Abstract
Background
Noonan syndrome (NS) is an autosomal dominant genetic condition that has a number of clinical features, including bleeding diathesis and a number of hematological abnormalities including clotting factor deficiencies, von Willebrand disease and abnormal platelet count/function.
Methods
We evaluated the frequency/types of bleeding disorders, and associated hematological laboratory findings, in patients with NS, using published data from 1965 to 2014.
Results
Of 45 studies identified, 31 included data for 428 patients with NS. Of these patients, 43% had reported bleeding, 26% had no reported bleeding and no bleed data was reported for 31%. Most patients (90%) had bleeding-related laboratory test abnormalities, but only 194 (45%) had a confirmed diagnosis of a specific bleeding disorder. Abnormal laboratory tests included: prolonged prothrombin time, activated partial thromboplastin time, and other platelet-related disorders. Of the 194 patients with a confirmed diagnosis of a specific bleeding disorder, 153 (79%) had single clotting factor deficiencies, von Willebrand disease or platelet-related disorders, and 41 (21%) had multiple deficiencies including platelet-related disorders.
Conclusion
As patients with NS can experience multiple bleeding disorders, including abnormal platelet function, clinical evaluations should be performed at diagnosis, after diagnosis, before any surgery is undertaken, and if patients become symptomatic.
Introduction
Noonan syndrome (NS) is an autosomal dominant genetic condition that affects one in 1,000–2,500 individuals. Typical signs of NS include characteristic facial features, short stature, congenital heart defect, skeletal and thoracic anomalies, developmental delay, and bleeding problems; these are seen in 30%–72% of patients with the condition.Citation1 Until recently, the molecular etiology of NS was unknown; this, coupled with the highly variable phenotype observed in these patients, made diagnosis difficult. It is now recognized, however, that ~50% of patients with NS demonstrate pathological variants of the PTPN11 gene. This results in the development of NS, or another disorder involving PTPN11 (such as LEOPARD syndrome), where cardiac defects also frequently manifest as pulmonary valve stenosis and hypertrophic cardiomyopathy.Citation2 A number of genes are known to play a role in NS; molecular testing of the four best-recognized genes in NS is now generally available and has identified mutations in PTPN11 in ~50%, KRAS in <5%, SOS1 in ~15%, and RAF1 in 3%–17% of patients with the disorder.Citation3
NS can be associated with an increased risk of bleeding and bruising, and a variety of bleeding abnormalities, with factor XI deficiency and platelet abnormalities being described most frequently.Citation4–Citation6 However, it is not clear whether there is any direct correlation between bleeding risk and results of coagulation tests so there is currently increased focus on evaluating bleeding risk in patients with NS. As a consequence of their condition, many patients undergo multiple surgeries, often starting in early childhood, so it is important to establish bleeding risk prior to any intervention. For example, pulmonary valve stenosis, which is often reported in patients with NS, is typically unsuitable for interventional balloon dilation due to bleeding risk, and usually requires surgery for correction. Also, the presence of a partial atrioventricular canal with outflow obstruction can result in a demanding surgical procedure to correct the malformation.Citation7 The aim of this systematic review was to identify the frequency and types of bleeding disorders, and to evaluate any links with associated laboratory findings, in patients with NS, with a view to providing physicians with guidelines on how to evaluate bleeding complications in patients with NS.
Methods and results
Publications from 1965 to 2014 were reviewed. They included trials, case reports/series, and reviews identified via MEDLINE®, EMBASE®, and Scopus®. Key search terms included: Noonan; bleed*3; hemorrhag*3; thrombocytop*enia; h*emostatic; h*emostasis; bleed* diathesis; platelet* disorder*.
Studies of patients with NS were included in the analysis only if the bleeding phenotype was described. Studies were excluded if NS was not present or not confirmed in all cases; the publication was a secondary analysis (review of other case reports with no new information); there was no patient-level bleeding phenotype information reported for any patients. All available patient data were abstracted, and included demographics, bleeding symptoms, laboratory abnormalities, bleeding score, and specific disorders reported. The numbers of patients in each study are shown in Table S1.
