Abstract
Treatment options are limited for multiple myeloma patients who have developed four/five drug-refractory disease. Selinexor (Sel) and belantamab mafodotin (belamaf) were recently approved by the US FDA for treatment of RRMM. The toxicity profile of these drugs is a concern since these agents are used in patients who have already undergone multiple lines of treatment. In this review, we discuss the toxicity profile and strategies for the management of toxicities of Sel and belamaf for the treatment of RRMM. We conducted a comprehensive literature search on PubMed, Embase, Cochrane, and Clinicaltrials.gov using the terms “selinexor”, “belantamab”, “belamaf”, and “multiple myeloma” without applying any limitations based on the date of the study, language, or country of origin. The most common hematological toxicity associated with these two drugs is thrombocytopenia. Cytopenias, constitutional symptoms, gastrointestinal effects, and hyponatremia are the major toxicities of Sel. Keratopathy and anemia are the major toxicities of belamaf. Treatment modifications and dose interruption are usually needed when side effects are more than grade II. As these are newer drugs with limited data, continuous surveillance and monitoring are warranted during the treatment course with early mitigation strategies.
Introduction
Multiple myeloma (MM) is the second most prevalent hematologic cancer that led to approximately 12,830 deaths in the US during 2020.Citation1 However, the advent of newer drugs has improved its five-year survival rate. A vast majority of MM patients require subsequent lines of therapy following relapses.Citation2 Treatment options are limited for those who develop the triple-class refractory disease (ie, refractory to immunomodulators (IMiDs), proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies) pressing the need for the development of newer drugs that can overcome this resistance to conventional therapy.Citation3
Overview of Selinexor
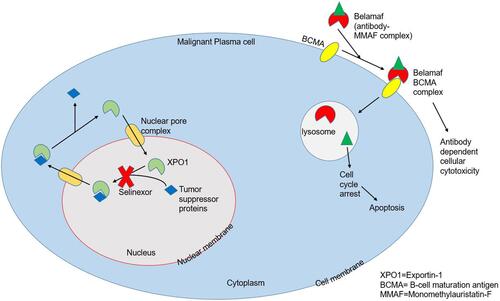
Selinexor (KPT-330) is an oral, reversible inhibitor of major nuclear exporter of tumor suppressor proteins (TSPs) known as Exportin-1 (XPO1) or chromosomal maintenance 1 (CRM1) (). XPO1 binds to the guanosine triphosphate (GTP)-binding nuclear protein called Ran and forms the XPO1/Ran GTP nucleocytoplasmic transport complex which is responsible for the transport of many TSPs out of the nucleus such as p53, breast cancer gene 1/2 (BRCA1/2), forkhead box-O (FOXO) and growth regulatory factors (c-myc, cyclins, Mouse Double Minute 2 homolog (MDM2)). When overexpressed, XPO1 causes an aberrant distribution of these regulatory proteins localizing them within the cytoplasm, increasing the translation of oncoprotein mRNAs and functionally inactivating TSPs which gives the malignant cells a chance to evade apoptosis and thus proliferate. As a selective inhibitor of nuclear export (SINE), selinexor (Sel) causes forced nuclear retention of these compounds with subsequent cell cycle arrest and cancer cell death, largely sparing the normal cells.Citation3–Citation5
Following the encouraging results of the pivotal Phase II STORM trial,Citation5,Citation6 Sel in combination with low-dose dexamethasone (Dexa) was approved by the Food and Drug Administration (FDA) of the United States in July 2019Citation7 for quadrefractory (refractory to at least 2 PIs and two IMiDs) or penta-refractory MM (quadrefractory+ refractory to anti-CD38 antibodies).Citation3,Citation8 The adverse events (AEs) reported in the STORM,Citation6 STOMPCitation3 and BOSTONCitation9 trials were mostly manageable with supportive measures. Cytopenias, constitutional and gastrointestinal symptoms, and hyponatremia were the most common AEs in these trials, with grade (G)-3 or severe thrombocytopenia occurring in 54% of patients.Citation5,Citation10
Overview of Belantamab Mafodotin
B-cell maturation antigen (BCMA), almost exclusively expressed on plasma cells, is an attractive drug target for the treatment of drug-resistant MM.Citation11,Citation12 Belantamab mafodotin (belamaf), an anti-BCMA agent, received FDA approval in August 2020 for the treatment of adults with refractory MM who have previously received at least four therapies including PIs, IMiDs, and anti-CD38 monoclonal antibody.Citation13 Belamaf is an antibody-drug conjugate (ADC) in which afucosylated humanized IgG1-antibody is conjugated to microtubule inhibitor monomethyl auristatin-F (MMAF) that kills myeloma cells through a multimodal mechanism (). This ADC complex targets BCMA and induces immunogenic cell death through antibody-dependent cellular cytotoxicity and cellular phagocytosis. The cytotoxic component of ADC, ie, MMAF when delivered to the target B-cells inhibits tubulin polymerization causing cell cycle arrest at the G2/M checkpoint and subsequent apoptosis.Citation11,Citation14,Citation15 The use of single-agent belamaf has produced encouraging results in the pivotal DREAMM 1 and 2 trials, with an overall response rate (ORR) of 60% in DREAMM-1, and 31% for 2.5 mg/kg vs 34% for 3.4 mg/kg cohorts of DREAMM-2.Citation15–Citation17 The most common AE, keratopathy, occurring in 27% and 16% of patients receiving 2.5 mg/kg and 3.5 mg/kg doses of belamaf respectively, was manageable with supportive care along with dose adjustments and resolved after treatment completion. Thrombocytopenia and anemia were the next most common AEs.Citation18,Citation19
This review aims to discuss the toxicities associated with the use of Sel and belamaf in the treatment of heavily pretreated RRMM (relapsed/refractory MM) and to evaluate the management of these toxicities in the light of available evidence.
