Abstract
We present the case of a 45-year-old man with atrial fibrillation and morbid obesity (weight 128 kg, height 168 cm, BMI 45.4) who was switched from Warfarin 5 mg once daily to Apixaban 5 mg twice daily because he did not achieve at least 60% of the time in therapeutic range. We performed serial determinations of apixaban plasma concentration (at 2, 6, 12, 24 hrs after intake) showing drug levels within reference range, even when the patient lose weight.
Introduction
Obesity and Atrial Fibrillation (AF) are major risk factors for ischemic stroke.Citation1 The increased risk of cerebrovascular events in obese patients may be not only related to the accompanying co-morbidities, but it may also be explained by a low-grade chronic inflammation, which is associated with a prothrombotic/procoagulant state.Citation2 In obese patients, the glomerular filtration rate (GFR) and renal plasma flow (RPF) exceeded the control value by 51 and 31%, respectively. Consequently, the filtration fraction may increase, enhancing the renal clearance of oral anticoagulants (OACs).Citation3 Obese patients require greater doses of vitamin K oral anticoagulants (VKAs) and longer lead-in periods may be necessary for achieving therapeutic INR values.Citation4 Non-vitamin K oral anticoagulants (NOACs) should be preferred over VKAs for long-term stroke prevention in patients with non-valvular AF, according to the better clinical performance both in clinical trialCitation5–Citation8 and in real-life setting.Citation9–Citation14 However, based on the lack of clinical data about the efficacy and safety of NOACs in morbidly obese patients, both the International Society on Thrombosis and Hemostasis (ISTH) and the NOACs summary of product characteristics do not recommend the use of NOACs in patients with a body mass index (BMI) >40 kg/m2 or a weight >120 kg, unless drug‐specific peak or trough levels fall within the usual on‐therapy range.Citation15
Clinical Case
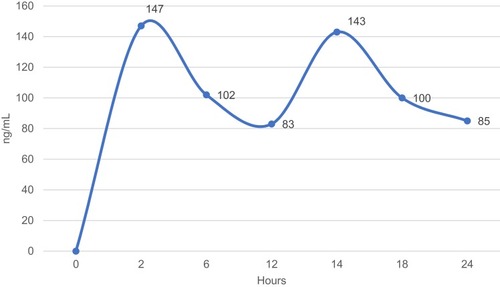
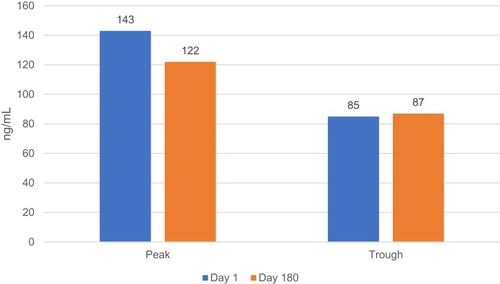
A 45-year-old man with dilated cardiomyopathy, morbid obesity (weight 128 kg, height 168 cm, BMI 45.4 kg/m2), arterial hypertension, diabetes mellitus, moderate sleep apnea syndrome and paroxysmal atrial fibrillation was admitted to our department for implantable cardioverter-defibrillator (ICD) implantation in primary prevention of sudden cardiac death. The mean blood pressure (BP) was 145/90 mmHg over the last two weeks and the glycated hemoglobin (HbA1c) was 8.3%. Trans-thoracic echocardiography showed dilated cardiomyopathy with an ejection fraction (EF), calculated by the Simpson’s biplane method, of 30% (n.v. >55%). The medical treatment included Bisoprolol 10 mg once daily (OD); Sacubitril/Valsartan 97/103 mg twice daily (TD), Canrenone 100 mg OD, Furosemide 25 mg TD, Amiodarone 200 mg OD, Metformin 1000 mg three times daily. For the high thromboembolic risk profile (CHA2DS2-VASc Score: 3), the patient was on anticoagulation therapy with Warfarin 5 mg OD; however, he did not achieve at least 60% of the time in therapeutic range (INR Target 2–3), assessed thought the Resendaal method (INR value range: 1.8–3.8). The serum creatinine level was 1.2 mg/dl and the estimated glomerular filtration rate, assessed by the Cockcroft-Gault equation, was 140.7 mL/min/1.73 m2. We switched the therapy from Warfarin to Apixaban (5 mg TD) and we performed serial determinations of apixaban plasma concentration (at 2, 6, 12.24 hrs after intake). The apixaban plasma level was detected by chromogenic anti-factor Xa assay according to the manufacturer’s recommendations. The upper reference limit was 141 ng/mL. The clinical biochemistry laboratory of our institution has implemented and maintains a quality management system which fulfils the requirements of the standard ISO 9001:2008 (registration number: IT-74072). The apixaban plasma level at peak (2 hrs after the first intake) was 147 ng/mL, at 6th, 12th (at trough), 14th (2 hrs after the first intake), 18th and 24th hour (at trough) was 102 ng/mL, 83 ng/mL, 143 ng/mL, 110 ng/mL and 85 ng/mL, respectively (). At three months from discharge the patient lost weight (weight 105 kg, height 168 cm, BMI 37.2), he showed well-controlled blood pressure (mean BP value 125/80 mmHg) and improved glycemic profile (HbA1c value <7.0%). The serum creatinine level was 1.1 mg/dl and the estimated glomerular filtration rate, assessed by the Cockcroft-Gault equation, was 125.9 mL/min/1.73 m2. The trans-thoracic echocardiography did not show a significant EF improvement (EF value of 33%). The effect of the weight loss, achieved by intragastric balloon procedure and strict diet, did not change the apixaban plasma level at peak (122 ng/mL) and at trough (87 ng/mL) (). The patient did not report any thromboembolic or hemorrhagic events or other side effects at twelve months follow-up. The adherence to the apixaban therapy was 95%. The written informed consent has been provided by the patient to have the case details published. We received institutional approval from the University of Campania – Monaldi Hospital Ethical Committee to publish these details.
