Abstract
The current COVID-19 pandemic emerged in December 2019, in China, affecting millions of people worldwide. COVID-19 is mainly a disease of the respiratory system, yet systematic complications have also been reported among SARS-CoV-2 infected patients. Thrombotic complications are one of the severe clinical outcomes of COVID-19, especially among critically ill patients, and are associated with poor prognosis. To date, many studies have concluded that COVID-19 increases the incidence of thrombotic events and coagulopathies; however, the exact mechanism behind such a disease outcome is not well known. Various pathophysiological mechanisms for thrombotic events in COVID-19 have been proposed, these include virus-induced endothelial cell damage, inflammation, and excess production of pro-inflammatory cytokines. As a result, most critically diseased COVID-19 patients are managed with prophylactic anticoagulant, yet some still develop thrombotic episodes. Therefore, better understanding of the mechanisms behind the thrombotic complications is needed to develop treatments that specifically target such pathways, which may aid in better disease management and improve the prognosis.
Introduction
In December 2019, the Chinese Center for Disease Control and Prevention identified the presence of a novel coronavirus in throat swab samples from a series of pneumonia cases of unknown etiology, that presented with dry cough, dyspnea, and fever.Citation1 The newly-identified virus was named as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), since it resembled SARS-CoV that emerged in 2002–2003 and resulted in high morbidities and mortalities. By March 2020, the World Health Organization declared a global pandemic, and the disease caused by the virus was named Coronavirus disease 2019 (COVID-19).Citation2 Subsequently, many countries around the world implemented various preventive strategies to minimize the rate of the virus spread. Yet, SARS-CoV-2 affected millions of different age groups worldwide. The latest statistics show the infected cases exceeded 158 million with more than 3 million deaths worldwide.
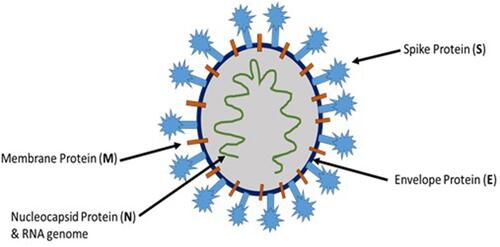
The novel coronavirus, SARS-CoV-2, belongs to the coronaviridae family, which are positive-sense single-stranded enveloped RNA viruses. There are four different genera for coronaviruses, and SARS-CoV-2 belongs to the β coronaviruses.Citation3 SARS-CoV-2 and coronaviruses in general have four essential structural proteins (), namely spike (S) glycoprotein, envelope (E) glycoprotein, membrane (M) glycoprotein, and nucleocapsid (N) protein. These proteins are necessary for successful viral attachment and penetration of the host cells, synthesis of viral proteins, maturation, and release of the viral progeny.Citation4 The viral S protein, a transmembrane protein that protrudes from the viral surface, has been considered as an important factor in the pathogenesis of coronaviruses including SARS-CoV-2. S glycoprotein aids in the viral attachment and fusion to the host cell membrane.Citation5
Thrombotic episodes are one of the severe complications of COVID-19, particularly in critically ill patients, which can be associated with poor prognosis. A growing body of evidence suggests that COVID-19 increases the incidence of thrombotic complications; however, the exact mechanisms are not fully elucidated. Therefore, the current review aims to provide an overview of the available literature to outline the current knowledge of the risks, pathogenesis, and the therapeutic interventions for thrombotic complications that are associated with the COVID-19 pandemic.