Materials and Methods
We conducted a comprehensive literature search on four databases including PubMed, Embase, Cochrane, and Clinicaltrials.gov. We used the search terms “selinexor”, “belantamab”, and “multiple myeloma”. We did not apply any limitations based on the date of publication, language, or country of origin. The initial search resulted in 430 total articles. After removing duplicates and screening manually to only include articles based on human studies and those that have reported on the safety profile or management of toxicity of the two drugs belamaf and Sel, 100 articles were selected for review, including six studies on Sel and two studies on belamaf. The selection of the articles was confirmed by two authors.
Results
Dose, Combination Regimens, and Toxicity Profile of Selinexor
The dose of Sel ranged from 3–85 mg/m2 in the first-in-human trial where Sel was used in 189 patients with advanced solid malignancies. The starting dose of 3 mg/m2 was extrapolated from non-human studies.Citation20 Chen et al. investigated the safety of Sel in heavily pretreated MM patients (n=84). They administered 3–60 mg/m2 of oral Sel either in eight doses or 10 doses per 28-day cycle in the dose-escalation phase (n=25). In the dose-expansion phase (n=59), they administered Sel 45 or 60 mg/m2 twice-weekly along with 20 mg of Dexa in a 28-day cycle vs Sel alone with the same flat doses in the 21-day cycle.Citation2 Sel-Dexa combination vs Sel alone showed better overall response rates (ORR), ie, 22% vs 4%, and lower rates of serious AEs (SAEs), ie, 39% vs 61%. Given the fewer dosage modifications, Sel 45 mg/m2 (~80 mg) twice-weekly with 20 mg Dexa emerged as an appropriate treatment regimen for future studies.Citation2 The STORM phase II trial parts 1 and 2 used the same regimen of Sel-Dexa, ie, 80 mg of Sel twice-weekly along with 20 mg of Dexa in a 28-day cycle in 79 patients and 122 patients, respectively, and yielded ORR of 21% and 26%.Citation5,Citation6 The STOMP phase Ib/2 study evaluated Sel-Dexa combination with bortezomib (Bort) in 42 RRMM patients. Once-weekly administration of Sel 100 mg, Dexa 40 mg, and Bort 1.3 mg/m2 per 35-day cycle was the most tolerable regimen vs other tested regimens, with an ORR of 58%. Those without PI refractoriness had an ORR of 84% vs 43% for PI-refractory MM.Citation3 The Phase III BOSTON trial used the same weekly regimen of Sel-Dexa-Bort/35-day cycle and compared it with the 21-day cycle of Bort (1.3 mg/m2)-Dexa (20 mg) twice-weekly for 8 weeks followed by Bort (1.3 mg/m2) once-weekly and Dexa 20 mg twice-weekly.Citation9 Though G-3/4 hematologic (thrombocytopenia: 39% vs 17%, anemia: 16% vs 10%) and G-3/4 non-hematologic AEs except peripheral neuropathy (fatigue: 13% vs 1%, nausea: 8% vs 0%, peripheral neuropathy: 5% vs 9%) were more common with Sel-Dexa-Bort vs Bort-Dexa, once-weekly combination of Sel-Dexa-Bort showed superior median PFS (HR: 0.7, 95% CI: 0.53–0.93) and ORR (OR: 1.96, 95% CI: 1.3–3.1) compared to Bort-Dexa.Citation3 Recently, Jakubowiak et al evaluated the twice-weekly combination of Sel-Dexa with carfilzomib (Carf) in 21 patients with RRMM. This was a dose-escalation trial with the Sel dose ranging from 20–60 mg twice-weekly along with Carf and Dexa.Citation21 The recommended twice-weekly doses in this trial were Sel 60 mg, Carf 20/27 mg/m2 and Dexa 20 mg. Overall, G-3/4 thrombocytopenia (71%) and infections (24%) were the most common hematologic and non-hematologic AEs in this trial.Citation21
The common G-3/4 hematological AEs as reported by these studiesCitation2,Citation3,Citation5,Citation9,Citation20,Citation21 were thrombocytopenia (39–71%), anemia (16–33%), leukopenia (8–33%) and neutropenia (9–33%) whereas common G-3/4 non-hematological AEs were hyponatremia (5–26%), fatigue (13–15%), diarrhea (5–10%), eye disorders (9–10%), musculoskeletal disorders (4–10%), elevated liver enzymes (10%), peripheral neuropathy (5%) and vomiting (2–4%). Gastrointestinal (GI) AEs such as nausea, vomiting, diarrhea, weight loss were mainly G-1/2 and usually reversible. The toxicities reported by these studies are summarized in . SAEs (27–63%) responsible for complications in Sel ±Dexa trials included infections (respiratory infections (n=16), sepsis (n=12), bacteremia (n=4)), fever with/without neutropenia (n=14), encephalopathy/delirium/confusion (n=11), anemia (n=6), hyponatremia (n=6), dehydration (n=6), renal failure (n=4), nausea/vomiting (n=4), thrombocytopenia (n=4), elevated LFTs (n=2), intracranial hemorrhage (ICH) (n=2) and GI bleeding (n=1).Citation2,Citation5,Citation6 SAEs due to Sel-Dexa-Bort were febrile neutropenia (n=2), full-thickness macular hole in eye (n=1), and pulmonary embolism (n=1).Citation3 Sel-Dexa-Carf use resulted in infections more commonly (n=7) as SAEs, GI bleeding (n = 1; unrelated to treatment), syncope (n = 1), thromboembolism (n = 1), pain related to progressive disease (n = 1) and systolic heart failure (n = 1).Citation21
