Abstract
Background
Biosimilar granulocyte colony-stimulating factor (G-CSF) has recently been introduced into clinical practice. G-CSFs are used to mobilize CD34+ cells and accelerate engraftment after transplantation. However, in Asia, particularly in Japan, data for peripheral blood stem cell (PBSC) mobilization by this biosimilar G-CSF are currently lacking. Therefore, the clinical efficacy and safety of biosimilar G-CSF for hematopoietic stem cell transplantation needs to be evaluated in a Japanese context.
Materials and methods
The subjects included two groups of patients with malignant lymphoma and multiple myeloma. All patients received chemotherapy priming for the mobilization of PBSCs. All patients were treated with chemotherapy followed by the administration of either the biosimilar G-CSF, filgrastim XM02 (FBNK), or the originators, filgrastim, or lenograstim.
Results
There were no significant differences among FBNK, filgrastim, and lenograstim treatments in the numbers of CD34+ cells in harvested PBSCs, the scores for granulocyte/macrophage colony forming units, or for malignant lymphoma and multiple myeloma patients evaluated as separate or combined cohorts. In addition, there were no significant differences in safety, side effects, complications, or the time to engraftment after autologous hematopoietic stem cell transplantation.
Conclusion
Biosimilar FBNK shows the same efficacy and safety as originator G-CSFs for facilitating bone marrow recovery in Japanese malignant lymphoma and multiple myeloma patients undergoing stem cell transplantation. In addition, it is less expensive than the originators, reducing hospitalization costs.
Introduction
Transplantation of mobilized peripheral blood stem cells (PBSCs) is used to facilitate hematologic recovery after high-dose chemotherapy.Citation1,Citation2 Although several methods for mobilization of PBSCs have been described,Citation3–Citation5 leukapheresis is a commonly used collection procedure. PBSC yield is enhanced if collection is performed at the time of recovery from chemotherapy, and while patients are receiving treatment with granulocyte colony-stimulating factors (G-CSFs).Citation5,Citation6
Recently, biosimilar G-CSF products have been introduced to the market. Some trials have revealed their noninferiority for treatment of neutropenia during chemotherapy, compared with the originator.Citation7–Citation10 The US Food and Drug Administration describes biosimilars as highly similar to a previously approved biological product, known as the biological reference product, and as having been shown to have no clinically meaningful differences from the reference product. Furthermore, published data from prospective randomized and retrospective studies on the use of biosimilar filgrastim for PBSC mobilization in an autologous setting suggest a substantially similar efficacy and safety in comparison with the originator.Citation11–Citation21 However, in Asia, particularly in Japan, there is currently a lack of data for PBSC mobilization by biosimilar G-CSF. Therefore, evaluation in a Japanese context is needed to assess the clinical efficacy and safety of bio-similar G-CSF for hematopoietic stem cell transplantation, including autologous stem cell transplantation (ASCT). Filgrastim BS-NK (FBNK) (Nippon Kayaku Co., Ltd., Tokyo, Japan) is a genetically modified biosimilar G-CSF drug and is the same as Tevagrastim® (XM02).Citation11 The production and sale of FBNK was approved in Japan in February, 2013. In this study, we evaluated the efficacy of FBNK compared with the originators for peripheral blood cell mobilization and transplantation.
Materials and methods
Study subjects
The subjects included two groups, treated with biosimilar or originator G-CSF. 1) Biosimilar group: from July 2014 to October 2015, 14 consecutive patients affected by malignant lymphoma (ML) and multiple myeloma (MM) were scheduled for collection of PBSCs for ASCT, after induction chemotherapy. None of the patients had previously received any mobilization therapy. 2) Originator group: 57 consecutive patients who underwent the same procedure between December 2006 and July 2013 were enrolled. The originator G-CSF was filgrastim for 34 patients and lenograstim for 23 patients.
The study protocol was approved by Institutional Review Board in Kansai Medical University and written informed consent was obtained from each patient. Patients’ characteristics and underlying diseases are shown in .