Pathogenesis of COVID-19 Infection
SARS-CoV-2 is a zoonotic virus that evolved in a way to be able to be transmitted from human-to-human.Citation6 SARS-CoV-2 can transmit from one individual to another either by direct transfer through aerosols or indirectly via contaminated surfaces. SARS-CoV-2 contains large RNA genomes flanked by 5′ and 3′ untranslated regions containing cis-acting secondary RNA structures necessary for RNA synthesis and replication. At the 5′ end, the viral RNA possesses two large open reading frames (ORFs; ORF1a and ORF1b) that occupy two-thirds of the nonstructural genome.Citation7 The currently proposed pathogenesis of SARS-CoV-2 is derived from the knowledge that is available on SARS-CoV, where the viral spike protein binds to host’s angiotensin-converting enzyme 2 (ACE2) receptor, which is differentially expressed on various tissues, including respiratory tract cells, gastrointestinal tract cells, cardiac muscle cells, and endothelial cells.Citation8
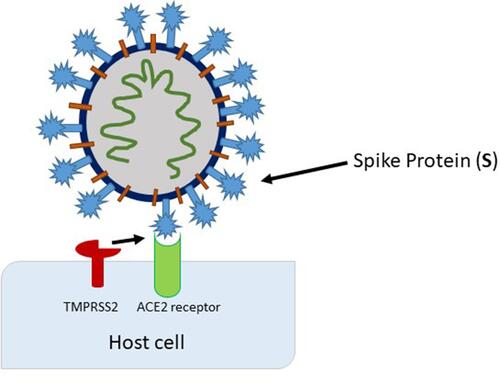
A successful binding of the spike protein to ACE2 and the internalization of the virus requires the presence of the specific protease transmembrane serine protease 2 (TMPRSS2) that cleaves and activates ACE2.Citation9 With the successful viral entry into the cells, host replication machinery is used, and viral progenies are produced, which can further infect adjacent cells (). Indeed, recent evidence shows TMPRSS2 is expressed in human endothelial cells obtained from the lungs which may explain the pulmonary complications of COVID-19.Citation10 Furthermore, smokers and patients with moderate-to-severe COPD had been found to have higher levels of ACE2 mRNA and protein levels in lung tissue. Furthermore, hypertensive patients were found to have the more severe form of the disease, suggesting antihypertensive therapy may play a role in developing serious COVID-19 infection due to enhanced ACE2 and SARS-CoV-2 interactions.Citation11
Figure 2 Activation of the spike protein by TMPRSS2 at (or close to) the cell surface, leading to fusion of the viral membrane with the plasma membrane.
Upon entry, the viral genome is released into the cytosol, this leads to the immediate translation of ORF1a and ORF1b which encode for 15–16 non-structural proteins. The majority of these non-structural proteins make up the viral replication and transcription complex (TRC). In turn, the TRC houses RNA-processing and modifying enzymes as well as a RNA proofreading mechanism that is essential for maintaining the integrity of the viral genome. Translation of ORF1a and ORF1b results in polyproteins that are co-translationally and post-translationally processed into the single non-structural proteins, which leads to the formation of the viral replication and transcription system. As non-structural proteins are formed and expressed, the synthesis of viral replication machinery also takes place, which consists of perinuclear double-membrane vesicles (DMVs), convoluted membranes (CMs), and small open double-membrane spherules (DMSs) forming a protective and suitable microenvironment for viral genomic RNA replication and transcription of subgenomic mRNAs. The newly synthesized viral genomic RNA results in budding into the lumen of secretory vesicular compartments, which are then secreted from the infected cell by exocytosis.Citation7,Citation12
The clinical presentations of COVID-19 are highly variable from one individual to another. COVID-19 ranges from asymptomatic to severe disease that could result in death. However, mild-to-moderate flu-like symptoms are the most common presentations among COVID-19 patients, including fever, dry cough, sore throat, runny nose, and, in some cases, involvement of the lower respiratory tract that may lead to acute respiratory distress syndrome (ARDS).Citation13 Other general symptoms such as weakness, headache, and gastrointestinal symptoms including diarrhea and vomiting have also been reported.Citation14 Moreover, to a lower extent some of the COVID-19 patients experienced loss of smell and taste.Citation15 Some affected individuals could further progress to severe forms of the disease, where they develop severe pneumonia, pulmonary edema, septic shock, and organ failure that would result in death.Citation16 Thromboembolic events and acute kidney injury have also been reported as COVID-19 complications.Citation13 Indeed, laboratory findings such as thrombocytopenia, increased prothrombin time, and activated partial thromboplastin time along with elevated D-dimer were reported among critically ill patients, which further supports the thrombotic and coagulopathy complications in COVID-19 patients. Together these findings indicate that COVID-19 is a systematic disease with serious thrombotic complications that require more attention.