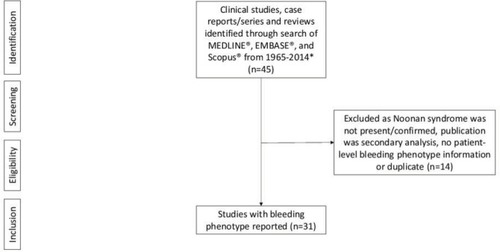
Of 45 studies identified,Citation1,Citation4–Citation6,Citation8–Citation48 31Citation4–Citation6,Citation8–Citation35 had relevant data from 428 patients with NS (). Of the 31 studies included, 13 were single case studies, five included <10 patients, and 13 included >10 patients. The largest cumulative study, including 151 patients,Citation18 had previously been reported with 72Citation5 and 31Citation33 patients; however, different sets of information are presented in each publication. Nearly half (49%) of the patients were male; 43% (183 patients) had reported bleeding, 26% (112 patients) had not reported bleeding, and for 31% (133 patients) there was no data on bleeding. Most patients (384; 90%) had some reported laboratory test abnormalities (platelet function and/or coagulation abnormalities). Of the patients with reported bleeding, abnormal laboratory tests included: prolonged prothrombin time (PT) (29 patients; 7%); activated partial thromboplastin time (aPTT) (71 patients; 17%); PT/aPTT (23 patients; 5%); and platelet-related disorders (42 patients; 10%). There were also reports of normal PT (104 patients; 24%) and aPTT (157 patients; 37%).
Figure 1 Prisma figure of studies for inclusion.
In one of the studies of 39 patients (and 28 controls), nearly 40% of patients with NS had a bleeding diathesis but >90% had platelet function and/or coagulation abnormalities.Citation9 Another study of 13 patients suggested that bleeding signs do not appear to be due to coagulation disorders.Citation22
Of the 428 patients evaluated, only 46% (195 patients) had a specific diagnosis of factor deficiency, von Willebrand disease, or platelet-related disorder. Of these patients, 154 (78%) had single-factor deficiencies or von Willebrand disease, and 42 (22%) had multiple factor deficiencies (). Of the factor deficiencies, factor XI (FXI) deficiency was most common (81 patients), followed by factor XII (FXII) (34 patients), and factor VIII (FVIII) (28 patients) (). Platelet-related disorders were reported in 46 patients, with thrombocytopenia and platelet aggregation abnormality being most commonly reported. In the 42 patients reporting multiple factor deficiencies, FXI+FXII combined (11 patients), and FVIII+ FXI combined (seven patients) were the most common ().
Table 1 Factor deficiencies and other bleeding disorders reported in patients with NS
Table 2 Multiple factor deficiencies and platelet-related disorders reported in patients with NS
Discussion
Physicians are diagnosing NS more readily; however, this literature review identifies a clear gap in the evaluation and diagnosis of bleeding diathesis and specific bleeding disorders in these patients. Indeed, several studies have highlighted that there is no correlation between coagulation study results and bleeding risk. In one study of 39 patients (and 28 controls), whilst only 40% of patients with NS had a bleeding diathesis, more than 90% had platelet function and/or coagulation abnormalities.Citation9 Another study of 13 patients suggested that bleeding signs do not appear to be due to coagulation disorders.Citation22 These findings suggest that screening needs to include tests beyond PT, aPTT and platelet count. Therefore, guidelines for clinical evaluation in NS should highlight the importance of comprehensive testing, as well as the need for specialist involvement by a pediatric hematologist in differential diagnosis, both at diagnosis of NS and pre-operatively, whenever screening test abnormalities are identified.Citation49
Although there is currently no consensus on the optimum strategy for diagnosis of bleeding in patients with NS, a review by Roberts et al in 2013 suggested that at diagnosis of NS a complete blood cell count (CBC) with differential and PT/aPTT should be undertaken. After diagnosis, repeat CBC with differential and PT/aPTT if aged 6–12 months at initial screen, and pre-operatively, CBC with differential and PT/aPTT, then in consultation with hematologist FIX, FXI and FXII concentrations, von Willebrand factor, and platelet aggregation. Furthermore, if symptomatic, PT/aPTT if bleeding is abnormal or persistent, then refer to a hematologist.Citation50
Due to the inconsistent reporting of platelet test results and platelet disorders in the studies included in this analysis, it does not appear that appropriate tests are being undertaken in clinical practice. Platelet aggregation, for example, needs to be conducted on a fresh specimen and is more likely to be offered at regional centers. It is therefore important to distinguish between abnormalities in platelet function suspected by screening tests (eg, platelet function analysis-100) and confirmed platelet function disorders based upon aggregometry patterns and confirmed with flow cytometry, electron microscopy, or genetic testing.
