects, with significant association of polymorphisms within the HLA-DPB1 gene being identified.Citation54–Citation56 The prevalence of these polymorphisms in other populations is not clear, although an earlier study in Polish subjects showed a similar result.Citation57 Taken together, these studies have demonstrated novel markers and factors that could be important in AERD pathogenesis, although further investigation will be needed to assess their role in disease pathogenesis and possible utility as biomarkers.
Current and future therapies
In addition to standard asthma therapies such as inhaled corticosteroids and β2-agonists, a keystone of AERD management involves leukotriene blockade. Two classes of medications are in clinical use – the leukotriene receptor antagonists, which include montelukast and zafirlukst, and the 5-LO inhibitor zileuton. Controlled, prospective, placebo-controlled studies with montelukast and zileuton have both shown efficacy in aspirin-sensitive asthma as measured by improved forced expiratory volume in 1 second (FEV1) scores, decreased use of rescue inhalers, and an increase in asthma quality-of-life measures.Citation58,Citation59 Based on their unique mechanism of actions, there is some thought that they may act in an additive way to ameliorate symptoms, although no prospective combined trials, or head-to-head trials, have been conducted in the AERD population. A single head-to-head trial in asthmatics, which did not address aspirin-sensitive asthma, specifically demonstrated modest superiority of zileuton compared to montelukast.Citation60 Leukotriene receptor antagonists are often used as first-line therapy based on practical considerations (as they are less expensive) and have fewer side effects; however, zileuton may have superior efficacy in AERD based on patient survey data.Citation61 Zileuton impairs all leukotriene production by virtue of 5-LO inhibition, whereas the clinically available leukotriene receptor antagonists selectively target CysLT1. As discussed previously, although CysLT1 is the high-affinity receptor for LTD4, leukotrienes also signal via CysLT2 and other LTE4 receptors. Given that CysLT2, like CysLT1, is upregulated in nasal polyps, this provides a mechanistic explanation as to why zileuton would have a broader antileukotriene activity than selective CysLT1 agents.Citation62 Taken together, CysLT2 and other recently described putative leukotriene receptors, such as GPR99 and P2Y12, are potential targets for future research efforts in AERD-directed therapeutics.
On the one hand, while aspirin can trigger acute respiratory symptoms, aspirin desensitization followed by daily aspirin therapy leads to improved long-term symptoms in AERD subjects. The protocol is conducted by starting with small doses of aspirin and gradually achieving doses of 650–1,300 mg daily. Notably, although the approach is similar to traditional allergy desensitization, which addresses IgE-mediated reactions, the pathophysiology is distinct. The most significant improvements with aspirin desensitization relate to upper airway symptoms including smell and decreased polyp formation; however, asthma severity, use of steroids, and hospitalizations are also lessened.Citation63–Citation66 The beneficial mechanisms of aspirin are not entirely clear, though aspirin likely modulates multiple pathways involved in AERD pathogenesis. Our group and others have shown that aspirin blocks IL-4-activated signal transducer and activator of transcription 6 (STAT6), which is a key transcriptional regulator of CysLT1 and has known binding sites in the LTC4S promoter.Citation43,Citation67 This corresponds with an earlier work, which showed downregulation of CysLT1 on leukocytes from nasal mucosa following aspirin desensitization.Citation31 Another study has shown downregulation of IL-4 and MMP-9 levels following desensitization.Citation68
Despite the use of leukotriene pathway inhibitors and aspirin desensitization, AERD remains a disease with high morbidity.Citation61 Advances in AERD pathophysiology, however, offer promising future targets to better address this disease. Multiple monoclonal antibodies targeting immune pathways are in development or have clinical approval for related conditions. Mepolizumab is an anti-IL-5 monoclonal antibody that has been approved for severe eosinophilic asthma and has been shown in a small study to decrease nasal polyposis.Citation69 As previously discussed, IL-5 is not a central mediator of AERD as compared to aspirin-tolerant chronic sinusitis; however, it is clearly elevated in AERD compared with healthy controls or those with chronic sinusitis without polyps, suggesting it may yet be a fruitful target.Citation40 Recently, another anti-IL-5 drug, reslizumab, has been approved and may offer benefit similar to mepolizumab. Dupilumab is an IL-4 α receptor antagonist that blocks both IL-4 and IL-13 signaling. Currently in clinical trials, it has shown benefit in a study of moderate-to-severe eosinophilic asthmatics in decreasing asthma exacerbations and improving FEV1.Citation70 Based on the demonstrated role of IL-4 in AERD, this represents a potentially promising new therapy for aspirin-sensitive asthmatics. Another biologic with proven efficacy in allergic asthma is the anti-IgE monoclonal antibody omalizumab. Interestingly, despite the fact that IgE does not feature prominently in AERD pathogenesis, there have been multiple case reports of omalizumab benefiting AERD patients.Citation71–Citation73 Although the mechanism of action is not clear, these case reports indicate that further prospective studies should be considered. In addition to these biologics already in clinical use or preclinical development, future research efforts targeting novel pathways such as IL-33 or TSLP signaling should also be explored.