Discussion
The optimal anticoagulant treatment for stroke prevention in obese patients with atrial fibrillation is still a matter of debate. On one hand, VKAs required an increased starting dosage and more time for achieving the international normalized ratio (INR) values within the therapeutic range;Citation16 on the other hand, no large randomized controlled trial has specifically investigated the efficacy and safety of NOACs in the obese population and the current guidelines recommends avoiding NOACs in morbidly obese patients (BMI >40 kg/m2 or weight >120 kg).Citation15 Several recent studies (weight-based post hoc analyses and retrospective cohort studies) investigated the clinical performance of NOACs in morbidly obese patients with AF.Citation17–Citation22 Despite the recommendations of the ISTH guidelines, we switched the oral anticoagulation therapy from warfarin to apixaban (5 mg TD) because of its low volume of distributionCitation19 and on the basis of the results of a recent weight-based post hoc analysis of the ARISTOTLE trial that investigated the efficacy and safety of apixaban versus warfarin in 1035 obese patients (>120 kg).Citation16 This post hoc analysis demonstrated that apixaban treatment was associated with a non-significant lower risk for stroke/systemic embolism (0.4% vs 1.18%; 95% CI 0.111–1.063, P value= 0.063) and for major bleeding (1.67% vs 2%; 95% CI 0.422–1.636, P value= 0.6) respect to VKAs therapy. The evaluation of apixaban serum level at peak and trough time demonstrated the therapeutic drug levels in our morbid obese patient and the stability of apixaban plasma levels even when the patient has lost weight.
The use of plasma level monitoring for NOAC dose-adjustment is discouraged for the vast majority of patients due to the lack of outcome data to support such an approach; however, in rare cases of potentially substantial drug–drug interactions;Citation23,Citation24 in special population in which the use of NOACs is still debated,Citation25,Citation26 or in case of not deferrable cardiac or non-cardiac interventional or surgical procedures,Citation27 a “patient-centered approach”, including the plasmatic drug evaluation, might be considered for choosing the more appropriate anticoagulant molecule.
Conclusions
The present clinical case describes a practice experience about the use of plasmatic drug evaluation to assess the apixaban therapeutic levels in morbid obese patients with atrial fibrillation, and suggests the apixaban therapy could be as a valid alternative to VKA therapy in this special population.
