Abstract
Purpose
Primary prophylaxis, using factor VIII replacement, is the recognized standard of care for severe hemophilia A. Recombinant factor VIII-Fc fusion protein (rFVIIIFc) and emicizumab, a humanized, bispecific antibody, are approved for routine prophylaxis of bleeding episodes in severe hemophilia A. These products have different mechanisms of action, methods of administration and treatment schedules. In the absence of head-to-head trials, indirect treatment comparisons can provide informative evidence on the relative efficacy of the two treatments. The aim of the study was to compare the approved dosing regimens for each product, rFVIIIFc individualized prophylaxis and emicizumab administered once every week (Q1W), every 2 weeks (Q2W) or every 4 weeks (Q4W), based on clinical trial evidence.
Patients and Methods
The comparison was conducted using matching-adjusted indirect comparison since clinical evidence did not form a connected network. Individual patient data for rFVIIIFc (A-LONG) were compared with data for emicizumab (HAVEN trial program) for mean annualized bleeding rate (ABR) and proportion of patients with zero bleeds. Safety data reported across the analyzed treatment arms were tabularized but not formally compared.
Results
After matching, no significant differences were observed between mean ABR for rFVIIIFc and emicizumab administered Q1W, Q2W or Q4W. The proportion of patients with zero bleeds was significantly higher with rFVIIIFc compared with emicizumab administered Q4W (51.2% versus 29.3%, respectively; odds ratio 2.53; 95% confidence interval 1.09–5.89); no significant differences noted when rFVIIIFc was compared with emicizumab administered Q1W or Q2W. The mean number of adverse events expressed per participant was 1.9 for individualized prophylaxis with rFVIIIFc and 3.7–4.0, 4.1 and 3.6 for emicizumab administered Q1W, Q2W or Q4W, respectively.
Conclusion
This indirect treatment comparison suggests that rFVIIIFc individualized prophylaxis is more efficacious than emicizumab Q4W, and at least as effective as more frequent emicizumab regimens, for the management of hemophilia A.
Introduction
Factor replacement products are the mainstay of treatment for individuals with hemophilia A,Citation1 a serious bleeding disorder characterized by frequent and spontaneous bleeding into joints and muscles.Citation2 Recurrent joint bleeding is a hallmark of severe hemophilia and a major cause of morbidity, leading to progressive irreversible joint damage and the development of hemophilic arthropathy.Citation3
Primary prophylaxis, using replacement factor VIII (FVIII), is the recognized standard of care for individuals with severe hemophilia A, and, initiated early in life, it can prevent joint damage and reduce the frequency of joint and other hemorrhages.Citation4,Citation5 Significant heterogeneity exists between patients with hemophilia with respect to bleeding phenotype and response to treatment; therefore, to optimize outcomes, treatment schedules should be flexible and tailored to individual patient’s needs.Citation6
Recombinant factor VIII-Fc fusion protein (rFVIIIFc) is approved for on-demand treatment and control of bleeding episodes, routine prophylaxis to reduce the frequency of bleeding episodes, and perioperative management of bleeding in pediatric, adolescent and adult patients with hemophilia A.Citation7,Citation8 The recommended dose for long-term prophylaxis according to the European label is 50 IU/kg every 3–5 days, which can be adjusted based on a patient’s response in the range 25–65 IU/kg,Citation8 thus providing an opportunity for adjusting dosing to the requirements of each individual patient.
The safety and efficacy of rFVIIIFc was established in two Phase 3 studies of previously treated pediatric (Kids A-LONG) and adult/adolescent (A-LONG) patients with severe hemophilia A.Citation9,Citation10 An individualized prophylaxis regimen, which aimed for trough levels of 1–3 IU/dL in adults/adolescents, provided clinically meaningful reductions in annualized bleeding rates (ABRs) compared with on-demand treatment.Citation9 These results were confirmed in an extension study (ASPIRE), with low ABRs and extended dosing intervals sustained for up to 5.9 years of treatment.Citation11
Emicizumab is a recombinant humanized, bispecific, monoclonal antibody that mimics the function of activated FVIII by bridging activated factors IX and X to induce coagulation at the site of bleeding.Citation12 Emicizumab is approved for routine prophylaxis of bleeding episodes in patients with severe hemophilia A with or without FVIII inhibitors.Citation13,Citation14 It is administered as a subcutaneous injection at the recommended dose of 3 mg/kg once weekly for the first 4 weeks (loading dose), followed by a maintenance dose of 1.5 mg/kg once weekly, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks. The safety and efficacy of emicizumab was investigated in the HAVEN clinical trial program.Citation15–Citation18
rFVIIIFc and emicizumab have different modes of action, methods of administration and treatment schedules, and despite the lack of head-to-head clinical trial evidence, a comparison of these therapeutic strategies would be beneficial. In this regard, the aim of this study was to compare the efficacy of rFVIIIFc individualized prophylaxis versus emicizumab for the treatment of patients with hemophilia A, based on clinical trial evidence.