Conclusion
AERD is a disease of the upper and lower airways, featuring prominent eosinophilia and eicosanoid dysregulation. In addition to traditional type 2 immune mediators, recent work points to a role for IFN-γ.Citation10 Current therapies include leukotriene modifiers and aspirin desensitization. Although AERD represents a minor constituent of all asthma, it represents a severe subset with high morbidity that warrants ongoing efforts at the level of basic research and clinical trials.
Novel aspects
AERD is a disease characterized by the overexpression and overresponsiveness of the cysteinyl leukotriene pathway. Additionally, there is underproduction of the protective prostaglandin E2. This creates a situation where following ingestion of aspirin or other NSAIDs, there is unconstrained release of inflammatory mediators that lead to symptoms associated with disease. Therapies that target the leukotriene pathway are somewhat effective in providing relief; however, their effect is not complete. Newer biologic therapies aimed at targeting the inflammatory cells themselves may offer enhanced benefit. Two of these that may have an immediate impact are the recently approved drugs mepolizumab and reslizumab, which target eosinophils in uncontrolled asthma. Although not approved for sinusitis, given the asthma component of AERD, these drugs will likely be prescribed, and it will be interesting to see if they improve sinus symptoms and disease severity as eosinophil numbers are decreased.
Disclosure
The authors report that John W Steinke is co-primary investigator on an NIH grant to study sinusitis. The authors report no other conflicts of interest in this work.
References
- SamterMBeersRFJrIntolerance to aspirin. Clinical studies and consideration of its pathogenesisAnn Intern Med19686859759835646829
- RajanJPWineingerNEStevensonDDWhiteAAPrevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literatureJ Allergy Clin Immunol20151353676.e1681.e125282015
- SzczeklikANizankowskaEClinical features and diagnosis of aspirin induced asthmaThorax200055S42S4410992556
- VallyHTaylorMLThompsonPJThe prevalence of aspirin intolerant asthma (AIA) in Australian asthmatic patientsThorax20025756957412096197
- MasciaKBorishLPatrieJHuntJPhillipsCDSteinkeJWChronic hyperplastic eosiniphilic sinusistis as a predictor of aspirin-exacerbated respiratory diseaseAnn Allergy Asthma Immunol20059465265715984597
- SteinkeJWBorishLFactors driving the aspirin exacerbated respiratory disease phenotypeAm J Rhinol Allergy2015291354025590316
- PayneSCEarlySBHuyettPHanJKBorishLSteinkeJWEvidence for distinct histologic profile of nasal polyps with and without eosinophiliaLaryngoscope2011121102262226721898422
- BachertCWagenmannMHauserURudackCIL-5 synthesis is upregulated in human nasal polyp tissueJ Allergy Clin Immunol1997998378429215253
- SampsonAPCowburnASSladekKProfound overexpression of leukotriene C4 synthase in bronchial biopsies from aspirin-intolerant asthmatic patientsInt Arch Allergy Immunol19971133553579130576
- SteinkeJWLiuLHuyettPNegriJPayneSCBorishLProminent role of interferon-γ in aspirin-exacerbated respiratory diseaseJ Allergy Clin Immunol2013132856.e3865.e323806637
- CowburnASSladekKSojaJOverexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthmaJ Clin Invest19981018348469466979
- Perez-NovoCAWateletJBClaeysCvan CauwenbergePBachertCProstaglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposisJ Allergy Clin Immunol20051151189119615940133
- SchmidMGodeUSchaferDWigandMEArachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmaticsActa Otolaryngol199911927728010320091
- PicadoCFernandez-MorataJCJuanMCyclooxygenase-2 mRNA is down expressed in nasal polyps from aspirin-sensitive asthmaticsAm J Respir Crit Care Med199916029129610390414
- GosepathJBriegerJMannWJNew immunohistologic findings on the differential role of cyclooxygenase 1 and cyclooxygenase 2 in nasal polposisAm J Rhinol20051911111615921208
- MurakamiMNakashimaKKameiDCellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2J Biol Chem200327839379373794712835322
- SturmEMSchratlPSchuligoiRProstaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptorsJ Immunol2008181107273728318981149