Table 1 Toxicity Profile of Selinexor
Management of Selinexor Toxicity
Hematologic Toxicity
Thrombocytopenia, the primary hematological toxicity of Sel, occurs via inhibition of thrombopoietin (TPO)-signaling in the megakaryocyte maturation phase and can be managed with the use of TPO agonists (eltrombopag or romiplostim), platelet infusions, and drug holidays. Those with baseline thrombocytopenia are more prone to develop high-grade thrombocytopenia thereby predisposing them though rarely, to life-threatening bleeding events such as ICH or GI bleeding.Citation2,Citation6 Therefore, Sel needs to be avoided unless the platelet count is ≥50,000/mm.Citation3,Citation6 On a similar note, patients who received platelet infusion within one week or TPO agents within 2 weeks prior to the first dose of Sel should not be administered Sel; those patients were excluded from the STORM trial part 2. Both in-vivo and in-vitro studies affirm that Sel-mediated thrombocytopenia is reversible with TPO-agonists ensuring that Sel has been washed out and there is no more ongoing Sel use. Otherwise, TPO-agents get antagonized by the TPO-blocking mechanism of Sel causing ongoing thrombocytopenia.Citation20 There are no strict guidelines about the TPO-agents’ use in Sel toxicity and their use is solely at the discretion of treating hematologists. In the BOSTON trial where TPO-agents were used in 18% of patients with thrombocytopenia, the events of dose reductions or interruptions were significantly reduced.Citation9 Sel-induced thrombocytopenia occurs in a dose-dependent fashion; one study looked at platelet drop at day 29 with different Sel doses and found evidence of less thrombocytopenia with its lower doses (50–70 mg) (n=36) vs higher doses >100 mg biweekly (n=28). The interruption of Sel dose for 8–21 days resulted in improvement in G-4 thrombocytopenia.Citation20 Those who are receiving FDA approved dose of Sel and develop G-4 thrombocytopenia without bleeding should stop taking Sel until at least G-3. When at G-3, Sel dose needs to be reduced from 80 mg twice-weekly (~160 mg/week) to 100 mg once weekly until thrombocytopenia improves to G-2. Following this, Sel can be resumed in two divided doses per week (total 100 mg/week), ie, 60 mg, 40 mg.Citation6
Adequate hematopoietic functions such as hemoglobin>8 gm/dl and absolute neutrophils >1000/mm3 should be ensured before Sel treatment, preferably in the absence of recent blood transfusions, erythropoietin (EPO) analogues or colony-stimulating factors (CSF). For G-3/4 anemia and neutropenia, Sel needs to be interrupted (counts can be boosted with EPO and CSF analogues per the discretion of the hematologist) as well until toxicity improves to G-2 and then Sel can be resumed at 60 mg twice-weekly. As it is critical to modify the Sel treatment with respect to hematologic toxicity, the most important aspect of managing its toxicity is to monitor blood cell counts ideally before each dose of Sel so that the subsequent dose could be modified. However, given twice-weekly dosing of oral Sel, performing blood counts twice weekly might appear cumbersome which needs to be balanced with the clinical utility of these labs. Therefore, we recommend checking complete blood counts at least once-weekly in the first two cycles of Sel as a majority of these AEs require treatment modifications during this timeframe. Venous thromboembolism (5% of the patients in one study), requires anticoagulation.Citation21
Gastrointestinal Toxicity
GI toxicity such as nausea, anorexia/decreased appetite, vomiting, diarrhea, and weight loss are usually centrally mediated and though low-grade, can limit the tolerability of Sel.Citation2 However, the addition of Dexa and the use of prophylactic antiemetics have improved its tolerance.Citation2 Most of the GI AEs, particularly vomiting are usually severe in the first 2 weeks and may decrease over time. Antiemetic use has been reported in 89–100% of the patients receiving Sel and many patients might need two (14–33%) or three antiemetics (5%).Citation3,Citation5,Citation6 For nausea/vomiting, 8 mg of ondansetron or equivalent antiemetics such as prochlorperazine, granisetron can be used before the first dose of Sel, and then ondansetron 8 mg should be given as needed twice daily or thrice daily at least for 2 days.Citation5,Citation6 Those with persistent nausea despite ondansetron or equivalent can be given olanzapine and neurokinin-1 receptor blockers such as aprepitant and rolapitant.Citation5,Citation22