Patients and Methods
Data Sources and Sample Selection
The pivotal trials, which provided efficacy and safety data for market authorization, were used as source data for comparison of the approved dosing regimens for each product (rFVIIIFc individualized prophylaxis;Citation7,Citation8 and emicizumab administered once every week [Q1W], every 2 weeks [Q2W] or every 4 weeks [Q4W])Citation13,Citation14, in the target population of adult/adolescents (≥12 years) with hemophilia A without inhibitors. Methodology and findings of these trials (A-LONG for rFVIIIFc; HAVEN clinical trial program for emicizumab) have been described previously.Citation9,Citation16,Citation17
Briefly, A-LONG was a phase 3 open-label, multicenter, partially randomized study of rFVIIIFc in patients aged ≥12 years of age with severe hemophilia A.Citation9 Enrolled patients were assigned to one of the three treatment arms: individualized prophylaxis (25–65 IU/kg every 3–5 days; n=118), weekly prophylaxis (65 IU/kg; n=24), or episodic treatment (10–50 IU/kg; n=23). Prior to enrollment, patients in the individualized prophylaxis arm could have received FVIII as prophylaxis or on-demand, while those recruited to the other two arms could only have received on-demand treatment. In the individualized prophylaxis arm, to maintain good control of breakthrough bleeding, each patient’s pharmacokinetic (PK) parameters were used to guide individual adjustments to dosing interval (down to 3 days or up to 5 days) and/or dose (up to 65 IU/kg) to target a steady-state FVIII trough level of 1–3 IU/dL. Adjustments were also made if a patient experienced two spontaneous bleeding episodes within an 8-week period.
For emicizumab, data were included from both HAVEN 3 and HAVEN 4. HAVEN 3 was a partially randomized study of emicizumab in patients aged ≥12 years of age with severe hemophilia A without current FVIII inhibitors (<0.6 Bethesda units per mL).Citation16 Participants receiving episodic therapy with FVIII were randomly assigned in a 2:2:1 ratio to receive emicizumab Q1W (1.5 mg/kg; group A; n=36) or Q2W (3.0 mg/kg group B; n=35), or to continue on-demand therapy with FVIII (group C, n=18). An additional 63 patients who received FVIII prophylaxis before study entry were allocated to group D and received prophylaxis with emicizumab Q1W (1.5 mg/kg). HAVEN 4 was a non-randomized, single-arm study of emicizumab in patients aged ≥12 years of age with severe hemophilia A (n=41), which included five patients with hemophilia A with inhibitors undergoing treatment with FVIII concentrates or bypassing agents.Citation17 All patients received a subcutaneous loading dose of emicizumab of 3 mg/kg Q1W for the initial 4 weeks, followed by emicizumab prophylaxis Q4W (6.0 mg/kg) for at least 24 weeks. Data from all prophylaxis arms of both studies were used in the analysis, which included patients treated with 1.5 mg/kg Q1W, 3.0 mg/kg Q2W and 6.0 mg/kg Q4W.Citation16,Citation17 HAVEN 1 and HAVEN 2 included patients with hemophilia A with inhibitors aged ≥12 years or <12 years of age, respectively, and were excluded from the analysis.Citation15,Citation18
Methodology of Indirect Comparisons
The pivotal trials included in the analysis had some similarities in their design. Both A-LONG and HAVEN 3 comprised randomized and non-randomized arms, while HAVEN 4 was a non-comparative, single-arm study. In both A-LONG and HAVEN 3, on-demand treatment was assessed as a reference regimen within the randomized arms of each study. Despite this, a network meta-analysis including the on-demand arms was considered unfeasible, since rFVIIIFc individualized prophylaxis was assessed within a separate, non-randomized arm of A-LONG and could not be assessed in relation to on-demand treatment. In the absence of head-to-head trials and/or a connected network of clinical evidence, the matching-adjusted indirect comparison (MAIC), which adjusts for differences in baseline characteristics between treatments, was selected as the most suitable method to compare rFVIIIFc with emicizumab for the prophylactic treatment of hemophilia and was performed according to guidelines