Thrombotic Complications of SARS-CoV-2 Infection
A number of studies have reported thrombotic and hypercoagulability complications among COVID-19 cases. These complications represent a serious worsening of the disease since it is associated with adverse outcomes. A recent systematic review reported that among 2,928 COVID-19 severely diseased patients, 56.3% developed thrombosis, and 34% of ICU admitted patients were found to have thrombotic complications, where 16.1% were reported with deep vein thrombosis and 12.6% with pulmonary embolism.Citation17 Many other independent studies have reported different rates of thrombotic complications among COVID-19 patients (). It is now established that critically ill COVID-19 patients were associated with a higher incidence of thrombotic events than other COVID-19 patients. This could be associated with higher levels of pro-inflammatory and dysregulated fibrinolytic states among severe COVID-19 cases.
Table 1 Incidence of Thrombotic Complications Among COVID-19 Patients
Indeed, it was reported in a cohort of 54 COVID-19 patients who died from the infection, it was found those patients had higher levels of D-dimer, troponin, and interleukin-6 (IL-6).Citation18 Furthermore, Wang et alCitation19 reported that, among 199 COVID-19 patients, elevated levels of D-dimer, fibrinogen degradation products, prolonged prothrombin time, and thrombin time were found to be higher among patients with severe disease manifestations. These findings indicate that COVID-19 is associated with abnormal coagulation and subsequently leads to thrombotic complications and severe disease outcome. Moreover, Harenberg et alCitation20 reported there was an initial increase in the prothrombin time and activated partial thromboplastin time, but later both are decreased due to consumption of coagulation factors. These findings were also associated with an increase in the platelet count, fibrinogen, and D-dimer. In addition, in a cohort of 248 COVID-19 cases, Yao et alCitation21 reported that D-dimer levels were higher among 185 patients, and it was significantly increasing with an increase in disease severity.
Interestingly, the ABO blood group system has also been suggested to contribute to susceptibility and severity of COVID-19 infection. Indeed, a study by Marcos et alCitation22 concluded that blood group O was associated with lower ICU admission and thrombotic complications when compared to other blood groups. Furthermore, blood group B had significantly higher rates of thrombotic complications and was associated with more ICU. A possible mechanism for this is that plasma levels of Von Willebrand factor ((VWF) a glycoprotein essential for thrombus formation) levels are lower in group O patients when compared to non-O blood group patients.
Pathogenesis of Thrombotic Complications of COVID-19
Various factors have been reported to be associated with increased incidence of coagulopathies among COVID-19 patients. These include patients’ age, and coexistence of chronic disease such as cardiovascular disease, diabetes, and hypertension.Citation23 Indeed, COVID-19 patents with chronic disease such as diabetes have been shown to have increased pro-inflammatory mediators IL-6, suggesting these patients are at higher risk of developing more serious complications of COVID-19.Citation24,Citation25 In addition, males have been shown to have been more at risk of developing thrombotic complications. Indeed, various COVID-19 reports have so far shown that elderly patients are severely affected by the infection and they account for most of the severe cases that requires ICU admission, with a higher incidence of thrombosis.Citation26
