Abstract
Background and Objectives
Candida albicans is a significant source of morbidity and mortality for patients with acute myeloid leukemia (AML). Prolonged use of fluconazole as empirical antifungal prophylaxis in AML patients leads to overexpression of efflux pump genes that resulted in the emergence of azole-resistant species. Consequently, the introduction of a new strategy to improve the management of C. albicans infections is an urgent need. Nonsteroidal anti-inflammatory drug (NSAID) ketorolac is associated with a reduction in cancer relapses. The present study was performed to investigate the use of ketorolac-fluconazole combination to reverse fluconazole resistance in C. albicans isolated from AML patients on induction chemotherapy.
Patients and Methods
One hundred and seventy AML patients were evaluated. Fifty C. albicans were isolated and subjected to disc diffusion assay and broth microdilution for fluconazole alone and combined with different concentrations of ketorolac. Efflux pump gene (CDR1, CDR2, and MDR1) expressions were quantified by real-time PCR.
Results
The tested ketorolac acted synergistically with fluconazole against resistant C. albicans with the minimum inhibitory concentration (MIC) of fluconazole decreased from >160 μg/mL to 0.3–1.25 μg/mL in (93.8%) of resistant isolates with fractional inhibitory concentration index (FICI) value of 0.25. The majority of the resistant isolates overexpressed CDR1 (71.1%) and MDR1 (60%).
Conclusion
Ketorolac-fluconazole in vitro combination would be a promising strategy for further clinical in vivo trials to overcome fluconazole resistance in AML patients on induction chemotherapy.
Introduction
Acute myeloid leukemia (AML) is a hematological disease caused by the clonal expansion of myeloblasts in the peripheral blood, bone marrow, or other tissues. It is characterized by various chromosomal abnormalities and gene mutations.Citation1 The typical clinical manifestations of AML are fever, fatigue, and bleeding caused by the expansion of blasts and decreased normal hematopoiesis in the bone marrow. Treatment of AML by combination chemotherapy results in persistent neutropenia, which further increases the risk of opportunistic infections.Citation2
Candida spp. is an important opportunistic human pathogen that causes oropharyngeal candidiasis (OPC), vulvovaginitis, and invasive infections in AML patients.Citation3 The of American Infectious Diseases Society has approved fluconazole as a primary drug of choice for prophylaxis and treatment of candidiasis.Citation4
Fluconazole has several advantages over other antifungal drugs including the safety, oral bioavailability, cost, and ability to cross the blood–brain barrier.Citation5 Fluconazole inhibits the cytochrome P450 enzyme lanosterol demethylase, a critical enzyme in the synthesis of ergosterol which is encoded by the ERG11 gene.Citation6 However, the extensive use of fluconazole as empirical therapy in cancer patients, especially in AML patients, has increased the incidence of resistance to the drug among different fungal strains, especially Candida albicans.Citation7 Fluconazole resistance has the potential to cross over to other azoles including voriconazole and itraconazole.Citation8
There are multiple mechanisms for azole resistance, the major one is overexpression of plasma membrane efflux pumps.Citation9 The ATP-binding cassette (ABC) pumps and the major facilitator superfamily (MFS) transporters are the two main families of efflux proteins. They differ in the source of energy used for their activity and encoded by Candida drug resistance (CDR1 and CDR2) and multidrug resistance (MDR1) genes. They are located in the plasma membrane and are responsible for pumping the drug out of the fungal cell decreasing its intracellular concentration which leads to treatment failure.Citation10
Several studiesCitation11,Citation12 have stated the ability of some drugs to reverse azole resistance in C. albicans. Generally, these drug concentrations required to reverse the azole resistance are above the therapeutic concentrations. Moreover, some of these drugs can result in serious side effects, such as those caused by cyclosporine and tacrolimus.Citation13 Remarkably, a few studies have reported the ability of some nonsteroidal anti-inflammatory drugs (NSAIDs) to act synergistically with different antifungals. For example, ibuprofen was found to exhibit synergistic activity with azoles against Candida spp.Citation14 NSAIDs have antipyretic, analgesic, and can be used alone or in combination with other drugs for the treatment of cancer. They also have direct and indirect antimicrobial effects.Citation15 The antifungal activities of NSAIDs against Candida spp. include the reduction of extracellular polysaccharide, hyphal, and biofilm formations.Citation16
To repurpose drugs and explore new leads in the field of antifungal drug discovery; we explored another nonsteroidal anti-inflammatory drug “ketorolac” to reverse azole resistance in C. albicans. Ketorolac is a potent analgesic, antipyretic, and moderate anti-inflammatory drug used in the treatment of severe cancer pain. The simple use of this safe and effective anti-inflammatory agent might eliminate most early cancer relapses.Citation17
In the current study, we investigate a new strategy to improve the management of C. albicans infections through the use of in vitro ketorolac-fluconazole in combination to reverse fluconazole resistance in C. albicans isolated from AML patients and overexpressing efflux pumps genes (CDR1, CDR2, and MDR1) assessed by quantitative real-time qRT-PCR.
