Abstract
Objective
The present investigation aims on the clinical attributes and haematological parameters between symptomatic (COVID–19 ICU) and asymptomatic (COVID–19 homes isolation) patients as predisposing sign for COVID-19 related mortality.
Materials and Methods
A retrospective cohort research was conducted of admitted patients to ICU, who were suffering from severe COVID-19 in Aseer Central Hospital, Abha, Kingdom of Saudi Arabia (KSA) from July 2020 until September 2020. The study included individuals with COVID −19 and ICU admission as symptomatic group and others who are COVID–19 positives with quarantine as asymptomatic group. Epidemiological, clinical and haematological laboratory data were retrospectively collected, analysed with control subjects.
Results
Of the 38 ICU patients studied, the most common symptoms were fever and respiratory distress (100%), cough (86.8%). Majority were of Saudi origin (78.9%). Eighteen (47.4%) COVID-19 ICU patients showed leukocytosis, 6 (15.8%) had severe thrombocytopenia (with most having thrombocytopenia), 18 (47.4%) were anaemic. A significant correlation was observed between the WBC, RBC, Hb, platelets, neutrophil and lymphocyte count between ICU inmates compared with quarantine (p < 0.001) and RBC, Hb, neutrophil and lymphocyte count with control groups (p < 0.001).
Conclusion
From the observations it is evident that, the blood tests have potential clinical value in predicting COVID-19 progression. Further, patient characteristics including age, leukocyte count, RBC, platelets and differential leukocyte counts may be significant predictors for monitoring the progression of the critical illness observed in SARS-COV-2 patients. Also, treatment procedures can be re-defined further to reduce COVID–19 mortalities in more critically ill COVID-19 individuals.
Introduction
A pandemic of coronavirus disease 2019 (COVID-19) is a viral infection which cause severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with a variety of disease presentation and severity.Citation1 COVID-19 has affected almost the whole world, with death of approximately more than 6.2 million worldwide and 500 million cases, since that fateful December month of 2019, and more than 9000 deaths and more than 7.5 lakh cases have been recorded in KSA.Citation2 Epidemiological researches have depicted that 6–10% of COVID-19 affected patients progress to a more critical stage and will be in dire need of admittance to the intensive care unit (ICU) on account of acute respiratory.Citation3 Invasive ventilation is usually required by most of these ICU admitted patients, due mostly to intensive lung injury and acute respiratory distress syndrome (ARDS).Citation4 Till now, most of these patients on invasive mechanical ventilation (MV) have depicted an increased mortality pattern.Citation5 Most ICU harbouring these COVID-19 patients have shown a mortality rate of 50–65% range.Citation6–8 Some ICU keeping COVID-19 patients on MV have reported a mortality rate as high as 97%.Citation9
Many studies have shown a significant alteration in blood investigations associated with SARS-CoV-2 as well as the disease outcomes.Citation10–13 In addition, very few studies provide correlation between peripheral blood WBC morphologic changes and disease outcomes.Citation14 Another study demonstrated the quantitative and qualitative of the peripheral blood changes in COVID-19 cases which showed reduction in T cell count.Citation15
In many studies, COVID-19 has shown a link with inflammation and thrombosis. It has been shown that serious cases had thrombotic complications in ICU COVID-19 patients. Another study showed the association between C-reactive protein (CRP) and infection with COVID-19.Citation16,Citation17 As platelets are critical in haemostasis, thrombosis, and inflammatory responses, one study showed that COVID-19 may induce production of autoantibodies which leads to destruction of platelets by immune responses which was approved in another study in patients infected with human immunodeficiency virus (HIV).Citation18 It is well known that during infection, the platelets are activated and adhered to the sub-endothelium, their hyperactivity leads to thrombosis, arterial ischemia and in many cases causes pulmonary embolisms. Many viral infections such as HIV, hepatitis C virus (HCV) and influenza virus can directly lead to hyperactivity of the platelets.Citation19–22 In a recent study COVID-19 has been found to induce hyperactivity of the platelets through binding of spike protein to ACE2 receptor. It has been approved that SARS-CoV-2 can induce platelet activation which may contribute in thrombus formation and inflammatory responses in COVID-19 patients.Citation23 One of the important inflammatory markers, which give an insight to the inflammation going on in various organs is the Platelet lymphocyte ratio (PLR).Citation24,Citation25 Some studies have also found it to be independent from either platelet count or lymphocyte count.Citation25
Fast and correct laboratory diagnosis of active COVID-19 infection is one of the keystones of pandemic control. If these COVID-19 serious cases can be identified early by haematological and other parameters, it can facilitate clinicians to take early appropriate remedial measures to control the morbidity and mortality of COVID-19 cases.Citation26 Some earlier literature on reporting on laboratory-confirmed COVID-19 cases have noticed changes in the haematological parameters, which included neutrophil count and lymphocyte count.Citation27,Citation28 With a view of the above, we undertook this particular research to further clarify the clinical attributes and haematological parameters in ICU admitted COVID-19 patients compared to COVID-19 positive patients without symptoms and healthy controls.