Ethics
Informed consent has been provided by the patient to have the case details published. The institutional approval was required to publish the case details by the University of Campania – Monaldi Hospital Ethical Committee.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We gratefully thank the nursing staff of Cardiology Unit Vanvitelli – Monaldi Hospital for the blood sample collection.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41(5):e418–e426. doi:10.1161/STROKEAHA.109.57696720299666
- Sonnenberg G, Krakower G, Kissebah A. A novel pathway to the manifestations of metabolic syndrome. Obes Res. 2004;12:180e186. doi:10.1038/oby.2004.2414981209
- Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817–F822. doi:10.1152/ajprenal.2000.278.5.F81710807594
- Wallace JL, Reaves AB, Tolley EA, et al. Comparison of initial warfarin response in obese patients versus non-obese patients. J Thromb Thrombolysis. 2013;36:96–101. doi:10.1007/s11239-012-0811-x23015280
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY steering committee and investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi:10.1056/NEJMoa090556119717844
- Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi:10.1056/NEJMoa100963821830957
- Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE committees and investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi:10.1056/NEJMoa110703921870978
- Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi:10.1056/NEJMoa131090724251359
- Russo V, Bianchi V, Cavallaro C, et al. Efficacy and safety of dabigatran in a “real-life” population at high thromboembolic and hemorrhagic risk: data from MonaldiCare registry. Eur Rev Med Pharmacol Sci. 2015;19(20):3961–3967.26531286
- Russo V, Rago A, Proietti R, et al. Efficacy and safety of the target-specific oral anticoagulants for stroke prevention in atrial fibrillation: the real-life evidence. Ther Adv Drug Saf. 2017;8(2):67–75. doi:10.1177/204209861667399028255434
- Russo V, Attena E, Mazzone C, et al. Real-life performance of edoxaban in elderly patients with atrial fibrillation: a multicenter propensity Score-Matched Cohort Study. Clin Ther. 2019;41(8):1598–1604. doi:10.1016/j.clinthera.2019.04.04131151813
- Russo V, Carbone A, Rago A, Golino P, Nigro G. Direct oral anticoagulants in octogenarians with atrial fibrillation: it’s never too late. J Cardiovasc Pharmacol. 2019;73(4):207–214. doi:10.1097/FJC.000000000000066130855404
- Verdecchia P, D’Onofrio A, Russo V, et al. Persistence on apixaban in atrial fibrillation patients: a retrospective multicentre study. J Cardiovasc Med (Hagerstown). 2019;20(2):66–73. doi:10.2459/JCM.000000000000074430540644
- Russo V, Rago A, Proietti R, et al. Safety and efficacy of triple antithrombotic therapy with dabigatran versus vitamin k antagonist in atrial fibrillation patients: a pilot study. Biomed Res Int. 2019;2019:5473240. doi:10.1155/2019/547324030895193
- Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308–1313. doi:10.1111/jth.1332327299806
- Self TH, Wallace JL, Sakaan S, Sands CW. Effect of body weight on dose of vitamin K antagonists. South Med J. 2015;108(10):637–643. doi:10.14423/SMJ.000000000000035626437201
- Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother. 2018;22:1060028018796604.
- Sandhu RK, Ezekowitz J, Andersson U, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37(38):2869–2878. doi:10.1093/eurheartj/ehw12427071819
- Hohnloser SH, Fudim M, Alexander JH, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation. 2019;139(20):2292–2300. doi:10.1161/CIRCULATIONAHA.118.03795530773022
- Piran S, Traquair H, Chan N, Bhagirath V, Schulman S. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: a retrospective study. Res Pract Thromb Haemost. 2018;2(4):684–688. doi:10.1002/rth2.1214630349887
- Boriani G, Ruff CT, Kuder JF, et al. Relationship between body mass index and outcomes in patients with atrial fibrillation treated with edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. Eur Heart J. 2019;40(19):1541–1550. doi:10.1093/eurheartj/ehy86130624719
- Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76(6):908–916. doi:10.1111/bcp.2013.76.issue-623488672
- Russo V, Bottino R, Rago A, et al. Atrial fibrillation and malignancy: the clinical performance of non-vitamin K oral anticoagulants – a systematic review. Semin Thromb Hemost. 2019;45(2):205–214. doi:10.1055/s-0038-166138630119139
- Russo V, Attena E, Mazzone C, et al. Nonvitamin K antagonist oral anticoagulants use in patients with atrial fibrillation and bioprosthetic heart valves/prior surgical valve repair: a multicenter clinical practice experience. Semin Thromb Hemost. 2018;44(4):364–369. doi:10.1055/s-0037-161526129304513
- Russo V, Rago A, Papa AA, et al. Use of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with malignancy: clinical practice experience in a single institution and literature review. Semin Thromb Hemost. 2018;44(4):370–376. doi:10.1055/s-0037-160743629220855
- Rago A, Papa AA, Cassese A, et al. Clinical performance of apixaban vs. Vitamin K antagonists in patients with atrial fibrillation undergoing direct electrical current cardioversion: a prospective propensity Score-Matched Cohort Study. Am J Cardiovasc Drugs. 2019;19(4):421–427. doi:10.1007/s40256-019-00341-930838557
- Russo V, Rago A, Papa AA, D’Onofrio A, Golino P, Nigro G. Efficacy and safety of dabigatran in patients with atrial fibrillation scheduled for transoesophageal echocardiogram-guided direct electrical current cardioversion: a prospective propensity score-matched cohort study. J Thromb Thrombolysis. 2018;45(2):206–212. doi:10.1007/s11239-017-1599-529260427