Materials and Methods
Ethical Statement
This study was approved by the Local Ethics Committee (no. 17100543), Faculty of Medicine, Assiut University in accordance with the provisions of the Declaration of Helsinki. Informed written consent was obtained from all patients before enrolment in the study.
Patients
This study included 170 AML patients admitted from October 29, 2016, to March 11, 2020, to the Department of Internal Medicine (Hematology Unit), and South Egypt Cancer Institute (SECI) in Assiut University, Assiut, Egypt. All newly diagnosed non-M3 AML patients (aged 18–69 years) were enrolled in this study. The patients were diagnosed according to the WHO criteria for AML.Citation18 Standard induction chemotherapy for 170 non-M3 AML patients in this study was idarubicin 12 mg/m2 per day for two to three days, and cytarabine 100 mg/m2/day for five to seven days. Patients received prophylactic treatment during the period of neutropenia following chemotherapy in the form of sulfamethoxazole (400 mg/trimethoprim 80 mg once or twice daily). Patients received empirical azole prophylaxis, fluconazole (400 mg PO/IV per day).Citation19 During induction chemotherapy, granulocyte colony-stimulating factor (G-CSF) was used in a few cases showing poor performance status and not applied routinely for all patients. The definition of treatment response generally followed the European Leukemia Network (ELN) 2010 recommendation.Citation20 Complete remission (CR) was defined as a blast count less than 5% in the bone marrow. Partial remission (PR) was defined as a decrease in bone marrow blasts by 50% but still remaining in a range of 5–25%. Resistant disease (RD) was referred to those who failed to achieve CR or PR in bone marrow examination after chemotherapy. The undefined response was referred to those who had no available results of bone marrow examination after chemotherapy. Exclusion criteria including AML (M3), AML with antecedent hematologic malignancy, Patients with possible risk factors of candidiasis, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) positive, diabetes, and autoimmune diseases. Patients with contraindication to fluconazole therapy as abnormal heart rhythm, prolonged QT interval on EKG, abnormal liver function tests, pregnancy, and chronic kidney disease: moderate to severe stage. Data were collected and included the following parameters: age, gender, and, cytogenetic results at diagnosis, induction regimens, and treatment response, .
Table 1 Clinical Data of 170 Patients with Newly Diagnosed Acute Myeloid Leukemia
Standard Control Strain
C. albicans ATCC 10231 was obtained from MIRCIN culture collection of the Faculty of Agriculture, Ain Shams University, Egypt.
Clinical Sample Collection and Processing
Samples collected from the patients according to their clinical presentation and different localizing symptoms. Vaginal swabs from patients with vaginal infection. Oropharyngeal swabs from patients with oral thrush were rubbed on the candidiasis lesion. Swabs were streaked on Sabouraud dextrose agar medium supplemented with chloramphenicol (0.5 g/L) (SDA, HiMedia, India) and incubated at 30°C for 48 h.Citation21 Candida spp. shows typical creamy white pasty colonies with characteristic yeasty odor. Each isolated Candida spp. was individually stored at −20°C with 20% glycerol.Citation22
Phenotypic Identification of Candida albicans Strains
Germ tube test: by inoculating yeasts in small tubes containing 0.5 mL of human serum (Sigma-Aldrich, Germany) containing 0.5% glucose and incubated at 37°C for two to three hours. It is positive for C.albicans or C. dubliniensis.Citation22
Corn meal agar (CMA): by inoculating yeasts on CMA containing Tween 80 (Difco, USA), for four to seven days to ensure production of chlamydospores. It is positive for C. albicans or C. dubliniensis.Citation22
CHROMagar® Candida medium: (CHROMagar, Paris, France) which allows selective isolation and identification of C. albicans, C. dubliniensis, C. tropicalis, and C. krusei by morphology and color reaction. The strains were identified as C. albicans or C. dubliniensis for green colonies, C. tropicalis for steel blue colonies, C. krusei colonies showing rose color.Citation21,Citation22
Growth at 45°C: Growth at 45°C has been considered a useful test for the differentiation of C. dubliniensis (no growth) from C. albicans (growth).Citation21,Citation22
Sugar assimilation test: using API 20C AUX (bioMérieux SA, France) according to the manufacturer’s instructions.Citation23
Genotypic Identification of Candida albicans Strains by Polymerase Chain Reaction (PCR)
The species specific primer pair sequence for the amplification of the 25S rRNA gene was described by Mannarelli and Kurtzman;Citation24 (5ʹTGTTGCTCTCTCGGGGGCGGCCG3ʹ and 5ʹ AGATCATTATGCCAACATCCTAGGTTAAA 3ʹ). It is specific for C. albicans and amplifies a 175-bp DNA fragment. DNA extraction was done by a commercial QIAamp DNA Mini Kit (Qiagen, Germany). DNA amplification performed using the Thermocycler T100 gradient system (BioRadT100, USA). PCR reaction mixture and PCR amplification conditions were performed according to the method described by Marinho et al.Citation22 Amplification product visualized by electrophoresis on 2% agarose gel using a 100-bp ladder molecular weight ladder (Gen Ruler 100bp DNA ladder plus).