Table 1 Patient demographics and indications for stem cell harvest
PBSC harvest
For mobilization of PBSCs, all patients received chemotherapy priming consisting of various regimens (). PBSCs from patients with ML were either harvested after cyclophosphamide and G-CSF treatment, or following a cycle of chemotherapy including rituximab, ifosfamide, etoposide, or cytarabin. The patients with MM were primed with cyclophosphamide and G-CSF. All patients were treated with chemotherapy followed by the administration of FBNK, filgrastim, or lenograstim (5 µg/kg). Leukapheresis was performed with a cell separator (COBE Spectra, Gam-bro K.K., Tokyo, Japan). The procedure was commenced when the white blood cell count recovered to 1×109/L, and continued until the number of harvested mononuclear cells exceeded 3×108/kg body weight. A total blood volume of 10 L was processed in each apheresis session. The apheresis product was enriched for mononuclear cells, cryopreserved in 6% hydroxyethyl starch, 5% dimethylsulfoxide, and 4% human albumin, and then stored at −80°C.
CD34 analysis
A sample of each leukapheresis product was analyzed according to the method in our previous reports.Citation22,Citation23 All samples were washed to remove platelets and were stained with an anti-CD34 monoclonal antibody (QBEND-10; Immunotech, Marseille, France). After staining, the erythrocytes were lysed with ammonium chloride. Flow cytometric analysis was performed using an Epics XLMCL (Beckman Coulter, Brea, CA, USA). It is very difficult to predict the quantity of CD34+ cells collected from patients or donors, because the collection efficiency of CD34+ cells differs between individuals. Therefore, we counted the numbers of CD34+ cells used, with 65,500 cells being analyzed per sample, according to previous reports.Citation24,Citation25 After gating for forward and right scatter, a second gate was drawn around the CD34+ lower right scatter population to determine the percentage of hematopoietic progenitors corrected for the control. The target dose of PBSCs to collect was >2×106 CD34+ cells/kg for patients affected by ML, and >4×106 CD34+ cells/kg for patients affected by MM.
Colony forming assay
Colony forming cells were assayed with methyl cellulose as the support medium. Mononuclear cells (2×105) were cultured in triplicate in semisolid medium (0.8% methylcellulose in α–medium) supplemented with 10 ng/ml recombinant G-CSF, 10 ng/mL recombinant human granulocyte/macrophage-CSF, 100 IU/mL recombinant human interleukin-3, 2 U/mL recombinant human erythropoietin, and 10% placental conditioned medium as a source of CSF. Culture plates were incubated for 14 days at 37°C in a humidified atmosphere (5% CO2 in air) and granulocyte/macrophage colony forming units (CFU-GM) were counted under an inverted microscope.
ASCT
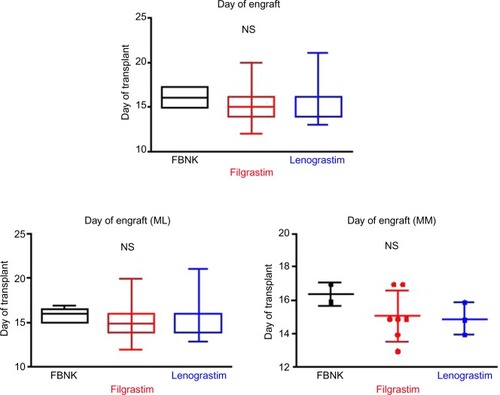
The choice of conditioning regimen was based on the patient’s diagnosis (). On day 0, all patients received an infusion of previously cryopreserved autologous PBSCs. Administration of recombinant human G-CSF (biosimilar or originator) was started on day 5 after the PBSC infusion, at a daily dose of 5 µg/kg administered subcutaneously, except for some patients for whom support therapy was anticipated because of their indications. To evaluate the impact of G-CSF on the ASCT, we calculated the median times to leucocyte engraftment, defined as an absolute neutrophil count of >0.5×109/L on the first 3 consecutive days, and to platelet recovery, defined as a platelet count of >20×109/L for 2 consecutive days without platelet transfusions.
Statistics
ML and MM patients were evaluated both as separate cohorts, and combined into a single cohort. Data were recorded as mean ± SD and were analyzed as appropriate. Welch’s t-test was used for the comparison of data for each case before and after G-CSF treatment. Between-group comparisons were analyzed using Scheffe’s test. All analyses were performed using the StatFlex program, version 6 (Artec Inc., Osaka, Japan).
Results
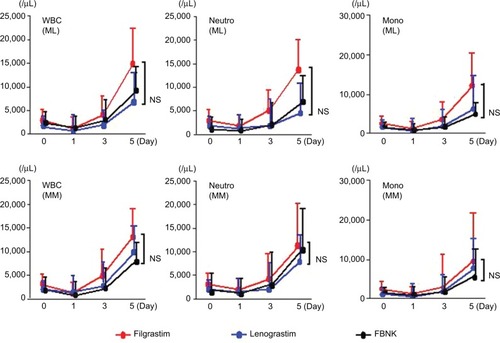
shows the change in leukocyte numbers before and after PBSC harvest. The numbers of all leukocytes, neutrophils, and monocytes peaked on day 5 after G-CSF treatment. There were no significant differences among the FBNK, filgrastim, and lenograstim groups.