developed by the National Institute for Health and Care Excellence Decision Support Unit.Citation19 Individual patient data (IPD), including baseline characteristics and effects observed in the individualized prophylaxis arm, were available for rFVIIIFc from the A-LONG trial. Data were anonymized and no information allowing individual patients to be identified was included. Each individual patient was assigned a weight calculated from the logistic regression model (see equation presented below), so that weighted mean baseline characteristics of the study population match the baseline characteristics reported for the comparator trial.Citation20
Where:
Xit = the covariate vector for the i-th individual receiving treatment t
Wit = weight assigned to the i-th individual receiving treatment t
The weights assigned to each patient individually can be interpreted as the estimated odds (relative propensity) of being in the comparator trials relative to the original study (A-LONG) and the weighted baseline characteristics of the A-LONG trial match the characteristics of the comparator population. The weights were used to recalculate the effect of the treatment in order to allow for the population-adjusted comparison with the estimates observed for the comparator.
Safety data reported across the analyzed treatment arms in the identified studies were tabularized but not formally compared using MAIC methodology since there was no unequivocal evidence for the interaction between baseline variables and the risk of adverse events (AEs).
Outcome Assessments
Efficacy outcomes assessed were mean ABR and proportion of patients with zero bleeds. These outcomes are clinically relevant and frequently reported treatment outcomes in clinical trials of hemophilia. The A-LONG protocol stipulated all bleeding events required administration of FVIII, regardless of severity; therefore, the estimates for the incidence of bleeding episodes reported for the A-LONG trial refer to all bleeding episodes. In the HAVEN 3 and HAVEN 4 trials, data were collected for all bleeds (treated and untreated) and treated bleeds; the clear algorithm defining which events qualified for FVIII treatment in the HAVEN program was not provided. For consistency with A-LONG, data for all bleeds were used in this analysis.
Data Analysis
IPD for rFVIIIFc from the A-LONG trial were weighted and matched to aggregated corresponding baseline characteristics for emicizumab in the comparator trials. Baseline variables included for adjustment were: age (mean, (standard deviation [SD]); target joint, including mean (SD) number of target joints (when available), or proportion of patients with 1 or ≥2 target joint(s); proportion of patients with prior prophylaxis; ethnicity (proportion of white patients); and treatment duration (mean, SD). Outcomes were recalculated using assigned weights; mean ABR was estimated using weighted negative binomial regression model (using R software v.3.5.5 with MASS package), which was consistent with the analysis in HAVEN trials. Odds of zero bleeds were calculated by dividing the reweighted number of patients with and without bleeding episodes. Weighted outcomes from A-LONG were statistically compared with observed values for emicizumab.
For each adjustment, the matched baseline characteristics are presented with the corresponding estimates of effective sample size for patients receiving rFVIIIFc. Recalculated ABR and the odds of patients with zero bleeds were compared with the estimates related to emicizumab. The odds ratio (OR) was calculated using the standard formula.Citation21 Relative treatment effects are presented as incidence rate ratios (IRR) with 95% confidence intervals (CI) for ABR, and ORs with 95% CI for the proportion of patients with zero bleeds. The IRR, together with the associated 95% CI, was calculated as the exponent of the difference between the log values of ABRs for rFVIIIFc and emicizumab.Citation21 A difference in IRR or OR was considered statistically significant when the associated 95% CI did not include 1.0. Statistical comparisons were conducted in R (R v.3.5.5 [https://www.r-project.org/]).