The exact mechanism responsible for COVID-19 induced thrombosis is not fully understood. However, several mechanisms have been put forward in an attempt to explain these complications among COVID-19 patients. Such mechanisms include increased platelet activation and activation coagulation cascade and/or decreased fibrinolysis, in addition to immune activation. However, a central mechanism involves virus-induced endothelial damage. Endothelial cells express the ACE2 receptor which is a SARS-CoV-2 target receptor. Ut has been suggested that viral penetration of endothelial cells inflicts tissue damage and subsequently exposure of collagen, a potent platelet agonist, leading to platelet adhesion and activation, furthermore, injured endothelial cells release tissue factor.Citation27,Citation28 Initially this leads to the activation and the recruitment of platelets, and further release of platelet granules content that aid in the platelet plug formation,Citation29 whilst tissue factor activates the extrinsic pathway of the coagulation cascade. Subsequently this leads to the activation of the intrinsic pathway of coagulation cascade, formation of thrombin that converts fibrinogen to fibrin which further stabilizes platelet plug, and forms thrombus.Citation28,Citation30 Evidence of increased ACE2 expression on the endothelial cells of SARS-CoV-2 infected patients has been reported, which suggest a higher probability of endothelial cells damage that leads to thrombotic outcome.Citation31
Abnormal fibrinolysis has also been suggested to contribute to thrombotic events during COVID-19 infection. Wright et alCitation32 reported that among 44 ICU admitted patients, 57% experienced a complete lack in the lysis of the clots, suggesting defective fibrinolysis. This was later supported by the increased levels of plasminogen activator inhibitor 1 among COVID-19 patients, which inhibits the plasminogen activator and leads to a decrease in the fibrin degradation.Citation33
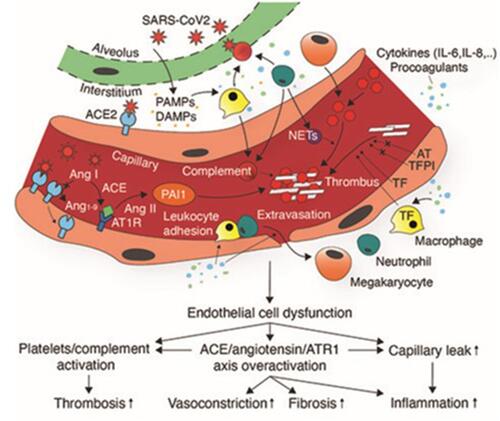
The immune system and homeostasis complement each other in order to defend against a pathogen and prevent further dissemination of the pathogen, by a process known as immuno-thrombosis. This physiological mechanism could be dysregulated, leading to the formation of excess thrombus formation.Citation34 When endothelial cells are infected with SARS-CoV-2, they undergo pyroptosis, a form of cell death.Citation35 This process is associated with the release of cellular content such as pathogen associated molecular pattern (PAMPs), and damage associated molecular patterns (DAMPs) causing inflammation. DAMPs and PAMPs interact with pattern recognition receptor and Toll-like receptor on innate immune cells triggering immune response and the release of pro-inflammatory cytokines and this enhances the expression of tissue factor.Citation35,Citation36 Therefore, some patients develop an induced immune response to SARS-CoV-2 that is defined as cytokine storm.Citation37 COVID-19 patients are found to have increased levels of specific chemokines and cytokines, which include IL-6, interferon-gamma, and IL-2.Citation38 IL-6 is reported to increase the production of platelets in COVID-19 patients, enhances the expression of tissue factor on monocytes and endothelial cells, and by itself can lead to further endothelial damage.Citation38 To a similar extent it has been reported that interferon-gamma also increases the production of platelets and causes endothelial dysfunction, creating a prothrombotic status. On the other hand, IL-2 in COVID-19 patients is reported to increase the production of plasminogen activator inhibitor-1, which reduces fibrinolysisCitation39 ().
Figure 3 Pathogenesis of thrombotic complications of COVID-19. SARS-CoV2 infection induces endothelial damage triggering endothelial release of cytokines, increasing capillary permeability. PAMPs and DAMPs induced activation of neutrophils, and macrophages results in localized production of cytokines, procoagulants, and complement activation, leading to further endothelial damage and tissue factor release. Endothelial damage exposes collagen and other prothrombotic mediators leading to thrombus formation.