- KayLJYeoWWPeachellPTProstaglandin E2 activated EP2 receptors to inhibit human lung mast degranulationBr J Pharmacol200614770771316432506
- SteinkeJWEditorial: Yin-Yang of EP receptor expressionJ Leukoc Biol20129261129113123204260
- SestiniPArmettiLGambaroGInhaled PgE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthmaAm J Respir Crit Care Med19961535725758564100
- FengCBellerEMBaggaSBoyceJAHuman mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responsesBlood20061073243325016357326
- SteinkeJWBradleyDArangoPCytseinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: importance to eosinophilia and asthmaJ Allergy Clin Immunol200311134234912589355
- BrocklehurstWEThe release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shockJ Physiol196015141643513804592
- ChristiePETagariPFord-HutchinsonAWUrinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjectsAm Rev Respir Dis1991143102510291850964
- SladekKSzczeklikACysteinyl leukotrienes overproduction and mast cell activation in aspirn-provoked bronchospasm in asthmaEur Respir J199363913998386106
- AustenKFMaekawaAKanaokaYBoyceJAThe leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implicationsJ Allergy Clin Immunol2009124340641419647860
- ArmJPO’HickeySSpurBWLeeTHAirway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthmaAm Rev Respir Dis19891401481532546469
- ChristiePESchmitz-SchumannMSpurBWLeeTHAirway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjectsEur Respir J19936146814738112440
- LaitinenLALaitinenAHaahtelaTVilkkaVSpurBWLeeTHLeukotriene E4 and granulocytic infiltration into asthmatic airwaysLancet19933419899908096945
- CorriganCMallettKYingSExpression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitisJ Allergy Clin Immunol2005115231632215696087
- SousaARParikhAScaddingGCorriganCJLeeTHLeukotriene-receptor expression on nasal mucosal inflammatory cells in asprin-sensitive rhinosinusitisN Engl J Med20023471493149912421891
- LynchKRO’NeillGPLiuQCharacterization of the human cysteinyl leukotriene CysLT1 receptorNature1999399673878979310391245
- ChristiePESmithCMLeeTHThe potent and selective sulfidopeptide leukotriene antagonist, SK&F 104353, inhibits aspirin-induced asthmaAm Rev Respir Dis19911449579581928974
- DahlenBTreatment of aspirin-intolerant asthma with antileukotrienesAm J Respir Crit Care Med2000161SupplS137S14110673243
- ParuchuriSTashimoHFengCLeukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptorJ Exp Med2009206112543255519822647
- KanaokaYMaekawaAAustenKFIdentification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligandJ Biol Chem201328816109671097223504326
- MaekawaAKanaokaYXingWAustenKFFunctional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptorsProc Natl Acad Sci U S A200810543166951670018931305
- KowalskiMLGrzegorczykJPawliczakRKornatowskiTWagrowska-DanilewiczMDanilewiczMDecreased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivityAllergy200257649350012028114
- VargaEMJacobsonMRMasuyamaKInflammatory cell populations and cytokine mRNA expression in the nasal mucosa in aspirin-sensitive rhinitisEur Respir J199914361061510543283
- StevensWWOcampoCJBerdnikovsSCytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory diseaseAm J Respir Crit Care Med2015192668269426067893
- ShomeGPTarboxJShearerMKennedyRCytokine expression in peripheral blood lymphocytes before and after aspirin desensitization in aspirin-exacerbated respiratory diseaseAllergy Asthma Proc200728670671018201436
- HsiehFHLamBKPenroseJFAustenKFBoyceJAT helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4J Exp Med200119312313311136826
- SteinkeJWCulpJAKropfEBorishLModulation by aspirin of nuclear phospho-signal transducer and activator of transcription 6 expression: possible role in therapeutic benefit associated with aspirin desensitizationJ Allergy Clin Immunol20091244724.e4730.e419767084
- ZhangNVan ZeleTPerez-NovoCDifferent types of T-effector cells orchestrate mucosal inflammation in chronic sinus diseaseJ Allergy Clin Immunol2008122596196818804271
- LiuTKanaokaYBarrettNAAspirin-exacerbated respiratory disease involves a cysteinyl leukotriene-driven IL-33-mediated mast cell activation pathwayJ Immunol201519583537354526342029
- GriseriTArnoldICPearsonCGranulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitisImmunity201543118719926200014
- LamasSMichelTBrennerBMMarsdenPANitric oxide synthesis in endothelial cells: evidence for a pathway inducible by TNF-alphaAm J Physiol19912614 Pt 1C634C6411656767
- TakabayashiTKatoAPetersATExcessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expressionAm J Respir Crit Care Med20131871495723155140
- LaidlawTMKidderMSBhattacharyyaNCysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytesBlood2012119163790379822262771
- StankovicKMGoldszteinHRehDDPlattMPMetsonRGene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthmaLaryngoscope2008118588188918391768
- KimMAIzuharaKOhtaSAssociation of serum periostin with aspirin-exacerbated respiratory diseaseAnn Allergy Asthma Immunol2014113331432025037608
- ParulekarADAtikMAHananiaNAPeriostin, a novel biomarker of TH2-driven asthmaCurr Opin Pulm Med2014201606524247042
- ShinSParkJSKimYJOhTAnSParkCSDifferential gene expression profile in PBMCs from subjects with AERD and ATA: a gene marker for AERDMol Genet Genomics2012287536137122457146
- KimJHParkBLCheongHSGenome-wide and follow-up studies identify CEP68 gene variants associated with risk of aspirin-intolerant asthmaPLoS One2010511e1381821072201
- ParkBLKimTHKimJHGenome-wide association study of aspirin-exacerbated respiratory disease in a Korean populationHum Genet2013132331332123180272
- KimSHChoBYChoiHThe SNP rs3128965 of HLA-DPB1 as a genetic marker of the AERD phenotypePLoS One2014912e11122025536158
- DekkerJWNizankowskaESchmitz-SchumannMAspirin-induced asthma and HLA-DRB1 and HLA-DPB1 genotypesClin Exp Allergy19972755745779179433
- DahlenSEMalmstromKNizankowskaEImprovement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trialAm J Respir Crit Care Med2002165191411779723
- DahlenBNizankowskaESzczeklikABenefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmaticsAm J Respir Critc Care Med199815711871194
- KubavatAHKhippalNTakSA randomized, comparative, multicentric clinical trial to assess the efficacy and safety of zileuton extended-release tablets with montelukast sodium tablets in patients suffering from chronic persistent asthmaAm J Ther201320215416222926233
- TaVWhiteAASurvey-defined patient experiences with aspirin-exacerbated respiratory diseaseJ Allergy Clin Immunol Pract20153571171825858054
- WuXHongHZuoKExpression of leukotriene and its receptors in eosinophilic chronic rhinosinusitis with nasal polypsInt Forum Allergy Rhinol201661758126332237
- Mrowka-KataKCzeciorEKataDNamyslowskiGDziechciarz-WerbowskaJSowaPCurrent view on nasal polyps management in Samter’s triad patientsOtolaryngol Pol201266637337823200555
- SweetJMStevensonDDSimonRAMathisonDALong-term effects of aspirin desensitization – treatment for aspirin-sensitive rhinosinusitis-asthmaJ Allergy Clin Immunol19908559652299107
- StevensonDDSimonRASelection of patients for aspirin desensitization treatmentJ Allergy Clin Immunol200611880180417030229
- StevensonDDHankammerMAMathisonDAChristiansenSCSimonRAAspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomesJ Allergy Clin Immunol1996987517588876550
- PerezGMMeloMKeeganADZamoranoJAspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6J Immunol200216831428143411801685
- KatialRKStrandMPrasertsuntarasaiTLeungRZhengWAlamRThe effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory diseasesJ Allergy Clin Immunol2010126473874420728206
- GevaertPVan BruaeneNCattaertTMepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposisJ Allergy Clin Immunol20111285989995.e1e821958585
- WenzelSFordLPearlmanDDupilumab in persistent asthma with elevated eosinophil levelsN Eng J Med20133682624552466
- BoboleaIBarrancoPFiandorACabanasRQuirceSOmalizumab: a potential new therapeutic approach for aspirin-exacerbated respiratory diseaseJ Investig Allergol Clin Immunol2010205448449
- YalcinADUcarSGumusluSStraussLGEffects of omalizumab on eosinophil cationic peptide, 25-hydroxyvitamin-D, IL-1β and sCD200 in cases of Samter’s syndrome: 36 months follow-upImmunopharmacol Immunotoxicol201335452452723841472
- BergmannKCZuberbierTChurchMKOmalizumab in the treatment of aspirin-exacerbated respiratory diseaseJ Allergy Clin Immunol Pract20153345946025648572