Results
Baseline Characteristics Before Matching
The analysis included 117 patients who received rFVIIIFc individualized prophylaxis in A-LONG, and 99 and 41 patients from HAVEN 3 and HAVEN 4, respectively, who received emicizumab. Median age was 29 years in A-LONG and ranged from 36 to 41 years in the two HAVEN trials. Across the trials, the length of treatment varied; in A-LONG median duration of rFVIIIFc treatment in the individualized prophylaxis arm was 32.1 weeks; the median duration of the efficacy period ranged from 29.6 to 33.7 weeks in HAVEN 3 and was 25.6 weeks in HAVEN 4.
Prior to study entry, the treatment regimen was prophylaxis in 73.7% of patients in the individualized prophylaxis arm in A-LONG, and 41.4% and 73.0% in HAVEN 3 and HAVEN 4, respectively. At baseline, the proportion of patients with ≥1 target joint was 68.5% in A-LONG. Overall, in HAVEN 3 and HAVEN 4, the proportion of patients with ≥1 target joint across the treatment arms was 41.3–94.4% and 61.0–86.0%, respectively. In A-LONG, the median number of bleeding events in the 12 months prior to study entry was 6.0 and 27.0 in patients receiving a prior prophylaxis or prior episodic regimen, respectively. In the HAVEN program, the proportion of bleeding events was reported for the prior 24 weeks only. In HAVEN 3 the proportion of patients with <9 bleeding events in the 24 weeks before trial entry was 25.0%, 14.3%, 22.2% and 84.1% in treatments arms A to D, respectively. The median number of bleeding events in the 24 weeks before study entry was 5.0 in HAVEN 4.
Matching of Baseline Characteristics
IPD for rFVIIIFc from the individualized prophylaxis arm of A-LONG were matched to the baseline characteristics of emicizumab Q1W (n=99; Supplementary Table 1), Q2W (n=35; Supplementary Table 2) and Q4W (n=41; Supplementary Table 3). The effective sample size (ESS) for rFVIIIFc for each comparison after matching was n=94 (Q1W), n=19 (Q2W) and n=36 (Q4W), respectively.
Annualized Bleeding Rate, All Bleeds
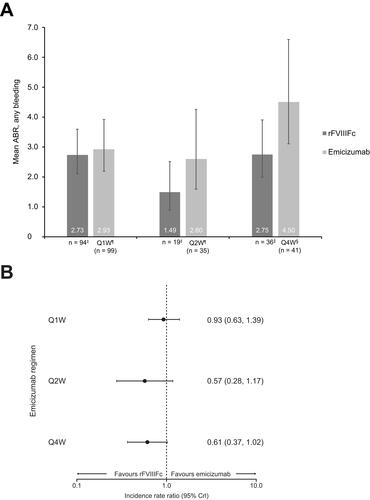
After matching, the mean ABR was 2.73 for individualized prophylaxis with rFVIIIFc and 2.93 for emicizumab administered Q1W. The difference in ABR between the two treatments was not statistically significant (IRR 0.93; 95% CI 0.63–1.39; ). Similarly, there was no statistically significant difference between mean ABR for individualized prophylaxis with rFVIIIFc and emicizumab administered Q2W (1.49 versus 2.60; IRR 0.57; 95% CI 0.28–1.17) and Q4W (2.75 versus 4.50; IRR 0.61; 95% CI 0.37–1.02).
Figure 1 (A and B) Mean ABR, any bleeding, after matching for all selected baseline variables;†forest plot represents relative treatment effects presented as IRR with 95% CI.
Proportion of Patients With Zero Bleeds
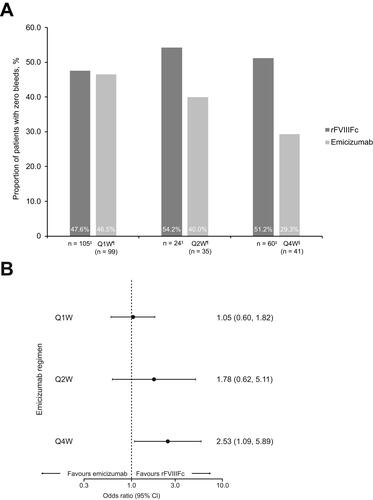
The proportion of patients with zero bleeds was significantly higher with individualized prophylaxis with rFVIIIFc compared with emicizumab administered Q4W (51.2% versus 29.3%, respectively; OR 2.53; 95% CI 1.09–5.89; ). There were no statistically significant differences in the proportion of patients with zero bleeds between rFVIIIFc and emicizumab administered Q1W (47.6% versus 46.5%; OR 1.05; 95% CI 0.60–1.82) or Q2W (54.2% versus 40.0%; OR 1.78; 95% CI 0.62–5.11).