In addition to these proposed mechanisms, the complement system has also been implicated in the development of thrombotic complications in COVID-19 patients. Indeed, in a study conducted by Magro et al,Citation40 it was found that there is a continuous activation of the alternative and the lectin pathways of the complement system, that was supported by the presence of terminal C5b-9 complement complex on the vessels of the affected individual’s lungs. The membrane attack complex, C5b-9, has the ability to activate platelets, endothelial damage, and enhance the release of VWF. Individual complement components, including C5a, have the ability to induce the production of tissue factor, plasminogen activator inhibitor-1 and IL-6, which further stimulate thrombus formation.Citation41 Furthermore, since SARS-CoV-2 invades the cells through an ACE2 receptor, this causes downregulation of the receptor and subsequent upregulation of angiotensin II, which is normally degraded by ACE2. High levels of angiotensin II cause vasocontraction,Citation42 increased levels of tissue factor, and plasminogen activator inhibitor, therefore favoring a hypercoagulable state.Citation43
Clinical Manifestation of Thrombotic Events in COVID-19 Patients
Thrombotic complications of COVID-19 have been reported from the start of this pandemic and are highly reported among severely ill patients.Citation17 Severe infection with SARS-CoV-2 has been reported to be associated with the development of microvascular and macrovascular thrombosis that eventually leads to an increase in the mortality rates. Macrovascular and microvascular thrombosis results from platelet activation and the activation of the coagulation cascade and subsequent thrombus formation in blood vessels of varying sizes that could cause partial or complete occlusion of the blood vessels.Citation44,Citation45 Venous thromboembolism and arterial thrombosis are among the macrovascular thrombotic complications of COVID-19. Both forms of venous thromboembolism, pulmonary embolism, and deep vein thrombosis have been reported among COVID-19 patients. It was reported that among 1,765 patients the rate of venous pulmonary embolism in COVID-19 patients was around 22%, with higher incidence rates among ICU admitted patients.Citation46 Whilst some patients experienced deep vein thrombosis. Zhang et alCitation47 reported that among 143 hospitalized COVID-19 patients, 66 (46.2%) developed deep vein thrombosis, and it was common in elderly patients and was associated with poor disease outcome and higher mortality rates. These rates could be even higher than the reported data, since not all COVID-19 patients undergo computerized tomography, mainly because of the risk of spreading the infection. Moreover, a meta-analysis involved 46,348 COVID-19 cases reported that around 7% had increased levels of troponin which is associated with myocardial injury is also reported among COVID-19 patients.Citation48 Identifying the risks and incidence rate of these complications among COVID-19 is essential for determining the best procedures for diagnosing such outcomes for best patient’s management. Furthermore, COVID-19 patients also experience coagulation abnormalities that are similar but not identical to thrombotic thrombocytopenic purpura, or hemolytic uremic syndrome or disseminated intravascular coagulation (DIC). Higher levels of D-dimer, thrombocytopenia and prolonged prothrombin time in COVID-19 patients suggest the occurrence of DIC, yet in DIC the levels of D-dimer is higher than that of COVID-19 and thrombocytopenia is more predominant. In addition, COVID-19 patients with thrombotic complications have high levels of ferritin and lactate dehydrogenase along with platelet rich plaques in the lungs and other organs, yet there is no schistocytes that are normally present in the case of thrombotic microangiopathies.Citation49 This indicates that thrombotic manifestation in COVID-19 have unique features and the mechanism behind this variation need to be investigated for better disease diagnosis and management.
Management of COVID-19 Associated Thrombotic Complications
Due to the rapidly evolving literature, there are no unified guidelines on how best to diagnose and manage thrombotic and hypercoagulability in COVID-19 patients. This is partly due to the variability in incidence among studies which can be attributed to differences in population. However, most of the published guidelines recommend using prophylactic anticoagulant for all hospitalized COVID-19 patients, to avoid such severe disease outcomes.Citation50 Most of the guidelines suggest the use of daily low-molecular-weight heparins or subcutaneous unfractionated heparin. Low-molecular-weight heparin is considered more beneficial than other prophylactic regimens, particularly in COVID-19 cases, as it has been previously reported that it has a longer half-life than unfractionated heparin,Citation51 acts as an inhibitor for viral attachment by binding to SARS-CoV-2 spike protein,Citation52 and it has immunomodulatory and anti-inflammatory effects.Citation53 Despite using thromboprophylaxis, some critically ill patients still developed thrombotic complications. This could be due to heparin resistance and lower levels of anti-activated factor X in these patients, which could be attributed to high levels of fibrinogen and reduced antithrombin levels in COVID-19 patients, yet the exact mechanism for heparin resistance among COVID-19 patients is not known.Citation54 Therefore, it is recommended to use higher doses of prophylactic anticoagulant, as it was reported that with higher doses the levels of anti-activated factor X are higher, which could aid in preventing thrombosis. Yet, this finding cannot be generalized since it is not known if all COVID-19 hospitalized subjects would benefit from higher doses of thromboprophylaxis without leading to additional complications.