Figure 1 White blood cell numbers after G-CSF administration and PBSC harvest in patients with ML and MM.
Abbreviations: WBC, white blood cell; Neutro, neutrophil; Mono, monocyte; ML, malignant lymphoma; MM, multiple myeloma; G-CSF, granulocyte colony-stimulating factor; PBSC, peripheral blood stem cell; SD, standard deviation; FBNK, filgrastim BS-NK; NS, not significant.
Different parameters to characterize the yield and composition of the grafted cells are displayed in as dot plots. The upper three graphs show the total CD34+ cell numbers, the CD34+ cells/kg and the CFU-GM scores for all patients. The lower graphs show the CD34+ cells/kg and the CFU-GM scores for ML versus MM patients. There were no significant differences in CD34+ cell numbers and CFU-GM scores among the FBNK, filgrastim, and lenograstim treatments, not only for the combined patient cohort, but also for ML and MM patients evaluated separately. In addition, there were no significant differences in the frequencies of side effects induced by the three G-CSF drugs ().
Figure 2 Comparison of PBSC mobilized by either the biosimilar or the originator (filgrastim or lenograstim).
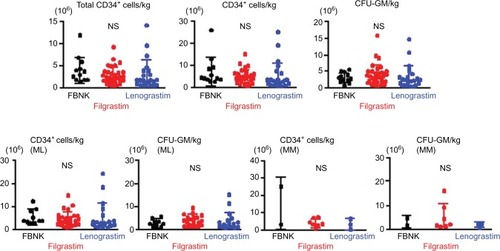
Table 2 Complications after ASCT among three G-CSF drugs
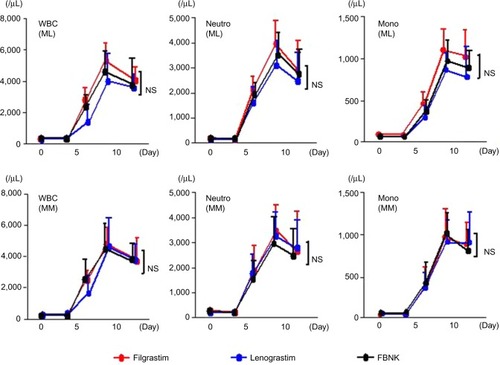
shows the leukocyte numbers before and after ASCT. The numbers of all leukocytes, neutrophils, and monocytes peaked on days 8–10 after transplantation. There were no significant differences among the FBNK, filgrastim, and lenograstim treatments. We could not study the number of CD34+ cells after transplantation.
Figure 3 Leukocyte numbers before and after ASCT in patients with ML and MM.
Abbreviations: WBC, white blood cell; Neutro, neutrophil; Mono, monocyte; ML, malignant lymphoma; MM, multiple myeloma; G-CSF, granulocyte colony-stimulating factor; SD, standard deviation; FBNK, filgrastim BS-NK; NS, not significant.
shows the times to engraftment for the combined, ML, and MM patient cohorts. Leukocyte numbers before and after ASCT are also shown. There were no significant differences in the time to engraftment among the FBNK, filgrastim, and lenograstim treatments, not only for the combined patient cohort, but also for ML and MM patients evaluated separately. In addition, there were no significant differences in complications after ASCT among the three drugs ().