Figure 2 (A and B) Proportion of patients with zero bleeds after matching for all selected baseline variables; †forest plot represents relative treatment effects presented as OR with 95% CI.
Safety
A summary of safety data from the individualized prophylaxis arm of A-LONG and all prophylaxis arms from HAVEN 3 and HAVEN 4 is presented in Supplementary Table 4. The mean number of AEs expressed per participant was 1.9 for individualized prophylaxis with rFVIIIFc, and 3.7–4.0, 4.1 and 3.6 for emicizumab administered Q1W, Q2W and Q4W, respectively. Injection site reactions were reported in 20–32% of patients receiving respective regimens of emicizumab prophylaxis, and thus were among the most frequently reported events. Injection site reactions were not reported among AEs in patients receiving individualized prophylaxis with rFVIIIFc in A-LONG. The proportions of patients reporting arthralgia, headache and upper respiratory tract infection were numerically lower with rFVIIIIFc individualized prophylaxis than those observed in the respective emicizumab arms. There was no evidence of differences regarding serious AEs between treatments.
Discussion
The results of this MAIC analysis indicate that rFVIIIFc is more efficacious than emicizumab Q4W and at least as efficacious as more frequent emicizumab regimens, for the management of patients with hemophilia A. Individualized prophylaxis with rFVIIIFc, the approved dosing regimen,Citation7,Citation8 was shown to be associated with a significantly greater proportion of patients with zero bleeds than emicizumab administered Q4W, while no statistically significant differences were observed in the proportion of patients with zero bleeds when rFVIIIFc was compared with emicizumab administered Q1W or Q2W. In addition, there were no statistically significant differences for mean ABR with individualized prophylaxis with rFVIIIFc and emicizumab administered Q1W, Q2W or Q4W. Although five of the six comparisons of bleeding events did not achieve statistical significance, clear trends in favor of rFVIIIFc were observed.
In the A-LONG study, the prophylactic dosing regimen of rFVIIIFc was not designed to optimize the prevention of bleeds; the protocol prescribed a PK-tailored dosing regimen aimed at targeting FVIII trough levels between 1 and 3 IU/dL. This relatively modest treatment target may make a comparison with emicizumab unfavorable for rFVIIIFc, as emicizumab more than likely reached its ceiling limit for optimal prophylactic efficacy with the published dosing regimens.
The results of the current study contrast with the publication by Reyes et al, the results of which suggested the superiority of prophylaxis with emicizumab over FVIII prophylaxis (combined comparator of four different FVIII concentrates, including rFVIIIFc) in patients with hemophilia A without inhibitors.Citation22 Importantly, the Reyes analysis included data from the weekly prophylaxis arm of A-LONG, which is not aligned to the approved dosing regimens of rFVIIIFc, and may have led to the overestimation of ABRs for rFVIIIFc, thus boosting the relative efficacy of emicizumab in comparison. Detailed analysis of the Reyes study has been described elsewhere.Citation23
For the current analysis, several methodologies were considered, including a network meta-analysis as utilized by Reyes et al,Citation22 a meta-analysis using the Bucher method and MAIC. Patients were not randomly assigned to the individualized prophylaxis arm with rFVIIIFc in A-LONG, and therefore, a connected network of evidence could not be formed between the rFVIIIFc individualized prophylaxis and emicizumab prophylaxis arms. As such, the standard meta- and network meta-analyses were considered not appropriate for these trial data. However, an indirect treatment comparison was still feasible, and it was important to adjust for differences in baseline characteristics between studies. MAIC is a validated method for the comparison of outcomes of interventions, which can overcome methodological limitations of indirect comparisons and network meta-analyses.Citation24 IPD from studies of one treatment are matched with aggregate data from published studies of another treatment, allowing treatment outcomes to be compared across balanced trial populations; thus, reducing observed cross-trial differences.Citation20 In the absence of head-to-head studies, we propose MAIC as the most robust method to compare treatments and the most appropriate for the current analysis.