In STORM trial part 1, eight doses of Sel were compared with six doses of Sel in a 28-day cycle, the rate of nausea was 82% vs 69%, respectively.Citation5 Sel should be interrupted in case of G-3 nausea until it improves to G-2 and then Sel can be resumed at 60 mg twice-weekly dosing.Citation6 GI toxicity is one of the most common causes of Sel termination. In one study, the treatment was terminated due to GI or constitutional AEs in 5/8 patients.Citation23 Prophylactic antiemetics can successfully avoid interruptions, dose reductions, or treatment terminations. It is of note that the once-weekly maximal dose of Sel of 100 mg along with the once-weekly Bort did not result in G-3/4 nausea, vomiting, or anorexia and was well tolerated with prophylactic antiemetics.Citation3
Anorexia and weight loss in cancer patients receiving Sel can be multifactorial and result from both chemotherapy and underlying malignancy itself. The use of appetite stimulants in addition to low-dose Dexa such as dronabinol, metoclopramide, and megestrol may improve appetite and cause modest weight gain.Citation3,Citation24 One randomized controlled trial comparing dronabinol with megestrol reported megestrol to be superior compared to dronabinol both for improving the appetite (75% vs 49%) and causing weight gain (11% vs 3%). The study found no difference when dronabinol-megestrol combination was compared to megestrol alone.Citation25 In a study by Jakubowiak et al., 100% of patients (n=21) received prophylactic megestrol acetate (160–400 mg daily) and 5-HT3 antagonist but 29% (n=6) of patients remained anorexic and 5% (n=1) patients experienced ongoing weight loss. The combination of antiemetics, however, was more effective in managing treatment interruptions since none (n=0/21) of the patients required treatment discontinuations due to GI AEs.Citation21 Another common AE of Sel is G-1/2 diarrhea that might require antidiarrheals. G-3/4 diarrhea was reported in 10% of patients by Jakubowiak et alCitation21 (n=2/21) and even less commonly (5–7%) by other investigators.Citation5,Citation23,Citation26 For ≥G2 diarrhea, Sel should be interrupted and resume when resolved to G1 but at 60 mg twice-weekly dose. One unrelated meta-analysis of eight randomized controlled trials found octreotide to be effective for severe cases of chemotherapy-induced diarrhea when compared to placebo (69% vs 54%).Citation27,Citation28 Dysgeusia reported in 10–17% of the patients in Sel studies is common with many other therapies but lacks evidence-based treatment strategies.Citation29
Renal Toxicity and Electrolyte Derangements
Dehydration and AKI are less common G-3/4 AEs with Sel and have a favorable outcome. These can be managed with outpatient fluid resuscitation or inpatient care depending upon severity.Citation30 Thus, far only one case of irreversible renal failure has been reported leading to treatment discontinuation.Citation6 G-3/4 hyponatremia is also common with Sel and should be managed with Sel interruption and dose reductions. Sel should be resumed at 60 mg twice weekly when the hyponatremia is at least G1 or resolved. Hyponatremia, even though G-3, is mostly asymptomatic and needs sodium replacement along with frequent lab monitoring. In the STORM trial part 1, only 6% of patients required salt tablets as compared to 22% of patients diagnosed with hyponatremia.Citation5 Chen et al. attributed a number of cases of delirium to hyponatremia. Delirium usually requires supportive care and correction of metabolic derangements such as hyponatremia.Citation2
Dose Adjustments
Early intervention with supportive care prevents the need for dose reduction and interruption. Treatment holidays and dose reductions were required in 52% and 37% of the study population in STORM part 1 and resulted in significantly less treatment termination (18%). This study had only three patients who received a higher dose of Sel 100 mg twice-weekly with all three patients requiring drug holidays or dose reduction.Citation5 In the STORM part 2, 80% of patients required dose modifications or holidays and the majority of those events occurred in the first 2 cycles, demanding an aggressive prophylactic treatment, and monitoring needs in the initial cycles.Citation6 About 17.2% of patients discontinued treatment due to treatment-related AEs. Bahlis et al. studied various dosing regimens for Bort and Sel in different combinations. There was no significant increase in G-3/4 AEs with Sel 100 mg weekly compared to 60 mg or 80 mg weekly regimen.Citation3 Jakubowiak et al reported 80% of patients receiving Sel needed a change in dosage or holiday from therapy but only 18% discontinued treatment due to AEs and 10% mortality (n=12/123) was attributed to major AEs. The rest recovered with conservative management.Citation21 Dose/treatment modifications to minimize adverse events are summarized in .