Antifungal Susceptibility Test
Disk Diffusion Method
Adopted by the Clinical and Laboratory Standards Institute (CLSI), the M44-A2 protocolCitation25 was used to evaluate the degree of fungal sensitivity for four common azoles. Antifungal discs were obtained from (HiMedia, India). The response to the antifungal agents was determined via the interpretive breakpointsCitation26 described in .
Table 2 Interpretative Breakpoints of Disk Diffusion Method for Fluconazole, Itraconazole, Miconazole, and VoriconazoleCitation26
Determination of MICs by Broth Microdilution
The MICs of fluconazole (Sigma, USA) and ketorolac (Sigma, USA) separately were identified by the protocol recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).Citation27 The test was conducted in 96-well microtiter plates with yeast (2.5×105 CFU/mL) in RPMI-1640 medium (PH 7.0) buffered with MOPS (Sigma, USA) and supplemented with glucose to a final concentration of 2%. RPMI-1640 containing wells were considered negative controls, and a drug-free well was set as growth control. After 24 h of incubation at 35°C, MIC values were determined with a spectrophotometer (wavelength of 450 nm; ETI System Fast Reader ELX, BioTek, US) as the lowest concentration of drug that resulted in >50% inhibition of growth relative to that of the growth control. MIC interpretive criteria of fluconazole for C. albicans were those described in the document E.DEF 7.3.1 [available on the EUCAST website: http://www.eucast.org]. MIC ≤2.0 mg/L was considered to be sensitive, MIC between 2.0 and 4.0 mg/L was considered to be intermediate and that >4.0 mg/L was considered resistant.
Fractional Inhibitory Concentration Index (FICI)
The intensity of interaction between ketorolac and fluconazole antifungals against azole-resistant C. albicans clinical isolates was determined briefly as follow, drugs were serially diluted 2-fold in RPMI-1640 medium, and 0.31–160 μg/mL fluconazole and 0.16–10 μg/mL ketorolac were added to the wells. Subsequently, yeast at a final concentration of 2.5×105 CFU/mL was added to each well. Wells containing only RPMI-1640 medium served as negative controls, and a drug-free well was set as a growth control. After 24 h of incubation at 35°C, MICs were determined as described above. To evaluate the intensity of the drug interactions, the fractional inhibitory concentration index (FICI) model was used to analyze the obtained data. The FICI model is based on the Loewe additivity theoryCitation28 and is expressed as FICI=FICA+FICB=(A/MICA)+(B/MICB) where A and B are the MIC of each drug in combination (in a single well), and MICA and MICB are the MIC of each drug individually. The drug interaction is interpreted as synergistic when FICI ≤0.5, indifferent when FICI >0.5–4.0, and antagonistic when FICI >4.0.Citation29
Efflux Pump Genes Expression Analysis by qRT-PCR
Reverse Transcriptase (RT)-PCR
The reverse transcription was performed using HiSenScript™ RH(-) cDNA Synthesis Kit (iNtRON, Cat. no. 25014) according to the manufacturer’s instructions. Reverse transcription was performed at 45°C for 60 min, followed by 80°C for 10 min. The cDNA products were stored at −20°C for later use as templates for quantitative real-time PCR (qPCR).