The surprisingly high incidence of multiple coagulation disorders suggests that work up in patients with NS needs to be more comprehensive. While FXI deficiency was most common individually and in combination, there were several multiple defects (both factor deficiencies and platelet function disorders) reported in the same patient.
Given that several germline mutations including PTPN11 and SOS1 are reported in patients with NS, there may be a possible correlation with bleeding phenotype. However, of the studies included in our analysis, only a few evaluated genetic mutations in relation to the reported bleeding disorder and results were not consistent. An evaluation of patients with NS reported PTPN11 gene mutations in 21 of 27 patients, and hematological disorders in nine of 27, with authors suggesting a near significant correlation.Citation12 Another study of 19 patients with NS showed that coagulation abnormalities were reported in patients with PTPN11, SOS1 and SOS1/RAF1 mutations and without a gene mutation, but they were not correlated with a specific gene mutation.Citation14 In addition, a study of 13 patients with NS (six with PTPN11 mutations) found that 12/13 had normal hematological assessment and only one had a platelet function disorder (storage pool disease).Citation22 One of the studies of 15 patients (14 with PTPN11 mutation and one with SOS1 mutation), showed that nine had a bleeding diathesis and complained of easy bruising, despite having normal platelet count, basic coagulation parameters, fibrinogen and antithrombin, and without a relevant reduction of coagulation factor activities. Furthermore, three of them had potentially acquired von Willebrand disease, which the authors suggested may explain the bleeding in those with pulmonary stenosis.Citation47 Further analysis of a large cohort of individuals with NS has suggested that PTPN11 gene mutations are more likely to be found in those with pulmonary stenosis, whereas hypertrophic cardiomyopathy is less prevalent among those with PTPN11 mutations.Citation2 Thus, bleeding disorders in NS do not appear to correlate with a particular genotype.
There are limitations to this analysis, which included mostly spontaneous case reports/series, because the reporting of symptoms, laboratory evaluations, and diagnosis may have been incomplete. Furthermore, while attempts were made to remove duplicate reporting of the same case in both an individual case report and prior compiled series/review, there may have been cases that were not explicitly referenced. When limited coagulation evaluation does not confirm to one or more specific diagnoses, it underscores the importance of referral to a pediatric hematologist so that the patient’s parents can give a detailed bleeding history. This should then be followed through with a differential diagnosis, using appropriate laboratory assays. In future case reports/series, there is an important need to ensure that symptoms, laboratory results, and ultimate diagnoses are tracked.
Conclusion
Patients with NS can experience multiple bleeding disorders, including platelet-related disorders. Comprehensive clinical evaluations should be carried out both at diagnosis, after diagnosis if patients are symptomatic, and prior to any surgical procedures, even if tests are normal, the risk for bleeding events should be carefully considered. Furthermore, as there is no current consensus on management of bleeding complications in patients with NS, it is important that physicians closely monitor these patients.
Acknowledgments
Some study information presented in this manuscript was previously presented as a poster at The American Society of Pediatric Hematology/Oncology (ASPHO) 2016 congress. Poster#549: “Evaluation of bleeding disorders in patients with Noonan syndrome: a systematic review”. Nugent D, Romano A, Sabharwal S, Germak J, Cooper DL. Editorial assistance for this manuscript was provided by PAREXEL, and funded by Novo Nordisk A/S.