Table 2 Adverse Events Leading to Modifications in Treatment Plan in Selinexor Studies
Toxicity Profile of Belantamab Mafodotin
Trudel et al. reported the first-in-human Phase I trial (DREAMM-1) of belantamab mafodotin (belamaf) which included a dose-escalation phase (part 1, n=38) and a dose-expansion phase (part 2, n=35). Based on the results of part 1, 3.4 mg/kg was the recommended dose in part 2.Citation16,Citation17 Lonial et al conducted a phase II study (DREAMM-2) with two dosing cohorts in 196 RRMM patients. Ninety-seven patients were treated with 2.5 mg/kg and 99 patients with 3.4 mg/kg of belamaf.Citation15 In part 1 of the DREAMM-1, no dose-limiting AEs were reported and there was no maximum tolerated dose. The most common G-3/4 AEs were thrombocytopenia [13/38 (34%)] and anemia [6/38 (16%)]. In part 2 of DREAMM-1, G-3/4 AEs were seen in 28/35 (80%) patients, the most common being thrombocytopenia [12/35 (34%)] and anemia [5/35 (14%)]. SAEs were seen in 40% of patients, the most common of which were infusion-related reaction (IRR) (n=2) and lung infection (n=2). Five patients had drug-related SAEs including IRR (n=2), ICH (n=1), lung infection (n=1) and pericardial effusion (n=1).Citation17
In the DREAMM-2, the most common G-3/4 AEs were keratopathy seen in 26/95 (27%) patients in the 2.5 mg/kg cohort and 34/99 (34%) patients in the 3.4 mg/kg cohort. Thrombocytopenia was seen in 19/95 (20%) and 33/99 (33%), respectively, for 2.5 mg/kg and 3.4 mg/kg cohorts and anemia in 19/95 (20%) and 25/99 (25%) respectively. Among those who received prophylaxis for IRR, 8/22 (2.5 mg/kg cohort) and 6/27 (3.4 mg/kg cohort) patients developed IRR. One patient in the 2.5 mg/kg cohort discontinued treatment due to G-3 IRR.Citation15 In part 1 of the DREAMM-1, dose reduction was required in 1/3 (33%) patients receiving 1.92 mg/kg, 1/8 (13%) patients receiving 2.50 mg/kg, 3/3 (100%) patients receiving 3.40 mg/kg and 5/6 (83%) in 4.6 mg/kg dose. Moreover, 1/4 (25%) patients receiving 1.92 mg/kg and 2/6 (33%) patients receiving 4.6 mg/kg dose in part 1 discontinued treatment due to AEs which included limbal cell defect, foreign body sensation in eyes plus thrombocytopenia, and hypercalcemia. The blurring of vision (40%) was the most common cause of interruption or delay in belamaf therapy in the DREAMM-1.Citation17 In part 2 of DREAMM-1, belamaf related AEs include IRR, thrombocytopenia, and corneal events. Two patients (6%) discontinued treatment and 7 (20%) required dose reduction/delays because of thrombocytopenia. AEs led to dose reduction in 23/45 (66%) and dose interruption/delay in 25/45 (71%) patients.Citation17 In DREAMM-2, 93/95 (98%) patients in 2.5mg/kg cohort and 99/99 (100%) patients in 3.4 mg/kg cohort had at least one AE. AEs led to dose delays in 54% (2.5mg/kg cohort) and 62% (in 3.4mg/kg cohort) patients while dose reduction was required in 29% (2.5mg/kg cohort) and 41% (3.4 mg/kg cohort) patients. About 8% (2.5 mg/kg cohort) and 10% (3.4mg/kg cohort) patients permanently discontinued the treatment due to AEs in DREAMM-2 the most common of which being keratopathy seen in 1 (2.5mg/kg cohort) and 3 (3.4 mg/kg cohort) patients.Citation15 One death occurred in part 1 of DREAMM-1 study which was attributed to disease progression. Three deaths occurred in part 2 owing to disease progression. No treatment-related deaths were reported in DREAMM-1.Citation17 A total of two potentially treatment-related deaths were reported in DREAMM-2, one due to sepsis in the 2.5 mg/kg cohort and one due to hemophagocytic lymphohistiocytosis in the 3.4 mg/kg cohort.Citation15
In part 1 of DREAMM-1, the frequency of G-3-4 corneal AEs increased with the increased dose of the drug. Corneal AEs were reported in 20/38 (53%) patients, most of which were mild G-1/2 seen in 18/38 (47%) but it resulted in treatment discontinuation in two patients. In part 2 of DREAMM-1, corneal events were seen in 22/35 (63%) patients comprising of mild-moderate (G-1/2) in 19 and G-3 in 3 (keratitis in 1, eye pain in 1, and dry eye in 1) patients. The median time to onset of corneal events was 23 days (range: 1–84 days) while the median duration of patients with a resolution date was 30 days (range: 5–224 days). Thirty-one (89%) patients had corneal findings on the ophthalmic examination which included superficial punctate keratitis 27/35 (77%), epithelial edema 22 (63%), stromal edema 5 (14%), and opacities 8 (23%). No patients discontinued treatment in part 2 due to corneal AEs.Citation17 In DREAMM-2, keratopathy was the most common cause of permanent treatment discontinuation with 1% (2.5mg/kg cohort) and 3% (3.4mg/kg cohort). It led to dose reduction in 23% (2.5 mg/kg cohort) and 27% (3.4mg/kg cohort) and dose delays in 27% (2.5mg/kg cohort) and 48% (3.4 mg/kg cohort) patients. The most common reported corneal symptoms were blurred vision and dry eye in two patients without keratopathy. In the ocular sub-study (n=30; 17 patients in 2.5 mg/kg cohort and 12 patients in 3.4mg/kg cohort), G-3 AEs were reported in 29% (2.5mg/kg cohort) and 42% (3.4mg/kg cohort) in treated eye and 18% (2.5mg/kg cohort) and 50% (3.4 mg/kg cohort) in untreated eye.Citation15 The toxicity profile of belamaf has been summarized in .