Materials and Methods
Study Design and Setting
A retrospective cohort study was conducted at Aseer Central Hospital, Abha, the largest government hospital in Abha, Aseer province. All critical care admissions from October to December 2020 presenting to any of the intensive care units of the hospital were included in the research. All selected ICU critically ill participants and quarantine individuals had established severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection which was earlier confirmed by positive report on polymerase chain reaction (PCR) investigation of a sample from nasopharynx or aspirate from trachea. The patients who were included in the present study had their clinical outcomes monitored until February 2021, the last date of the research follow-up.
Study Participants
Thirty-eight patients aged sixteen years to eighty-three years, admitted to the ICU with COVID-19 were included in this study. Laboratory confirmation that the patients were SARS-CoV-2 infected was based on positive reverse-transcriptase-polymerase chain-reaction (RT-PCR) assay.Citation29 They were designated as group-1. Those were compared with twenty-nine COVID-19 quarantined patients who were designated as group-2 and twenty healthy individuals who have not been infected by COVID-19 as group-3.
Exclusion and Inclusion Criteria
Exclusion criteria included previously known participants having coagulopathy, pregnancy, antiplatelet therapy, current use of systemic anticoagulants, vitamin K antagonists and patients who presented with serious cardiac problems. Quarantined patients, who fitted the criteria, were randomly selected from records.
Ethical Consent
Our study complies with the Declaration of Helsinki. Ethical approval has been provided from Regional Committee for Research Ethics [No of Registration (H-06-8-091)] vide REC-NO: REC-03-09-2020, Ministry of Health, Directorate Health Affairs - Aseer Region, KSA. Informed consent was obtained from each study participant after describing the objective and procedures of the study. Every response of the participants was kept confidential.
Detection of COVID-19 by Real Time-PCR and the Conditions of the Test
A nasopharynx samples were collected from assigned groups and proceeded for DNA and RNA extraction using magna pure DNA and viral NA kit (Roche). The extracted samples were amplified by the RealStar® SARS-CoV-2 RT-PCR Kit 1.0 (Altona diagnostic), to detect S gene for SARS-CoV-2 and E gene for B-βCoV. For quality control check, positive control contains both targets, B-βCoV and SARS-CoV-2 and negative controls (H2O) were used per test. After that, samples were analysed by Light Cycler 480 II (LC480II, Roche) and performed according to the manufacturer’s instructions. Samples with a CT value below 35 were considered positive amplification of the SARS-CoV-2 S gene.
Data Collection
All study data were retrieved from ICU records after proper permission. Collected clinical variables included demographics, comorbidities, and haematological parameters after ICU admission.
Retrospective Laboratory Data Analysis
All data were collected from the patient records after adequate permission and then entered in IBM SPSS version 23 [IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 22.0. Armonk, New York, USA] for further analysis. The data were subjected to general analysis like mean, standard deviation, standard error, kurtosis, skewness, etc, and then comparison was done with the help of non-parametric test, ie, Mann–Whitney test where p-value (2-tailed), Wilcoxon W, Mann–Whitney U, etc were analysed. PLR was calculated by dividing the platelet count by the lymphocytes.
Results
ICU patients had a mean±SD of 15.11±11.612 × 109/L for WBC, with a minimum of 2.15 × 109/L and maximum of 47.49×109/L (). RBC count was 4.11±1.26 million cells per mcL with a minimum of 2.38 million cells per mcL and maximum of 8.12 million cells per mcL. The Hb was 11.30±3.12 g/L, with minimum being 6.00 g/L and maximum being 20.80g/L. Platelets’ mean±SD was 184.95±123.3× 109/L, with a minimum of 18.00×109/L and 553.00×109/L maximum. Neutrophil count was (mean±SD) 13.34±11.38 × 109/L with a minimum of 0.50×109/L and a maximum of 43.80×109 /L. Lymphocyte count had a mean±SD of 0.97±0.66 × 109/L, the minimum being 0 and maximum being 2.78×109/L. In the case of monocytes, it (mean±SD) was 0.63±0.46× 109/L, with a minimum of 0.10 × 109/L and a maximum of 1.97×109/L. The Eosinophil count had a mean±SD of 0.12±0.23× 109/L with minimum being 0 and maximum of 0.97×109/L. Basophils were in the range of 0.039±0.04× 109/L (mean±SD) with maximum of 0.19 × 109/L and minimum of 0 (). PLR was 189.895.