Supplementary materials
Table S1 Case reports/series on bleeding disorders in patients with NS
References
- ArgyrouAMarinakisTKalofoliasNPapazoglouSThrombotic thrombocytopenic purpura in a young patient with Noonan syndrome and systemic lupus erythematosusArch Hell Med2010273545548
- ArtoniASelicorniAPassamontiSMHemostatic abnormalities in Noonan syndromePediatrics20141335e1299e130424753526
- BertolaDRCarneiroJDD’AmicoEAHematological findings in Noonan syndromeRev Hosp Clin Fac Med Sao Paulo20035815812754583
- González-CasadoIBarreda BonisASalamanca FresnoLNoonan syndrome and hemato-oncological anomaliesHorm Res Paediatr201176Suppl 2167168
- de HaanMVd KampJJBriëtEDubbeldamJNoonan syndrome: partial factor XI deficiencyAm J Med Genet19882922772823354599
- FlickJTSinghAKKizerJLazarchickJPlatelet dysfunction in Noonan’s syndrome. A case with a platelet cyclooxygenase-like deficiency and chronic idiopathic thrombocytopenic purpuraAm J Clin Pathol19919557397421902619
- GambaGMarabottoFLosaLCo-agulation factor deficiencies and abnormal bleeding in Noonan’s syndromeHorm Res Paediatr201176Suppl 232121952409
- KitchensCSAlexanderJAPartial deficiency of coagulation factor XI as a newly recognized feature of Noonan syndromeJ Pediatr198310222242276822926
- KoçAKösecikMTatlıMMAtasAEmiroğluHHBernard-Soulier Syndrome like platelet defect in a patient with noonan syndrome; a case reportTurk J Haematol200118319119327264256
- MassaranoAAWoodATaitRCStevensRSuperMNoonan syndrome: coagulation and clinical aspectsActa Paediatr19968510118111858922080
- NunesPAguilarSPradoSNPalaréMJFerrãoAMoraisASevere congenital thrombocytopaenia − first clinical manifestation of Noonan syndromeBMJ Case Rep20122012bcr1020114940
- PatrickKMakrisMImages in haematology. Noonan syndrome associated with bleeding disordersBr J Haematol2010151211720738302
- SharlandMBurchMMcKennaWMPatonMAA clinical study of Noonan syndromeArch Dis Child19926721781831543375
- SharlandMPattonMATalbotSChitolieABevanDHCoagulation-factor deficiencies and abnormal bleeding in Noonan’s syndromeLancet1992339878419211345952
- StaudtJMvan der HorstCMPetersMMelisPBleeding diathesis in Noonan syndromeScand J Plast Reconstr Surg Hand Surg200539424724816208790
- StoffmanJMChodirkerBNIsraelsSJCoagulation abnormalities in patients with Noonan Syndrome − a single centre case seriesBlood20041041110351035
- TanakaYMasunoMIwamotoHNoonan syndrome and cavernous hemangioma of the brainAm J Med Genet199982321221410215542
- TroianoMGottliebSReyRNoonan Syndrome: assessment of bleeding disordersHorm Res Paediatr201176Suppl 221
- VortiaEMahajanLKaplanBDuodenal hematoma complicating upper endoscopy with biopsy in two pediatric patients with Noonan’s syndrome: what pediatric gastroenterologists need to knowAm J Gastroenterol2011106Suppl 2S400
- WaespeNPraderSKroissSKnirschWSpeerOSchmugeMClinical and laboratory manifestations of bleeding diathesis in Noonan syndromeHämostaseologie2013331A74
- WittDRMcgillivrayBCAllansonJEBleeding diathesis in Noonan syndrome: a common associationAm J Med Genet19883123053173232698
- CaralisDGCharFGraberJDVoigtGCDelineation of multiple cardiac anomalies associated with the Noonan syndrome in an adult and review of the literatureJohns Hopkins Med J197413463463554427400
- EvansDGLonsdaleRNPattonMACutaneous lymphangioma and amegakaryocytic thrombocytopenia in Noonan syndromeClin Genet19913932282322036745
- GrangeCSHeidRLucasSBRossPLDouglasMJAnaesthesia in a parturient with Noonan’s syndromeCan J Anaesth19984543323369597207
- HumbertJAHammondKBHathawayWETrimethylaminuria: the fish-odour syndromeLancet1970276767707714195988
- KompDM“Car. factor” deficiency revisited”Pediatric Res197594184189
- PhillipsWGDunnillMGKurwaARBlackMMOrbital oedema: an unusual presentation of Noonan’s syndromeBr J Dermatol199312921901927654583
- SgourosSNKaramanolisGPapadopoulouEPostbiopsy intramural hematoma of the duodenum in an adult with Noonan’s syndromeJ Gastroenterol Hepatol200419101217121915377306
- SharlandMPattonMAChitolieATalbotSBevanDCoagulation factor abnormalities in Noonan syndromeJ Med Genet19902710646
- SingerSTHurstDAddiegoJEBleeding disorders in Noonan syndrome: three case reports and review of the literatureJ Pediatr Hematol Oncol19971921301349149742
- SugarAWEzsiasABloomALMorcosWEOrthognathic surgery in a patient with Noonan’s syndromeJ Oral Maxillofac Surg19945244214258133379
Disclosure
Alicia A Romano is a consultant for Novo Nordisk and Genentech, and is on the speaker bureau for Novo Nordisk, Genentech, and Genzyme Corporation. Shreya Sabharwal and David L Cooper are employees of Novo Nordisk. The authors report no other conflicts of interest in this work.