Table 3 Toxicity Profile of Belantamab
Management of Toxicity of Belantamab Mafodotin
Cytotoxic payload and linker instability are postulated to cause the ocular toxicity associated with ADC.Citation31 Previously many clinical trialsCitation31–Citation33 of refractory hematologic malignancies have used MMAF cytotoxin and maleimidocaproyl linker and have reported similar ocular toxicity as reported in DREAMM-1 and DREAMM-2 trials due to belamaf. Ophthalmic steroid drops in DREAMM-1 were used to mitigate the ocular toxicity given the established side effects of MMAF.Citation16 However, ocular toxicity (blurring of vision, eye pain, and dryness, keratitis, and photophobia) still occurred, especially with the increasing doses of the drug.Citation17 Permanent discontinuations due to corneal events were rare in this trial and the majority of these AEs were successfully managed with dose reductions, interruptions, or delays. About 50% of individuals with corneal events showed a resolution within about 35 days.Citation17 On follow-up interviews of 17 patients from the second part of DREAMM-1 trial at the end of treatment, 76% of patients had reported of blurred vision while on treatment but 62% either had resolution or ongoing improvement in the complaint. The majority of those who participated in this interview never considered treatment discontinuation.Citation34 Ophthalmic evaluation while on belamaf plays a crucial role in the early detection of keratitis, epithelial and stromal edema. Therefore, serial ophthalmic evaluation at baseline and before subsequent doses have a critical role as a mitigation strategy and may prompt treatment adjustments. Popat et al reported a case series of 5 patients from the DREAMM-1 trial and shared experience from their center at a median follow-up of 32.6 months. When corneal AEs occurred, they increased the frequency of topical steroids (prednisolone eye drops 1–2 drops up to four times a day), used preservative-free artificial tears (such as artificial tears 1–2 drops twice a day as needed), and interrupted the next dose (median 14 days, range: 7–98 days). These patients developed increased intraocular pressure, infection, and secondary cataract formation given the excessive use of topical steroids and required topical antibiotics and cataract extractions. Therefore, Popat et al. recommended against the long-term use of topical steroids and proposed dose modifications or interruptions as the main strategy to deal with corneal side effects.Citation35
DREAMM-2 trial investigators made ophthalmic evaluations a part of their protocol. The reports of changes in visual acuity were also subject to strict follow-up. Those patients with visual changes experienced ultimate resolution and none of them had permanent vision loss. An ocular substudy, part of the DREAMM-2, verified no role of topical steroids in preventing corneal side effects. Dose reductions or delays were the most effective strategies. DREAMM-2 trial, therefore, recommends 25% dose reduction if G-2 corneal events have been experienced and interruption or delay of the dose if G-3/4 corneal events have been experienced. The dose should be delayed until the corneal event is improved to G-2 and then belamaf with a 25% reduced dose should be given. In the United States, belamaf has been available since August 2020 under a Risk Evaluation and Mitigation Strategy (REMS) given ocular toxicity, called BLENREP REMS.Citation13 Dosage modifications of belamaf in RRMM can be made based on the Keratopathy and Visual Acuity (KVA) scale documented in the prescribing information of BLENREP ().Citation13,Citation19,Citation36 This scale was developed by GlaxoSmithKline (GSK) upon the recommendation of the FDA.
Table 4 Management of Ocular Toxicity of Belamaf
In DREAMM-1, onset of thrombocytopenia was observed for 50% at 7.5 days and 50% had resolution after 8 days of onset. Only 6% had discontinuation of therapy due to thrombocytopenia. These side effects can be managed with modification in the treatment plan.Citation16,Citation17 However, in DREAMM-2, thrombocytopenia was considered self-limiting. A total of 22 patients (11%) reported bleeding of G-2 or worse.Citation15 Serious infections such as pneumonia or lung infection might require dose interruptions or delays.
Discussion
Despite advancements in MM treatment over decades, with many active drugs and the use of hematopoietic stem cell transplantation/autologous stem cell transplantation, it remains incurable, and invariably patients with MM relapse and require therapies for the treatment of relapse.Citation37 During the course of illness, a considerable number of patients develop a refractory disease to three classes of commonly used drugs (PIs, IMiDs, and monoclonal antibodies). Overall survival in three-class, quad, or penta refractory disease is short.Citation6 MAMMOTH study reported outcomes of MM patients who were refractory to anti-CD38-monoclonal antibody and other agents. The median overall survival after anti-CD38 antibodies refractoriness was 8.6 months, 11.2 months in patients who were not simultaneously refractory to one IMiD and one PI, and 5.6 months in patients who were refractory to anti-CD-38 antibodies, two PIs, and two IMiDs (penta-refractory).Citation38 After multiple lines of treatment exposures, at the time of relapses, such patients have underlying marrow suppression and cumulative toxicities. Therefore, it becomes essential that they maintain a good quality of life while we use newly approved drugs such as Sel and belamaf.
After multiple prior lines of therapy, the selection of the appropriate next line of therapy is crucial in the context of prior toxicities, including profound thrombocytopenia. Sel at recommended doses (80 mg twice weekly) may not be an appropriate treatment choice for such patients due to the risk of ICH and GI bleeding depending on the severity of thrombocytopenia and should be avoided unless the platelet count is at least 50,000. However, thrombocytopenia is reversible with drug interruption and the use of TPO agents.Citation39 For severe thrombocytopenia, platelet transfusions have been shown to be effective in quickly increasing the platelet levels. TPO agonists (romiplostim or eltrombopag) can be used to increase platelet counts over two to 3 weeks while continuing the treatment with selinexor. TPO agonists should be used when platelet counts fall below 25,000/mm3 until the count rises to ≥50,000/mm3.Citation40 Frequent monitoring of platelet counts during Sel treatment is highly recommended. Sel should be interrupted for G4 thrombocytopenia and the dose should be reduced for G3/2 thrombocytopenia. Any life-threatening bleeding event history such as ICH should be carefully weighed against the re-induction of treatment and its benefits and reintroduction should be avoided if at all possible.