Venous thromboembolism is mainly managed by the use of therapeutic anticoagulants. The best choice of the anticoagulant as a treatment depends on patients’ renal and hepatic functions, gastrointestinal function, and thrombocytopenia. Furthermore, for patients admitted to hospital and receiving direct care it is preferred to use low-molecular weight heparin, unfractionated heparin (UFH), or fondaparinux (particularly for patients with heparin-induced thrombocytopenia), but for out-patients it is better to use direct oral anticoagulants. In addition, a number of drugs have been studied to be used as a treatment for sepsis-induced thrombotic complications, and it is suggested that they might have a therapeutic role in COVID-19 and these include; Danaparoid, that weakens thrombin production, Sulodexide, which potentiates anti-proteolytic activity of anti-thrombin and heparin co-factor, antithrombin, that inactivates coagulation enzymes, and thrombomodulin, which acts as a co-factor for thrombin.Citation55
Conclusion
SARS-CoV-2 infection rates are still increasing with an increase in the morbidity and mortality rates. There is accumulating evidence that COVID-19 is associated with various systematic complications, including thrombotic and hypercoagulability. Yet, the exact mechanism of such complication is not fully understood. However, a central mechanism that has been postulated is SARS-CoV-2 induced endothelial damage via ACE2 receptor. Endothelial injury and inflammation result in a series of reactions leading to the activation of the coagulation pathways and platelet. Moreover, increased levels of pro-inflammatory mediators complement cascade activation and shut down of anti-thrombotic pathways have been shown to play a role in the pathogenesis of thrombotic complications. However, the available data are limited, and further investigations are required for better understanding of the disease mechanisms.
Acknowledgment
Open access funding was provided by Qatar National Library.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi:10.1002/jmv.25678
- Sohrabi C, Alsafi Z, O’Neill N, et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/j.ijsu.2020.02.034
- Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect Genet Evol. 2020;79:104211. doi:10.1016/j.meegid.2020.104211
- Satarker S, Nampoothiri M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch Med Res. 2020;51(6):482–491. doi:10.1016/j.arcmed.2020.05.012
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi:10.1007/978-1-4939-2438-7_1
- Wong ACP, Li X, Lau SKP, Woo PCY. Global epidemiology of bat coronaviruses. Viruses. 2019;11(2):174. doi:10.3390/v11020174
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi:10.1038/s41579-020-00468-6
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi:10.1007/s11684-020-0754-0
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. doi:10.1016/j.cell.2020.02.052
- Matarese A, Gambardella J, Sardu C, Santulli G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8(11):462. doi:10.3390/biomedicines8110462
- Sardu C, Maggi P, Messina V, et al. Could anti‐hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID‐19 infection? data from centers of southern Italy. J Am Heart Assoc. 2020;9(17):e016948. doi:10.1161/JAHA.120.016948
- Sanyal S. How SARS-CoV-2 (COVID-19) spreads within infected hosts—what we know so far. Emerging Topics Life Sci. 2020;4(4):383–390. doi:10.1042/ETLS20200165
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/s0140-6736(20)30183-5
- Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4
- Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi:10.1016/j.mayocp.2020.05.030
- Tabata S, Imai K, Kawano S, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the diamond princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20(9):1043–1050. doi:10.1016/S1473-3099(20)30482-5
- Jenner WJ, Kanji R, Mirsadraee S, et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;51(3):595–607. doi:10.1007/s11239-021-02394-7
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
- Wang J, Zhang H, Qiao R, et al. Thrombo-inflammatory features predicting mortality in patients with COVID-19: the FAD-85 score. J Int Med Res. 2020;48(9):300060520955037. doi:10.1177/0300060520955037
- Harenberg J, Favaloro E. COVID-19: progression of disease and intravascular coagulation - present status and future perspectives. Clin Chem Lab Med. 2020;58(7):1029–1036. doi:10.1515/cclm-2020-0502
- Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8(1):49. doi:10.1186/s40560-020-00466-z
- Marcos SZ, Antelo ML, Galbete A, Etayo M, Ongay E, García-Erce JA. Infection and thrombosis associated with COVID-19: possible role of the ABO blood group. Medicina Clínica (English Edition). 2020;155(8):340–343. doi:10.1016/j.medcle.2020.06.013
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi:10.1016/S2213-2600(20)30116-8
- Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol. 2020;19(1):1–4. doi:10.1186/s12933-020-01047-y