References
- BriggsBJDickermanJDBleeding disorders in Noonan syndromePediatr Blood Cancer201258216717222012616
- TartagliaMKalidasKShawAPTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneityAm J Hum Genet20027061555156311992261
- TurnerAMNoonan syndromeJ Paediatr Child Health20145010E14E2021771153
- MassaranoAAWoodATaitRCStevensRSuperMNoonan syndrome: coagulation and clinical aspectsActa Paediatr19968510118111858922080
- SharlandMPattonMATalbotSChitolieABevanDHCoagulation-factor deficiencies and abnormal bleeding in Noonan’s syndromeLancet1992339878419211345952
- KitchensCSAlexanderJAPartial deficiency of coagulation factor XI as a newly recognized feature of Noonan syndromeJ Pediatr198310222242276822926
- FormigariRMichielonGDigilioMCGenetic syndromes and congenital heart defects: how is surgical management affected?Eur J Cardiothorac Surg200935460661419091590
- ArgyrouAMarinakisTKalofoliasNPapazoglouSThrombotic thrombocytopenic purpura in a young patient with Noonan syndrome and systemic lupus erythematosusArch Hell Med2010273545548
- ArtoniASelicorniAPassamontiSMHemostatic abnormalities in Noonan syndromePediatrics20141335e1299e130424753526
- BertolaDRCarneiroJDD’AmicoEAHematological findings in Noonan syndromeRev Hosp Clin Fac Med Sao Paulo20035815812754583
- de HaanMVd KampJJBriëtEDubbeldamJNoonan syndrome: partial factor XI deficiencyAm J Med Genet19882922772823354599
- González-CasadoIBarreda BonisASalamanca FresnoLNoonan syndrome and hemato-oncological anomaliesHorm Res Paediatr201176Suppl 2167168
- FlickJTSinghAKKizerJLazarchickJPlatelet dysfunction in Noonan’s syndrome. A case with a platelet cyclooxygenase-like deficiency and chronic idiopathic thrombocytopenic purpuraAm J Clin Pathol19919557397421902619
- GambaGMarabottoFLosaLCo-agulation factor deficiencies and abnormal bleeding in Noonan’s syndromeHorm Res Paediatr201176Suppl 232121952409
- KoçAKösecikMTatlıMMAtasAEmiroğluHHBernard-Soulier syndrome like platelet defect in a patient with Noonan Syndrome; a case reportTurk J Haematol200118319119327264256
- NunesPAguilarSPradoSNPalaréMJFerrãoAMoraisASevere congenital thrombocytopaenia−first clinical manifestation of Noonan syndromeBMJ Case Rep20122012bcr1020114940
- PatrickKMakrisMImages in haematology. Noonan syndrome associated with bleeding disordersBr J Haematol2010151211720738302
- SharlandMBurchMMcKennaWMPatonMAA clinical study of Noonan syndromeArch Dis Child19926721781831543375
- StaudtJMvan der HorstCMPetersMMelisPBleeding diathesis in Noonan syndromeScand J Plast Reconstr Surg Hand Surg200539424724816208790
- StoffmanJMChodirkerBNIsraelsSJCoagulation abnormalities in patients with Noonan Syndrome − a single centre case seriesBlood20041041110351035
- TanakaYMasunoMIwamotoHNoonan syndrome and cavernous hemangioma of the brainAm J Med Genet199982321221410215542
- TroianoMGottliebSReyRNoonan Syndrome: assessment of bleeding disordersHorm Res Paediatr201176Suppl 221
- VortiaEMahajanLKaplanBDuodenal hematoma complicating upper endoscopy with biopsy in two pediatric patients with Noonan’s syndrome: what pediatric gastroenterologists need to knowAm J Gastroenterol2011106Suppl 2S400
- WaespeNPraderSKroissSKnirschWSpeerOSchmugeMClinical and laboratory manifestations of bleeding diathesis in Noonan syndromeHämostaseologie2013331A74
- WittDRMcGillivrayBCAllansonJEBleeding diathesis in Noonan syndrome: a common associationAm J Med Genet19883123053173232698