When considering belamaf, it is of utmost importance that the treating hematologist is aware of its ocular toxicity and its management strategies as it may have dire visual consequences. Belamaf is currently available under REMS program that requires special certification for prescribers. About 76% of the patients in the DREAMM-1 reported some ocular complications.Citation16 A baseline ophthalmic evaluation and proper documentation of any visual problems using a KVA scale should be performed. Following the baseline evaluation, findings should be documented before each dose to monitor any change and tailor treatment according to the findings. It is prudent for the treating hematologist to discuss ocular toxicity with the ophthalmologist and request findings based on the KVA scale as it is a relatively newer drug, and many ophthalmologists might have limited experience. The patient on belamaf should be strictly advised to use preservative-free eye drops four times a day. The documentation of the use of contact lenses should be made and, if possible, avoided as it may worsen the keratitis. The DREAMM-2 ocular substudy data did not demonstrate a clinical benefit of prophylactic topical steroids and therefore should be avoided.Citation15 Preferably a strategy of dose interruption and reductions based on the KVA scale should be employed in the management of ocular toxicity. Ocular toxicity also becomes important if the patient has received previous treatment with Sel, as Sel has shown to contribute to blurred vision in 10–11% of patients and cataract formation in 4%.
Another toxicity worth watchful monitoring with both Sel and belamaf treatment is thromboembolism but is not commonly reported in clinical trials.Citation16,Citation21 Thromboembolism prophylaxis may be warranted but is not required. Non-hematological toxicities related to both Sel and belamaf can be managed with standard treatment guidelines. Nausea and vomiting related to Sel may predispose patients to develop AKI. An antiemetic should be added to Sel due to its high emetogenic potential and nausea should be addressed promptly.Citation3 Since Dexa is added to the Sel treatment, the treating physician may find that patients do not experience nausea during the initial days of treatment. Instead, they experience delayed nausea and vomiting that will require the additional use of antiemetics.
Sel is also related to neurotoxicity and hyponatremia. The treatment for hyponatremia is usually not required until when G3 or 4. Hyponatremia should be expected when patients report unexplained fatigue or slow thought process. Early identification of hyponatremia is crucial as it may worsen with nausea, vomiting, diarrhea, and reduced oral intake. Sodium levels should be monitored at baseline and throughout the treatment. Sel-related hyponatremia usually occurs on day eight or afterward, therefore other hyponatremia causes should be ruled out if it occurs earlier during the course. Neurotoxicity usually develops in the third or fourth week of Sel treatment and manifests as syncope, dizziness, cognitive difficulties, and mental status changes. The treatment should be interrupted and other causes of mental status changes should be ruled out.Citation2,Citation6,Citation21
As these are newer drugs with limited data, continuous surveillance and monitoring are strictly warranted during the treatment course with early mitigation strategies. The common AEs and their management strategies have been summarized in . Various ongoing clinical studies of these two drugs have been summarized in .
Table 5 Management of Toxicities of Selinexor and Belamaf
Table 6 Ongoing Clinical Studies for Selinexor and Belantamab (Source: Clinicaltrials.gov)
Conclusion
Cytopenias, constitutional symptoms, gastrointestinal effects, hyponatremia, and anemia are the major toxicities of Sel and belamaf. Managing Sel toxicities require frequent monitoring for blood counts and basic metabolic panel along with prophylactic use of antiemetics, and appetite stimulants as needed and colony-stimulating factors/hematopoietic growth factors in addition to dose interruptions and modifications to manage neutropenia and cytopenia. We recommend following REMS program guidelines for close monitoring and evaluation of belamaf toxicities and early ophthalmic intervention. The physician should be aware of thrombocytopenia and its management as well as belamaf ocular toxicity which is best managed with dose reduction and dose delays but if missed could have serious complications.
Acknowledgments
Authors thank Ms. Marsha Halajian and Laeth George, MD for providing English language editing services and proofreading.
Disclosure
F. Anwer reports personal fees from Bristol Myers Squibb as a speaker and fee from Janssen pharmaceutical as an advisory board member, this fee was not related to the submitted work. Without receiving direct funding, served as the local principal investigator for Allogene Therapeutics, Celgene, GlaxoSmithKline, and Bristol Myers Squibb; has a consulting or advisory role for Seattle Genetics, Incyte Corporation Speakers’ Bureau, Company: Incyte Corporation; receives travel and accommodations expenses from Seattle Genetics, Incyte; receives honoraria from Incyte, Company: Seattle Genetics; and received research funding from Seattle Genetics, Company: Celgene, Acetylon Pharmaceuticals, Millennium, Astellas Pharma and AbbVie; and reports no other potential conflicts of interest for this work. The other authors report no conflicts of interest for this work.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
- Chen C, Siegel D, Gutierrez M, et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and waldenstrom macroglobulinemia. Blood. 2018;131(8):855–863. doi:10.1182/blood-2017-08-797886
- Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132(24):2546–2554. doi:10.1182/blood-2018-06-858852
- Syed YY. Selinexor: first global approval. Drugs. 2019;79(13):1485–1494. doi:10.1007/s40265-019-01188-9
- Vogl DT, Dingli D, Cornell RF, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(9):859–866. doi:10.1200/JCO.2017.75.5207
- Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738. doi:10.1056/NEJMoa1903455
- US-FDA. FDA grants accelerated approval to selinexor for multiple myeloma. 2019. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-selinexor-multiple-myeloma. Accessed December, 2020.
- Walker JS, Garzon R, Lapalombella R. Selinexor for advanced hematologic malignancies. Leuk Lymphoma. 2020;61(10):2335–2350.
- Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, Phase 3 trial. Lancet. 2020;396(10262):1563–1573. doi:10.1016/S0140-6736(20)32292-3
- Malandrakis P, Ntanasis-Stathopoulos I, Gavriatopoulou M, Terpos E. Clinical utility of selinexor/dexamethasone in patients with relapsed or refractory multiple myeloma: a review of current evidence and patient selection. Onco Targets Ther. 2020;13:6405–6416. doi:10.2147/OTT.S227166
- Abramson HN. B-Cell Maturation Antigen (BCMA) as a target for new drug development in relapsed and/or refractory multiple myeloma. Int J Mol Sci. 2020;21(15):5192. doi:10.3390/ijms21155192
- Cho SF, Lin L, Xing L, et al. BCMA-targeting therapy: driving a new era of immunotherapy in multiple myeloma. Cancers. 2020;12(6):1–29. doi:10.3390/cancers12061473
- US-FDA. FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. 2020. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma. Accessed November 25, 2020.
- Markham AJD. Belantamab mafodotin: first approval. Drugs. 2020;1–7. doi:10.1007/s40265-019-01241-7
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, Phase 2 study. Lancet Oncol. 2020;21(2):207–221. doi:10.1016/S1470-2045(19)30788-0
- Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion Phase 1 trial. Lancet Oncol. 2018;19(12):1641–1653. doi:10.1016/S1470-2045(18)30576-X
- Trudel S, Lendvai N, Popat R, et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9(4):37. doi:10.1038/s41408-019-0196-6
- Trudel S, Cohen AD, Richardson PG, et al. Safety and tolerability of single-agent belantamab mafodotin in heavily pre-treated patients with relapsed/refractory multiple myeloma: pooled data from DREAMM-1 and DREAMM-2. HemaSphere. 2020;4:429–430.
- Farooq AV, Degli Esposti S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 Study. Ophthalmol Ther. 2020;9(4):889–911.
- Razak ARA, Mau-Soerensen M, Gabrail NY, et al. First-in-class, first-in-human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34(34):4142.
- Jakubowiak AJ, Jasielec JK, Rosenbaum CA, et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br J Haematol. 2019;186(4):549–560. doi:10.1111/bjh.15969
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. doi:10.1056/NEJMoa1515725
- Bahlis N, Chen C, Sebag M, et al. A phase 1B/2 study of selinexor in combination with backcone therapies for treatment of relapsed/refactory multiple myeloma. Haematologica Conf. 2016;101:84.
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;2013(3):Cd004310.
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20(2):567–573. doi:10.1200/JCO.2002.20.2.567
- Chen CI, Bahlis N, Gasparetto C, et al. Selinexor, pomalidomide, and dexamethasone (SPd) in patients with relapsed or refractory multiple myeloma. Blood. 2019;134.
- Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15(10):e447–e460. doi:10.1016/S1470-2045(14)70006-3
- Sun JX, Yang N. Role of octreotide in post chemotherapy and/or radiotherapy diarrhea: prophylaxis or therapy? Asia Pac J Clin Oncol. 2014;10(2):e108–e113. doi:10.1111/ajco.12055
- Hovan AJ, Williams PM, Stevenson-Moore P, et al. A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18(8):1081–1087. doi:10.1007/s00520-010-0902-1
- Park SE, Hwang JH, Choi JH, et al. Incidence, risk factors, and clinical outcomes of acute kidney injury caused by palliative chemotherapy in lung cancer. J Cancer. 2019;10(22):5332–5338. doi:10.7150/jca.28399
- Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody–drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31(10):589–604. doi:10.1089/jop.2015.0064
- Fathi AT, Chen R, Trippett TM, et al. Interim Analysis of a Phase 1 Study of the Antibody-Drug Conjugate SGN-CD19A in Relapsed or Refractory B-Lineage Acute Leukemia and Highly Aggressive Lymphoma. Washington, DC: American Society of Hematology; 2014.
- Moskowitz CH, Forero-Torres A, Shah BD, et al. Interim Analysis of a Phase 1 Study of the Antibody-Drug Conjugate SGN-CD19A in Relapsed or Refractory B-Lineage Non-Hodgkin Lymphoma. Washington, DC: American Society of Hematology; 2014.
- Eliason L, Opalinska J, Martin ML, et al. Dreamm-1: patient perspectives from the first-in-human study of single-agent belantamab mafodotin for relapsed and refractory multiple myeloma (RRMM). HemaSphere. 2020;4:936.
- Popat R, Warcel D, O’Nions J, et al. Characterisation of response and corneal events with extended follow-up after belantamab mafodotin (GSK2857916) monotherapy for patients with relapsed multiple myeloma: a case series from the first-time-in-human clinical trial. Haematologica. 2020;105(5):e261. doi:10.3324/haematol.2019.235937
- Accessdata.fda.gov. BLENREP (belantamab mafodotin-blmf) for injection, for intravenous use. 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761158s000lbl.pdf. Accessed February 1, 2021.
- Goldschmidt H, Ashcroft J, Szabo Z, Garderet L. Navigating the treatment landscape in multiple myeloma: which combinations to use and when? Ann Hematol. 2019;98(1):1–18. doi:10.1007/s00277-018-3546-8
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275. doi:10.1038/s41375-019-0435-7
- Machlus KR, Wu SK, Vijey P, et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood. 2017;130(9):1132–1143. doi:10.1182/blood-2016-11-752840
- Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020;34(9):2430–2440. doi:10.1038/s41375-020-0756-6