Prophylaxis with FVIII replacement products is the standard of care for the management of hemophilia A; treatment has evolved and is focused on increasing protection by raising factor levels above previous targets. This has the potential to allow a more active lifestyle with improved outcomes, including prevention of bleeding and joint disease progression and thus, potentially a better quality of life.Citation25 The HAVEN trial program demonstrated the efficacy of emicizumab for the treatment of patients with hemophilia A both with and without inhibitors.Citation16,Citation17 However, long-term data are not currently available and there are some safety concerns associated with a risk of thrombosis in combination with other procoagulant drugs.Citation1 Of note, during the HAVEN 4 study, 61% of patients received at least one concomitant dose of FVIII concentrates or bypassing agents and 39% received these treatments prior to activities that may lead to bleeding.Citation17 This indicates that the ABR with emicizumab prophylaxis alone is likely to be underestimated. A pre-specified sub-group analysis of HAVEN 4 concluded that emicizumab efficacy (6 mg/kg Q4W) was unaffected by FVIII inhibitor status, presence of target joints, or type of previous FVIII or bypassing agent treatment regimen (episodic versus prophylactic).Citation17 This reinforces the validity of the current analysis, as based on this conclusion, the ABRs in patients treated with emicizumab are not influenced by the presence of inhibitors, although the number of patients with inhibitors in HAVEN 4 is very low (expansion cohort, n=5).
The study has the following limitations. The outcomes assessed in our analysis were restricted to mean ABR and proportion of patients with zero bleeds. It would have been interesting to compare both interventions in terms of additional outcomes, such as FVIII utilisation to prevent bleeds before physical activity; however, relevant information was not provided in the HAVEN trials. In addition, there was a loss of sample size when comparing rFVIIIFc with emicizumab Q2W (ESS=19) and Q4W (ESS=36), which was largely due to the discrepancies in the proportion of patients with previous prophylaxis (73.5% versus 0%) and treatment duration (32.6 weeks versus 26.6 weeks), respectively. Furthermore, the adjustment for other confounding factors, eg weight, geographic region, FVIII genotype, which may also have an influence on the findings reported here, were considered; unfortunately, this was not possible as relevant information was not provided in the HAVEN trials. Safety outcomes reported across the treatment arms included in this analysis are presented; the mean number of AEs reported per participant was numerically lower for rFVIIIFc than for emicizumab (1.9 versus 3.6–4.1). Emicizumab was also associated with frequent injection site reactions, which were not reported for patients receiving individualized prophylaxis with rFVIIIFc in A-LONG. The proportions of patients reporting arthralgia, headache and upper respiratory tract infection were also numerically lower with rFVIIIFc than for those observed in the respective emicizumab arms. These AEs can potentially increase the burden associated with long-term emicizumab prophylaxis. However, no formal statistical analysis was carried out and this should be considered when interpreting these data. In addition, safety outcomes from the long-term follow-up studies should be considered for the full characterization of safety profiles.
Conclusion
This indirect treatment comparison indicates that rFVIIIFc individualized prophylaxis is more efficacious than emicizumab administered Q4W for the proportion of patients with zero bleeds. Similar efficacy was observed for mean ABR with rFVIIIFc individualized prophylaxis compared with emicizumab administered Q1W, Q2W and Q4W, with trends in favor of rFVIIIFc.
Abbreviation
ABR, annualized bleeding rate; CI, confidence interval; ESS, effective sample size; FVIII, factor VIII; IPD, Individual patient data; IRR, incidence rate ratio; rFVIIIFc, Recombinant factor VIII-Fc fusion protein; MAIC, matching-adjusted indirect comparison; OR, odds ratio; PK, pharmacokinetic; Q1W, once every week; Q2W, every 2 weeks; Q4W, every 4 weeks; SD, standard deviation.
Author Contributions
PW, SA, ZH and JN collected and interpreted the data. RK, FD, LAF, SL, ES, MDT interpreted the data. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The project was funded by Swedish Orphan Biovitrum AB (Sobi). Medical writing and editorial support, funded by Sobi, was provided by Rachel Bell, PhD, Bioscript Medical, Macclesfield, UK.