Table 1 Characteristics of the Blood Parameters of ICU Patients with COVID-19, Quarantine Patients with COVID-19
Quarantined group had a mean±SD of 5.7±0.48×109/L for WBC of with a minimum of 2.18×109/L and maximum of 14.92×109/L (). RBC count was 5.79±0.55 million cells per mcL with a minimum of 4.87 million cells per mcL and maximum of 6.83 million cells per mcL. The Hb was 16.0±1.97 g/L, with minimum being 8.20 g/L and maximum being 20.20 g/L. Platelets’ mean±SD was 306.76±106.77 × 109/L, with a minimum of 156.00×109/L and 567×109/L maximum. Neutrophil count was (mean±SD) 2.54±1.96×109/L with a minimum of 0.66×109/L and a maximum of 10.94×109/L. Lymphocyte count had a mean±SD of 2.519±0.82×109/L, the minimum being 0.88 and maximum being 4.26×109/L. In the case of monocytes, the mean±SD was 0.49±0.21×109/L, with a minimum of 0.14×109/L and a maximum of 1.12×109/L. The Eosinophil count had a mean±SD of 0.15±0.174×109/L with minimum being 0 and maximum of 0.79×109/L. Basophils were in the range of 0.04±0.022×109/L (mean±SD) with maximum of 0.08×109/L and minimum of 0 (). PLR was 121.746.
Considering WBC, the mean±SD for the control group was 6.53+1.96×109/L with a minimum of 3.58×109/L and maximum of 11.65×109/L (). RBC count was 5.31±0.31 million cells per mcL with a minimum of 4.62 million cells per mcL and maximum of 5.98 million cells per mcL. Hb level was 15.5+1.18g/L, with minimum being 12.70g/L and maximum being 17.60g/L. The mean±SD for Platelets was 286.65+86.54× 109/L, with a minimum of 156×109/L and 546×109/L maximum. Considering neutrophil count, it was (mean±SD) 3.2+1.32× 109/L with a minimum of 1.56×109/L and a maximum of 6.56×109/L. The mean±SD for lymphocyte count was of 2.54+0.785× 109/L, the minimum being 1.34 and maximum being 4.94×109/L. In the case of monocytes, the mean±SD was 0.53+0.126× 109/L, with a minimum of 0.37×109/L and a maximum of 0.9×109/L. The Eosinophil count had a mean±SD of 0.22+0.178× 109/L with minimum being 0.07 and maximum of 0.76×109/L. Basophils were in the range of 0.039+0.0236× 109/L (mean±SD) with maximum of 0.09× 109/L and minimum of 0.09 (). PLR was found to be 112.766.
A significant correlation was seen between the RBC (), Hb (), Neutrophil count () and Lymphocyte count () count (p < 0.001) of ICU patients with COVID-19 and healthy controls. However, no such correlation was seen between Monocyte count (), Eosinophil count () and Basophil () count (p = 0.294, p = 0.116 and p = 0.996).
Figure 1 Comparison of RBC count in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers).
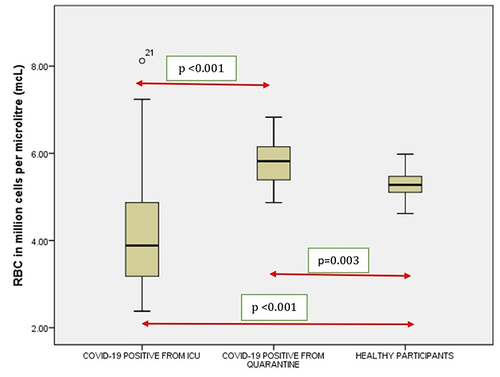
Figure 2 Comparison of hemoglobin in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers). *The asterisk is used for higher observed data points outside the boundary of the whiskers.
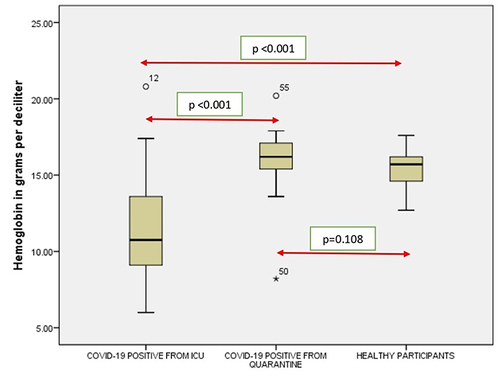
Figure 3 Comparison of neutrophils in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers). *The asterisk is used for higher observed data points outside the boundary of the whiskers.