Real-Time PCR
The procedures of qRT-PCR analysis were described in a previous study.Citation30 Expression levels of the target genes (CDR1, CDR2, and MDR1) and the housekeeping gene (ACT1, used as a normalizing gene) were assessed by quantitative real-time RT-PCR (qRT-PCR). The primers (analysis) used tested through the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST) and listed in .Citation31 The expression of target genes was carried out using SensiFAST SYBR® No-ROX (Bioline, USA) using a 7500 Fast Real-Time PCR System (Applied Biosystems). The fold change in expression of these genes was calculated according to the 2^(-ΔΔCT) method using ACT1 as a control gene to normalize cDNA levels.Citation32 Fluconazole-susceptible C. albicans clinical isolate (MIC, 0.5 mg/L) was used as a reference isolate for gene expression analysis. Real-time PCR reaction mix for (CDR1, CDR2, MDR1 and ACT1) contained 0.5 μL of cDNA,1.5 μL of 2 μm primer and 7.5 μL of 2×SYBR Green Master Mix in a final volume of 15 μL and qRT-PCR was performed using the following cycling conditions: 95°C for 10 min, 40 cycles of 95°C for 15 seconds, 50°C for 15 seconds for CDR1, CDR2, and ACT1, or 55°C for 15 seconds for MDR1, and 60°C for 30 seconds.
Table 3 Gene-specific Primers Used for RT- qPCRCitation31
Statistical Analysis
Data entry and data analysis get done using SPSS (Statistical Package for Social Science) version 19. Data were presented as a number, percentage, mean, median, standard deviation, and standard error. Chi-squared test and Fisher’s exact test were used for comparing qualitative variables. The Mann–Whitney U‐test was used to compare nonparametric tests. P-value considered statistically significant when P<0.05.
Results
Patient Population
The demographic and clinical characteristics of 170 non-M3-AML patients who received induction chemotherapy admitted to Clinical Hematology Unit, Internal Medicine Department, Assiut University Hospital, and South Egypt Cancer Institute (SECI) presented in . The median age was 49 years (range: 18–69 years). Female patients’ were 72 (42.35%) while male patients were 98 (57.65%). The diagnosis was performed according to the WHO criteria for AML. There were mainly AML M4, AML M2, AML M1 and AML M5 (51 (30%), 49 (28.82%), 33 (19.41%) and 19 (11.17%) respectively. A total of 39 patients (22.9%) had favorable cytogenetic risk, 74 (43.52%) intermediate cytogenetic risk, and 41 (24.11%) unfavorable risk.
The collected samples were 76 oropharyngeal swabs, 42 vaginal swabs. Seventy-five samples were diagnosed as yeast infection from which 50 isolates were diagnosed as Candida albicans by phenotypic tests and PCR.
Antifungal Susceptibility Test
Disk Diffusion Method
The pattern of antifungal resistance to tested azoles for Candida albicans isolates was relatively high 94% for both fluconazole and voriconazole, 74% for miconazole and itraconazole as shown in .
Table 4 Resistance Patterns of 50 C. albicans Using a Kirby–Bauer Disk Diffusion Method for Azoles
Ketorolac Acted Synergistically with Fluconazole Against Resistant C. albicans in vitro
The minimal inhibitory concentrations (MICs) of ketorolac and fluconazole against resistant C. albicans are listed in . The MIC of fluconazole was all >160 μg/mL for 94% of tested C. albicans isolates, indicating strong resistance of these C. albicans isolates. The MIC of ketorolac was >10 μg/mL. However, when used in combination with fluconazole, ketorolac could significantly decrease the MICs of fluconazole from >160 μg/mL to 0.3–1.25 μg/mL, indicating a significantly increased sensitivity of resistant C. albicans to fluconazole caused by ketorolac. When the MIC of FLC was decreased to <2 μg/mL, the concentration of ketorolac required was 2.5 μg/mL. Moreover, the FICI values obtained from the FICI model were <0.5, showing a strong synergism induced by ketorolac plus fluconazole.