- Sardu C, D’Onofrio N, Balestrieri ML, et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020;63(11):2486–2487. doi:10.1007/s00125-020-05216-2
- Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–e18. doi:10.1016/j.jinf.2020.03.005
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
- Gale AJ. Continuing education course# 2: current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273–280. doi:10.1177/0192623310389474
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. doi:10.1016/j.blre.2014.10.003
- Bautista-Vargas M, Bonilla-Abadía F, Cañas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis. 2020;50:479–483. doi:10.1007/s11239-020-02172-x
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi:10.1016/j.clim.2020.108427
- Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203. doi:10.1016/j.jamcollsurg.2020.05.007
- Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e82. doi:10.1016/S2352-3026(20)30216-7
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45. doi:10.1038/nri3345
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi:10.1038/s41577-020-0311-8
- Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. New Engl J Med. 2020;383(6):590–592. doi:10.1056/NEJMc2011400
- Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi:10.1007/s11427-020-1643-8
- Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS‐CoV‐2 infection: review of 3939 COVID‐19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41. doi:10.1002/JLB.3COVR0520-272R
- Du F, Liu B, Zhang S. COVID-19: the role of excessive cytokine release and potential ACE2 down-regulation in promoting hypercoagulable state associated with severe illness. J Thromb Thrombolysis. 2020;51:1–17.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi:10.1016/j.trsl.2020.04.007
- Fletcher-Sandersjöö A, Bellander B-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36–41. doi:10.1016/j.thromres.2020.06.027
- Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738.
- Celi A, Cianchetti S, Dell’Omo G, Pedrinelli R. Angiotensin II, tissue factor and the thrombotic paradox of hypertension. Expert Rev Cardiovasc Ther. 2010;8(12):1723–1729. doi:10.1586/erc.10.161
- Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. doi:10.1001/jamacardio.2020.1017
- Becker RC. COVID-19 update: covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54-67. doi:10.1007/s11239-020-02134-3.
- Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection a scoping review. Thromb Res. 2020;192:152–160. doi:10.1016/j.thromres.2020.05.039
- Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi:10.1161/circulationaha.120.046702
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi:10.1016/j.ijid.2020.03.017
- Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi:10.1016/S2352-3026(20)30145-9
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thrombosis Haemostasis. 2020;18(5):1023–1026. doi:10.1111/jth.14810
- Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):15–31. doi:10.1007/s11239-015-1314-3
- Kim SY, Jin W, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. doi:10.1016/j.antiviral.2020.104873
- Thachil J. The versatile heparin in COVID-19. J Thrombosis Haemostasis. 2020;18(5):1020–1022. doi:10.1111/jth.14821
- White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287–291. doi:10.1007/s11239-020-02145-0
- Ortega‐Paz L, Capodanno D, Montalescot G, Angiolillo Dominick J. Coronavirus disease 2019–associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021;10(3):e019650. doi:10.1161/JAHA.120.019650
- Chen S, Zhang D, Zheng T, Yu Y, Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis. 2021;51(1):33–39. doi:10.1007/s11239-020-02181-w
- Shah A, Donovan K, McHugh A, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Critical Care. 2020;24(1):1–10. doi:10.1186/s13054-020-03260-3
- Gibson CJ, Alqunaibit D, Smith KE, et al. Probative value of the d-dimer assay for diagnosis of deep venous thrombosis in the coronavirus disease 2019 syndrome. Crit Care Med. 2020;48(12):e1322–e1326. doi:10.1097/ccm.0000000000004614
- Monfardini L, Morassi M, Botti P, et al. Pulmonary thromboembolism in hospitalised COVID-19 patients at moderate to high risk by Wells score: a report from Lombardy, Italy. Br J Radiol. 2020;93(1113):20200407. doi:10.1259/bjr.20200407
- Avruscio G, Camporese G, Campello E, et al. COVID‐19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci. 2020;13(6):1108–1114. doi:10.1111/cts.12907
- Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi:10.1182/blood.2020006520
- Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi:10.1016/j.thromres.2020.05.018
- Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi:10.1016/j.jacc.2020.08.070
- Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and respiratory system disorders: current knowledge, future clinical and translational research questions. Arterioscler Thromb Vasc Biol. 2020;40(11):2586–2597. doi:10.1161/ATVBAHA.120.314515