Discussion
G-CSF has become widely used to treat therapy-induced neutropenia, for mobilization of PBSCs, and to accelerate engraftment after hematopoietic stem cell transplantation.Citation26,Citation27 In addition, several forms of biosimilar nonglycosylated recombinant human G-CSF have recently been clinically developed. These have been approved by the European Medicines Agency.Citation28–Citation31 In Japan, applications to approve the production of biosimilars are progressed according to guidelines for efficacy and security notified by the Ministry of Health, Labour, and Welfare. Three preparations of biosimilar G-CSF have been approved in Japan. FSK0808 is a domestic product, and the others, TKN732 and EP2006, are imported from foreign countries. The FBNK used in the present study corresponds to TKN732, an imported filgrastim biosimilar product sold in Europe as XM02. Therefore, the safety data of 541 overseas phase III randomized controlled trials can be extrapolated to this FBNK product.Citation28–Citation31
There are several publications regarding the use of bio-similar G-CSF for PBSC mobilization.Citation11–Citation21,Citation32 However, the World Marrow Donor Association does not recommend the use of biosimilars for stem cell mobilization in healthy adult donors, because the long-term safety is considered poor.Citation33 In the present study, there were no significant differences in CD34+ cell numbers and CFU-GM scores among FBNK, filgrastim, and lenograstim treatments for the total patient, ML and MM patient groups. Similarly, there were no significant differences in the time to engraftment after ASCT among the FBNK, filgrastim, and lenograstim treatments. Lefrère et alCitation12 reported that no significant difference was found between the biosimilar G-CSF Zarzio® and Neupogen® for stem cell mobilization in 40 patients, compared with an historical control. Andreola et alCitation11 also showed successful mobilization and engraftment using Plerixafor and XM02 in a study of 14 patients. Furthermore, Publicover et alCitation14 and Bassi et alCitation19 reported the same results using other biosimilars. These previous results support the present study.
The use of biosimilars needs to consider safety in addition to efficacy. The chief physician should monitor drug dosage and patient response, and strict safety monitoring of pharmaceutical products should be performed after marketing.Citation28 Previous phase III randomized controlled trials conducted internationally in many facilities reported that the adverse effects and tolerability were similar between the originator and G-CSF biosimilars.Citation28–Citation30 In the present study, there were no significant differences in the frequencies of side effects such as bone pain and fever among the three G-CSF drugs (FBNK, filgrastim, and lenograstim), and there were no significant differences in complications after ASCT.
A study of the effect on medical expenses reported that biosimilar G-CSF is advantageous in this regard in Europe.Citation11 These results appear to be compatible with Japan. However, an original evaluation is necessary, because the Japanese medical system is different from the European medical system. ASCT requires a higher dose of G-CSF than chemotherapy. The use of biosimilars may offer significant cost savings and help reduce health care costs. However, their long-term safety remains unclear. Therefore, further observation will be very important to establish the clinical use of FBNK.
Conclusion
In comparison with the originator G-CSFs, biosimilar FBNK shows the same efficacy and safety for facilitating bone marrow recovery in Japanese patients with ML and MM who undergo stem cell transplantation. In addition, it reduced the patients’ hospitalization costs because it is less expensive than the originators.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This study was partly supported by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research, a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a Grant (13670760 to SN) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- JuttnerCAToLBHaylockDNAutologous blood stem cell transplantationTransplant Proc1989211 Pt 3292929312565063
- HaasRHoADBredthauerUSuccessful autologous transplantation of blood stem cells mobilized with recombinant human granulocyte-macrophage colony-stimulating factorExp Hematol199018294981968009
- EliasADAyashLAndersonKCMobilization of peripheral blood progenitor cells by chemotherapy and granulocyte-macrophage colony-stimulating factor for hematologic support after high-dose intensification in breast cancerBlood19927911303630441350229
- BruggerWBrossKJFrischMobilization of peripheral blood progenitor cells by sequential administration of interleukin-3 and granulocyte-macrophage colony-stimulating factor following polychemotherapy with etoposide, ifosfamide, and cisplatinBlood1992795119312001371415
- SheridanWPBegleyCGJuttnerCAEffect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapyLancet199233987946406441371817
- TeplerICannistraSAFreiE3rdUse of peripheral-blood progenitor cells abrogates the myelotoxicity of repetitive outpatient high-dose carboplatin and cyclophosphamide chemotherapyJ Clin Oncol1993118158315918101563