Table 5 In vitro Interaction of Ketorolac with Fluconazole Against Resistant C. albicans
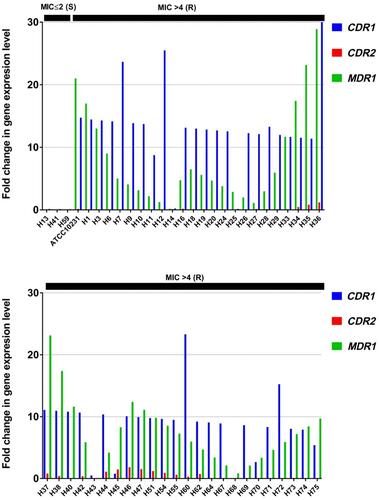
Efflux Pump Genes Expression Analysis
CDR1, CDR2, and MDR1 gene expression levels were quantified for all 50 isolates and normalized relative to the housekeeping gene, ACT1. This study found that the MDR1 gene showed the most gene overexpression (88%) followed by CDR1 gene (84%), and finally CDR2 gene (12%). When comparing the relationship between fluconazole MICs and the expression of efflux-related genes (), it was clear that isolates with higher fluconazole MIC (>4.0 mg/L) showed higher expression levels of CDR1 and MDR1, . These results confirm those expression levels of efflux-related genes CDR1 and MDR1 agree with fluconazole MICs in the C. albicans isolates.
Table 6 The Level of the Efflux Pumps Gene Expression in Resistant and Sensitive Strains in the Human Study Group
Discussion
In Egypt, leukemia comprises (10%) all malignancies, with AML representing (16.9%).Citation33 This study included 170, non-M3 (acute promyelocytic) AML patients. The ages ranged from 18 to 69 years, with a mean of 49 years. A slight male predominance was noted (female to male ratio was 1:1.36). The commonest FAB subgroup was M4 followed by M2. Another study at National Cancer Institute (NCI)-Egypt on 82 adult AML patients showed epidemiological characteristics of AML Egyptian patients, slightly similar to our data. Their ages ranged between 18 and 68 years with a median of 34 years. The male to female ratio was 1.05:1, and the commonest FAB subgroups reported were M1 and M2.Citation34
Another Egyptian study included ninety AML patients. The ages ranged from 18 to 76 years, with a mean of 37.8 years. A slight female predominance was noted (male to female ratio was 1:1.3). The commonest FAB subgroup was M2 followed by M5.Citation35
Response of induction chemotherapy in the current study, were complete remission (CR), partial remission, resistant disease and undefined response: 81 (47.64%), 20 (11.76%), 46 (27.05%) and 23 (13.52%), respectively. The time elapsed between presentation and start of treatment ranged from 4 to 85 days, with a mean of 13 days. The treatment delay in most patients of our study was mainly due to uncontrolled infection. An Egyptian study showed CR achievement in (57.5%) of AML patients, which is a slightly higher rate than that of our study.Citation35 It can be explained that we adopted treatment strategy interruption with neutropenic fever onset, which reduced treatment-related mortality at the expense of response rate. Also, slightly longer duration from onset of presentation to treatment beginning when compared with such study.
A retrospective study on 1317 AML patients had time elapsed between presentation and start of treatment with a median of four days, and a range of 1–78 days. The longer time was associated with worse CR in patients younger than 60 years, and this effect was more pronounced with duration of five days or more.Citation36 It is crucial that public health systems in developing countries (DC), including Egypt, turn to larger epidemiological studies to better understand how the disease characteristics interact with socioeconomic factors. An appropriate health system must shorten the time from diagnosis to treatment to ensure a better outcome of induction therapy and more successful result in AML.
C. albicans is an important cause of morbidity and mortality for AML patients. The rise of multidrug resistant organisms causes a challenge in the treatment of infective diseasesCitation37 Resistance among C. albicans represents a serious therapeutic problem that is mainly attributed to the overexpression of efflux pump genes encoded by CDR1, CDR2 (related to azole cross-resistance), and MDR1 genes (confined to selective resistance to fluconazole).Citation38
In our study, in vitro susceptibility testing for C. albicans isolated from AML patients to four azoles using disc diffusion method showed a high level of resistance pattern that was 94% for both fluconazole and voriconazole, 74% for miconazole and itraconazole. Also, the MIC of fluconazole was all >160 μg/mL for 94% of tested C. albicans isolates, indicating a very high resistance of these C. albicans isolates, which is consistent with 86.2% and 75.9 fluconazole resistance among C. albicans isolated in two recent local studies in Egypt, respectively.Citation39,Citation40
All the MICs obtained to support the antibiogram of isolates to fluconazole, which indicates that disk diffusion has a good correlation with MIC. Pfaller et al showed similar results.Citation41
Many researchers indicated that prolonged therapy and increased use of antifungals for prophylaxis or treatment of recurrent candidiasis are the most common risk factors to azole resistance.Citation42 To overcome fungal resistance, research on antifungal sensitizers has attracted considerable attention.Citation43 The need for novel antifungal regimens to overcome resistance prompted us to study the activity of ketorolac, which has a superior effect on pain control in cancer patients that suffer from frequent and recurrent Candida infections which showed a high level of resistance to the most common group of antifungals, the azoles, especially fluconazole.