- CaralisDGCharFGraberJDVoigtGCDelineation of multiple cardiac anomalies associated with the Noonan syndrome in an adult and review of the literatureJohns Hopkins Med J197413463463554427400
- EvansDGLonsdaleRNPattonMACutaneous lymphangioma and amegakaryocytic thrombocytopenia in Noonan syndromeClin Genet19913932282322036745
- GrangeCSHeidRLucasSBRossPLDouglasMJAnaesthesia in a parturient with Noonan’s syndromeCan J Anaesth19984543323369597207
- HumbertJAHammondKBHathawayWETrimethylaminuria: the fish-odour syndromeLancet1970276767707714195988
- KompDM“Car. factor” deficiency revisited”Pediatric Res197594184189
- PhillipsWGDunnillMGKurwaARBlackMMOrbital oedema: an unusual presentation of Noonan’s syndromeBr J Dermatol199312921901927654583
- SgourosSNKaramanolisGPapadopoulouEPostbiopsy intramural hematoma of the duodenum in an adult with Noonan’s syndromeJ Gastroenterol Hepatol200419101217121915377306
- SharlandMPattonMAChitolieATalbotSBevanDCoagulation factor abnormalities in Noonan syndromeJ Med Genet19902710646
- SingerSTHurstDAddiegoJEBleeding disorders in Noonan syndrome: three case reports and review of the literatureJ Pediatr Hematol Oncol19971921301349149742
- SugarAWEzsiasABloomALMorcosWEOrthognathic surgery in a patient with Noonan’s syndromeJ Oral Maxillofac Surg19945244214258133379
- CharFRodriguez-FernandezHScottCBorgaonkarDBellDRoweDThe Noonan Syndrome − a clinical study forty-five casesBirth Defects: Original Article Series1972VIII5110118
- CollinsETurnerGThe Noonan syndrome − a review of the clinical and genetic features of 27 casesJ Pediatr19738369419504148394
- DerbentMÖncelYTokelKClinical and hematologic findings in Noonan syndrome patients with PTPN11 gene mutationsAm J Med Genet A2010152A112768277420954246
- HathawayWEBleeding disorders due to platelet dysfunctionAm J Dis Child 1960197112121271345100795
- HilgartnerMEngleAMRedoSFCardiac surgery in patients with plasma thromboplastin antecedent (P.T.A.) deficiencyJ Thorac Cardiovasc Surg19654997498114295685
- CalvertGDTrimethylaminuria and inherited Noonan’s syndromeLancet1973177983203214119196
- NoonanJAHypertelorism with Turner phenotype. A new syndrome with associated congenital heart diseaseAm J Dis Child 1960196811643733804386970
- SharathkumarAABleeding disorders and Noonan syndromePediatric Blood Cancer2012593592 author reply 593. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/pbc.24151Accessed September 18, 201822566384
- SmpokouPTworog-DubeEKucherlapatiRSRobertsAEMedical complications, clinical findings, and educational outcomes in adults with Noonan syndromeAm J Med Genet A2012158A123106311123165751
- TofilNMWinklerMKWattsRGNoonanJThe use of recombinant factor VIIa in a patient with Noonan syndrome and life-threatening bleedingPediatr Crit Care Med20056335235415857538
- YoshidaRHasegawaTHasegawaYProtein-tyrosine phosphatase, nonreceptor type 11 mutation analysis and clinical assessment in 45 patients with Noonan syndromeJ Clin Endocrinol Metab20048973359336415240615
- WiegandGHofbeckMZenkerMBuddeURauchRBleeding diathesis in Noonan syndrome: is acquired von Willebrand syndrome the clue?Thromb Res20121305e251e25422985731
- BissTTBlanchetteVSClarkDSEvaluation of bleeding symptoms in children with an inherited mucocutaneous bleeding disorderBlood20091142212921292
- RomanoAAAllansonJEDahlgrenJNoonan syndrome: clinical features, diagnosis, and management guidelinesPediatrics2010126474675920876176
- RobertsAEAllansonJETartagliaMGelbBDNoonan syndromeLancet2013381986333334223312968