- MellstedtHNiederwieserDLudwigHThe challenge of biosimilarsAnn Oncol200819341141917872902
- SörgelFLerchHLauberTPhysicochemical and biologic comparability of a biosimilar granulocyte colony-stimulating factor with its reference productBioDrugs201024634735720873878
- NiederwieserDSchmitzSBiosimilar agents in oncology/haematology: from approval to practiceEur J Haematol201186427728821175852
- GasconPPresently available biosimilars in hematology-oncology: G-CSFTarget Oncol20127Suppl 1S293422258705
- AndreolaGBabicARabascioCNegriMMartinelliGLaszloDPlerixafor and Filgrastim XM02 (Tevagastrim) as a first line peripheral blood stem cell mobilisation strategy in patients with multiple myeloma and lymphoma candidated to autologous bone marrow transplantationEur J Haematol201288215415821992403
- LefrèreFBrignierACElieCFirst experience of autologous peripheral blood stem cell mobilization with biosimilar granulocyte colony-stimulating factorAdv Ther201128430431021400232
- SivginSKarakusEKaynarLThe comparison of Filgrastim (Neupogen®), biosimilar filgrastim (Leucostim®) and Lenograstim (Granocyte®) as a first line peripheral blood stem cell mobilization strategy in autologous hematopoieitic stem cell transplantation: a single center experience from TurkeyTransfus Apher Sci201348331532023611684
- PublicoverARichardsonDSDaviesAUse of biosimilar granulocyte colony-stimulating factor for peripheral blood stem cell mobilization: an analysis of mobilization and engraftmentBr J Haematol2013162110711123614650
- SchmittMXuXHilgendorfIMobilization of PBSC for allogeneic transplantation by the use of the G-CSF biosimilar XM02 in healthy donorsBone Marrow Transplant201348792292523318540
- MankoJWalter-CroneckaAJawniakDA clinical comparison of the efficacy and safety of biosimilar G-CSF and originator G-CSF in haematopoietic stem cell mobilizationPharmacol Rep201466223924224911076
- SchmittMPublicoverAOrchardKHBiosimilar G-CSF based mobilization of peripheral blood hematopoietic stem cells for autologous and allogeneic stem cell transplantationTheranostics20144328028924505236
- CesaroSTridelloGPreteABiosimilar granulocyte colony-stimulating factor for mobilization of autologous peripheral blood stem cell in pediatric hematology-oncology patientsTransfusion201555224625225070657
- BassiSStroppaEMMoroniCFSafety and efficacy of granulocyte colony-stimulating factor biosimilars in engraftment after autologous stem cell transplantation for haematological malignancies: a 4-year, single institute experience with fifferent conditioning regimensBlood Transfus201513347848325761321
- PhamTPatilSFlemingSComparison of biosimilar filgrastim with originator filgrastim for peripheral blood stem cell mobilization and engraftment in patients with multiple myeloma undergoing autologous stem cell transplantationTransfusion201555112709271326173921
- MarchesiFVaccaMGumenyukSBiosimilar filgrastim (Zarzio®) vs. lenograstim (Myelostim®) for peripheral blood stem cell mobilization in adult patients with lymphoma and myeloma: a single center experienceLeuk Lymphoma2016571–4489492
- KatsuraKNomuraSXieGLPlatelet procoagulant activity during peripheral blood stem cell harvestClin Appl Thromb Hemost19973124128
- NomuraSInamiNKanazawaSIwasakaTFukuharaSElevation of platelet activation markers and chemokines during peripheral blood stem cell harvest with G-CSFStem Cells200422569670315342934
- BenderJGUnverzagtKWalkerDGuidelines for determination of CD34+ cells by flow cytometry: application to the harvesting and transplantation of peripheral blood stem cellsWunderESovalatHHenonPHematopoietic Stem Cells: The Mulhouse ManualDayton, OHAlphaMed Press19943143
- MercolinoTJConnellyMCMeyerEJImmunologic differentiation of absolute lymphocyte count with an integrated flow cytometric system: a new concept for absolute T cell subset determinationsCytometry19952248597587734
- AaproMSCameronDAPettengellREORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumoursEur J Cancer200642152433245316750358
- SmithTJKhatcheressianJLymanGH2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guidelineJ Clin Oncol200624193187320516682719
- del GiglioAEniuAGanea-MotanDTopuzovELubenauHXM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapyBMC Cancer2008833219014494
- EngeltAGriskeviciusLZyuzginYLubenauHdel GiglioAXM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapyLeuk Lymphoma200950337437919347726
- GatzemeierUCiuleanuTDediuMGanea-MotanELubenauHDel GiglioAXM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapyJ Thorac Oncol2009473674019404210
- LubenauHBiasPMalyAKPharmacokinetic and pharmacodynamic profile of new biosimilar filgrastim XM02 equivalent to marketed filgrastim Neupogen: single-blind, randomized, crossover trialBioDrugs2009231435119344191
- FerroHHJuniMBelloRVidalADiezRAPavlovskySUtilization study of filgrastim (Neutromax) during autologous haematopoietic precursor transplantation for myeloma and lymphoma patientsTransfus Apher Sci2009412879319699152
- ShawBE1ConferDLHwangWYPamphilonDHPulsipherMAConcerns about the use of biosimilar granulocyte colony-stimulating factors for the mobilization of stem cells in normal donors: position of the World Marrow Donor AssociationHaematologica201196794294721719883