Disclosure
RK reports research funding and honoraria for consulting and lectures from Bayer, Biomarin, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Takeda/Shire and Sobi. PW, SA and FD are employees of Creativ-Ceutical a consultancy company that received funding from Sobi for this research. ZH, JN, LAF, SL and ES are employees of Sobi. MDT reports speaking and consultancy fees from Amgen, HemaBiologics, Pfizer, Principia, Takeda, Grifols, Octapharma and Biomarin; consultancy fees from Novo Nordisk, Genetech and Roche; and trial investigator for Takeda and Spark Therapeutics. He has a private practice that offers in and out-patient consultation services and the CEO and CFO for Bleeding and Clotting Disorders Institute. The authors report no other conflicts of interest in this work.
References
- Aledort L, Mannucci PM, Schramm W, Tarantino M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479–486. doi:10.2450/2019.0211-19
- National Organization for Rare Disorders (NORD®). Haemophilia A. Available from: https://rarediseases.org/rare-diseases/hemophilia-a/. Accessed October 2020.
- Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8(9):1895–1902. doi:10.1111/j.1538-7836.2010.03962.x
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi:10.1056/NEJMoa067659
- Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM, Group ES. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700–710. doi:10.1111/j.1538-7836.2011.04214.x
- Morfini M, Benson G, Jimenez-Yuste V, et al. Tailoring care to haemophilia patients’ needs: which specialty and when? Blood Transfus. 2015;13(4):644–650. doi:10.2450/2015.0302-14
- Bioverativ Therapeutics Inc. ELOCTATE® Prescribing Information; 2019. Available from: https://www.fda.gov/media/88746/download. Accessed October 2020.
- Swedish Orphan Biovitrum AB (publ). ELOCTA® summary of product characteristics; 2019. Available from: https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf. Accessed October 2020.
- Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. doi:10.1182/blood-2013-10-529974
- Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967–977. doi:10.1111/jth.12911
- Nolan B, Mahlangu J, Pabinger I, et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemophilia. 2020;26(3):494–502. doi:10.1111/hae.13953
- Franchini M, Marano G, Pati I, et al. Emicizumab for the treatment of haemophilia A: a narrative review. Blood Transfus. 2019;17(3):223–228. doi:10.2450/2019.0026-19
- Genetech Inc. Hemlibra® prescribing information; 2018. Available from: https://www.gene.com/download/pdf/hemlibra_prescribing.pdf. Accessed October 2020.
- Roche Pharma AG Hemlibra® summary of product characteristics; 2019. Available from: https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf. Accessed October 2020.
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. doi:10.1056/NEJMoa1703068
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia a without inhibitors. N Engl J Med. 2018;379(9):811–822. doi:10.1056/NEJMoa1803550
- Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–e305. doi:10.1016/S2352-3026(19)30054-7
- Young G, Liesner R, Chang T, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127–2138. doi:10.1182/blood.2019001869
- Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU Technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE; 2016. Available from: http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf. Accessed October 2020.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi:10.1016/j.jval.2012.05.004
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). Chochrane; 2011. Available from: www.training.cochrane.org/handbook. Accessed October 2020.
- Reyes A, Revil C, Niggli M, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia A without inhibitors: network meta-analysis and sub-group analyses of the intra-patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2019;35(12):2079–2087. doi:10.1080/03007995.2019.1649378
- Jain N, Lethagen S, Reyes A RE, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia A without inhibitors: network meta-analysis and sub-group analyses of the intra-patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2020;36(7):1125–1127. doi:10.1080/03007995.2020.1744549
- Batt K, Gao W, Ayyagari R, et al. Matching-adjusted indirect comparisons of annualized bleeding rate and utilization of BAY 94-9027 versus three recombinant factor VIII agents for prophylaxis in patients with severe hemophilia A. J Blood Med. 2019;10:147–159. doi:10.2147/JBM.S206806
- Jimenez-Yuste V, Auerswald G, Benson G, et al. Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: the past, the present and the future. Blood Transfus. 2014;12(3):314–319. doi:10.2450/2014.0298-13