In vitro, we found that ketorolac acted synergistically with fluconazole against tested C. albicans isolates, as interpreted by the FICI as it decreased the MIC of fluconazole by >4 folds against 93.6% of resistant isolates (new finding). This is superior to the recently reported 60.9% reversal of fluconazole resistance using ibuprofen by Sharma et al.Citation44
The MIC of ketorolac was >10 μg/mL. However, when used in combination with fluconazole, ketorolac could significantly decrease the MICs of fluconazole from >160 μg/mL to 0.3–1.25 μg/mL, indicating a significantly increased sensitivity of resistant C. albicans to fluconazole caused by ketorolac. When the MIC of FLC was decreased to <2 μg/mL, the concentration of ketorolac required was 2.5 μg/mL. Similar study on the combination of ibuprofen with fluconazole showed synergic activity in 8/12 of studied Candida spp. including four of the five fluconazole-resistant strains. The MICs of fluconazole in fluconazole-resistant Candida spp. decreased 2 to 128-fold when the drug was associated with ibuprofen. Also the MICs for ibuprofen decreased 64-fold for the 12 studied Candida spp. They reported the practicability of using ibuprofen in combination with fluconazole in the treatment of Candida infections.Citation14
The continuous emergence of resistance to conventional drugs through efflux pumps leads to increasing efforts directed toward discovering efflux inhibitory molecules.Citation45 This study found that the MDR1 gene was the gene that showed the most overexpression (88%) followed by CDR1 gene (84%), and finally CDR2 gene (12%). This finding is suggesting that CDR1 protein contributes more than CDR2 protein in resistance by ABC family of efflux pump which agreed with the finding of Tsao et al,Citation46 and Holmes et alCitation47 who conclude that in C. albicans Cdr1p efflux activity makes a greater contribution than Cdr2p to resistance to fluconazole and Cdr1p was present in greater amounts (2 to 20-fold) than Cdr2p. This result was contradictory with Chau et alCitation30 who found that CDR2 was overexpressed in the majority of the patient isolates. This can be explained by that reported by Niimi et alCitation48 who found that the strains hyperexpression CDR2 showed decreased susceptibility to caspofungin in agar plate drug resistance assays because ABC transporters confer resistance to a wide range of structurally unrelated xenobiotics so CDR2 may be related to other antifungal resistance.
In three C. albicans isolates, although their resistance profile to fluconazole, they exhibited downregulation of genes of efflux pump which suggested different azole resistance mechanisms as those belonging to ERG11 which was described.Citation49
Conclusion
To our knowledge, the current study is the first in vitro report on the use of ketorolac in reverting fluconazole resistance in C. albicans isolated from AML patients. Resistance of C. albicans to azole antifungals is associated with overexpression of efflux pump genes especially CDR1 and MDR1. Ketorolac concentration as low as (2.5 μg/mL) was able to revert resistance in 93.8% of tested strains, so the current study recommends for the next step to run clinical studies based on the in vivo ketorolac-fluconazole combination therapy for AML patients.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We acknowledge the Medical Research Center, Faculty of Medicine, Assiut University, for providing the necessary laboratory equipment for carrying out the experiments.
Disclosure
Dr Shereen A Sayed and Dr Hayam H Mohammed report grants from Faculty of Medicine Assiut University, during the conduct of the study. Dr Ehsan AB Hassan reports that a grant was obtained from Faculty of Medicine, Assiut University, for all the authors in this study. The authors declare no other potential conflicts of interest in this work.
Additional information
Funding
References
- Grove CS, Vassiliou GS. Acute myeloid leukemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941–951.
- Ohtake S, Miyawaki S, Fujita H, et al. Randomized study of induction therapy comparing standard dose idarubicin with high dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML 201 study. Blood. 2011;117:2358–2365. doi:10.1182/blood-2010-03-273243
- Wang ES. Common fungal infection in patients with leukemia. Clin Adv Hematol Oncol. 2017;5(15):352.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guidline for the management of candidiasis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;62(4):e1–e50.
- Shao PL, Huang LM, Hsueh PR. Recent advances and challenges in the treatment of invasive fungal infection. Int J Antimicrob Agents. 2007;30(6):487–489. doi:10.1016/j.ijantimicag.2007.07.019
- Paul S, Kannan I, Mohanram K. Eextensive ERG11 mutation associated with fluconazole- resistant Candida albicans isolated from HIV infected patients. Curr Med Mycol. 2019;5(3):1.
- Goncalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. Epidemiology and molecular mechanism of antifungal resistant in Candida and Aspergillus. Mycoses. 2016;59(4):198–219.
- Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanism and clinical impact. Clin Infect Dis. 2008;46(1):120–128. doi:10.1086/524071
- Rogers PD, Barker KS. Genome wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob Agents Chemother. 2003;47(4):1220–1227. doi:10.1128/AAC.47.4.1220-1227.2003
- Akins RA. An update on antifungal target and mechanism of resistance in Candida albicans. Med Mycol. 2005;43(4):285–318. doi:10.1080/13693780500138971
- Liu S, Yue L, Gu W, Li X, Zhang L, Sun S. Synergesic effect pffluconazole and calcium channel blocker against resistant Candida albicans. PLoS One. 2016;11(3):e0150859. doi:10.1371/journal.pone.0150859
- Chen X, Ren B, Chen M, et al. ASDCD:Antifugal synergistic drug combination database. PLoS One. 2014;9(1):e86499. doi:10.1371/journal.pone.0086499
- Rodrgues- Diez R, Gonzales-Guerrero C, Ocana-Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep. 2016;6(1):1–16. doi:10.1038/s41598-016-0001-8
- Pina-vaz C, Sansonetty F, Roderigues AG, Martinez-de-oliveria J, Fonseca AF, Mardh P-A. Antifungal activity of ibuprofen alone, and in combination with fluconazole against candida species. J Med Microbiol. 2000;49(9):831–840. doi:10.1099/0022-1317-49-9-831
- Hersh E, Hammond B, Fleury A. Antimicrobial activity of flurbirofen, and ibuprofen in vitro against six common periodontal pathogens. J Clin Dent. 1991;3(1):1–5.
- Ahangarkani F, Khodavaisy S, Mahmoudi S, et al. Indifferent effect of non-steroidal anti-inflammatory drugs (NSAIDs) combined with fluconazole against multidrug resistant Candida auris. Curr Med Mycol. 2019;5(3):26.
- Retsky M, Rogers R, Demicheli R, et al. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer replace: particular relevance to triple negative subgroup. Breast Cancer Res Treat. 2012;134(2):881–888. doi:10.1007/s10549-012-2094-5
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC; 2008:130–139.
- Rotstein S, Bow EJ, Laverdiere M, et al. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian fluconazole prophylaxis study group. Clin Infect Dis. 1999;28:331–340. doi:10.1086/515128
- Dohner H, Estey EH, Amadori S, et al. Dianosis and management of acute myeloid leukemia in adults: recommendation from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–474.
- Power D, Johnsen J. Difco TM, BBL TM, Manual: Manual of Microbiological Culture Media. Sparks: Becton Dickinson and company; 2009.
- Marinho SA, Teixeira AB, Santos OS, et al. Identification of Candida spp. by phenotypic tests and PCR. Braz j Microbiol. 2010;41(2):286=294. doi:10.1590/S1517-83822010000200004
- Pincus D, Orenga S, Chatellier S. Yeast identification past present and future methods. Med Mycol. 2007;45(2):97–121. doi:10.1080/13693780601059936
- Mannarelli BM, Kurtzman CP. Rapid identification of Candida albicans and other human pathogenic yeast by using short oligonucleotides in a PCR. J Clin Microbiol. 1998;1(36):1634–1641. doi:10.1128/JCM.36.6.1634-1641.1998
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeast; Approved Guideline. M44=A2 ed. Wayne, PA: Clinical and laboratory standards Institute; 2009.
- Ellis D. Antifungal susceptibility profile Australian antifungal susceptibility data for Candida isolates from recurrent vulvovaginal candidiasis (2007–2009) using the CLIS M44-A2 disc susceptibility standard for yeast. Mycology online. Adelaide, Australia: The University of Adelaide; 2011.
- Meletiadis J, Mouton J, Lagrou JW, Hamal K, Petr Guinea J; EUCAST. Subcommittee on Antifungal susceptibility testing of the ESCMID European committee for Antimicrobial susceptibility testing. Def. 2017;7(1).
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
- Ghen Y-L, Lehman VN, Averette AF, Perfect JR, Heitman J. Posaconazole exhibits In vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PLoS One. 2013;3:e57672.
- Chau AS, Mendrick CA, Sabatelli FJ, Loebenberg D, Mcnicholas PM. Application of real time quantitative PCR to molecular analysis of Candida albicans strain exhibiting reduced susceptibility to azoles. Antimicrob Agents Chemother. 2004;48:2124–2131. doi:10.1128/AAC.48.6.2124-2131.2004
- Rocha MFG, Banderia SP, De Alencar LP, et al. Azole resistance in Candida albicans from animals: highlights on efflux pump activity and gene over-expression. Mycoses. 2017;60:462–468. doi:10.1111/myc.12611
- Pfaffi MW. A new mathematical model for relative quantification in real time PCR. Nucleic Acids Res. 2001;29(9):e45–e. doi:10.1093/nar/29.9.e45
- Ibrahim AS, Khaled HM, Mikhail NM, Baraka H, Kamel H. Cancer Incidence in Egypt: resultsof the National Population –Based Cancer Registry Program. J Cancer Epidemiol. 2014;2014:1–8. doi:10.1155/2014/437971
- Acharya UH, Kanaan MN, Cui H, Roe DJ. Does the region of diagnosis correlate with overall survival in patients with Acute Myeloid Leukemia (AML)-a SEER review of AML diagnosis from 2004–2007. Blood. 2014;124:1291. doi:10.1182/blood.V124.21.1291.1291
- Zawam H, Salama R, Alsirafy SA, Bishr MK. Treatment outcome of Acute Myeloid Leukemia in Egypt: adeveloping country perspective. Int J Cancer Treat. 2018;1(1):53–59.
- Sekeres MA, Elson P, Kalaycio ME. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113:28–36. doi:10.1182/blood-2008-05-157065
- Donadu MG, Usai D, Marchetti M, et al. Antifungal activity of oil macerates of North Sardinia plants against Candida species isolated from clinical patients with candidiasis. Nat Prod Res. 2020;34(22):3280–3284. doi:10.1080/14786419.2018.1557175
- Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2(2):73–85. doi:10.1016/S1473-3099(02)00181-0
- Farhan MA, Moharram AM, Salah T, Shaaban OM. Relation between antifungal resistance and enzymatic activity of yeast causing oral and vaginal mycosis. Assiut Univ J Bot Microbiol. 2019;48(1):1–16.
- Farhan MA, Moharram AM, Salah T, Shaaban OM. Types of yeasts that cause vulvovaginal candidiasis in chronic users of corticosteroids. Med Mycol. 2019;57(6):681–687. doi:10.1093/mmy/myy117
- Pfaller M, Diekema DJ, Ostrosky-Zeichner L, et al. Correlation of MIC with outcome of Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interactive MIC breakpoint. J Clin Microbiol. 2008;46(8):2620–2629. doi:10.1128/JCM.00566-08
- Ehrstrom S, Yu A, Rylander E. Glucose in vaginal secretion before and after oral glucose tolerance testing in women with and without recurrent vulvovaginal candidiasis. Obstet Gynecol. 2006;108(6):1432–1437. doi:10.1097/01.AOG.0000246800.38892.fc
- Lu M, Yan H, Yu C, Yuan L, Sun S. Proton pump inhibitors act synergistically with fluconazole against resistant Candida albicans. Sci Rep. 2020;10(1):498. doi:10.1038/s41598-019-57174-4
- Sharma M, Biswas D, Kotwal A, et al. Ibuprafen- mediated reversal of fluconazole resistance in clinical isolates of Candida. J Clin Diagn Res. 2015;9(1):DC20.
- Usai D, Donadu M, Bua A, et al. Enhancement of antimicrobial activity of pump inhibitor associating drugs. J Infect Dev Ctries. 2019;13(02):162–164. doi:10.3855/jidc.11102
- Tsao S, Rahkhoodaee F, Raymond M. Relative contribution of the candida albicans ABC tr ansporters Cdr1p and Cdr2p to clinical azole rsistence. Antimicrob Agents Chemother. 2009;53(4):1344–1352. doi:10.1128/AAC.00926-08
- Holmes AR, Lin Y-H, Niimi K, et al. ABC transporters Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole–resistant Candida albicans clinical isolates. Antimicrob Agents Chemother. 2008;52(11):3851–3862. doi:10.1128/AAC.00463-08
- Niimi K, Maki K, Ikeda F, et al. Overexpression of Candida albicans CDR1, CDR2, orMDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50(4):1148–1155. doi:10.1128/AAC.50.4.1148-1155.2006
- Xiang M-J, Liu J-Y, Ni P-H, et al. Erg11 mutations associated with azoles resistence in clinical isolates of candida albicans. FEMS Yeast Res. 2013;13(4):386–393. doi:10.1111/1567-1364.12042