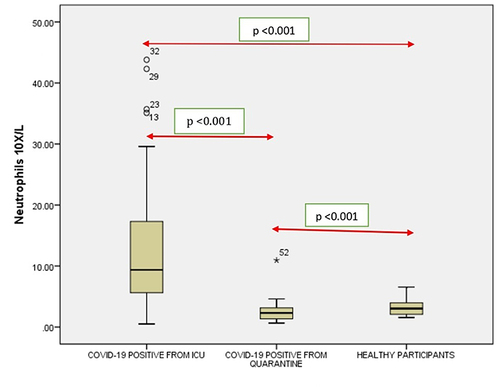
Figure 4 Comparison of lymphocytes in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers).
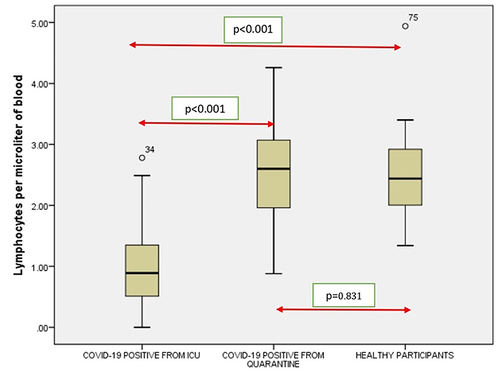
Figure 5 Comparison of monocytes in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers).
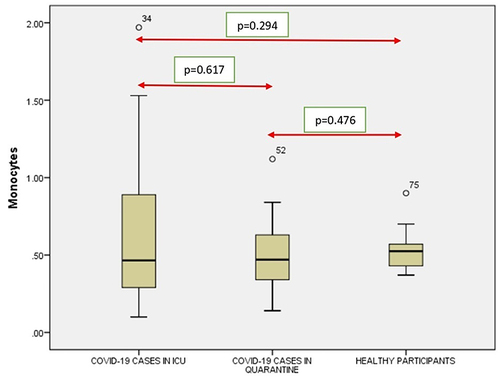
Figure 6 Comparison of eosinophils in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers). *The asterisk is used for higher observed data points outside the boundary of the whiskers.
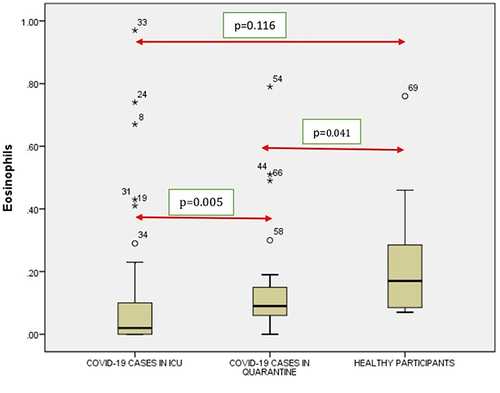
Figure 7 Comparison of basophils in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers). *The asterisk is used for higher observed data points outside the boundary of the whiskers.
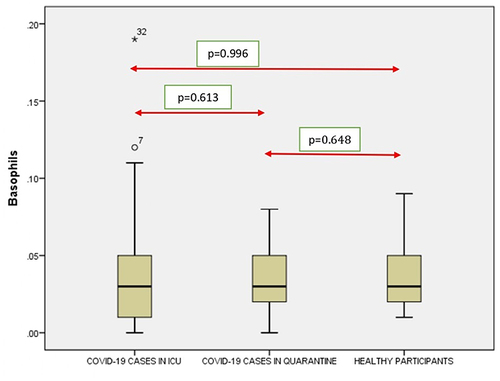
It was seen that there is a significant correlation between the WBC (), RBC (), Hgb (), Platelets (), Neutrophil count () and Lymphocyte count () of the ICU patients with COVID-19 and Quarantined patients with COVID-19 (p < 0.001). But no such relationship was seen between Monocyte () count (p = 0.617), Eosinophil () count (p = 0.005) and Basophil () count (p = 0.613) ().
Figure 8 Comparison of WBC count in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers). *The asterisk is used for higher observed data points outside the boundary of the whiskers.
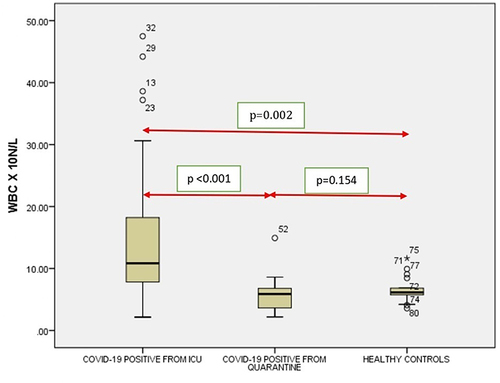
Figure 9 Comparison of Platelets in COVID-19 positive cases of ICU patients, quarantined patients with COVID-19 and healthy participants. Red double direction arrow: used to compare the p value between various groups. °Outlier (observed data points outside the boundary of the whiskers).
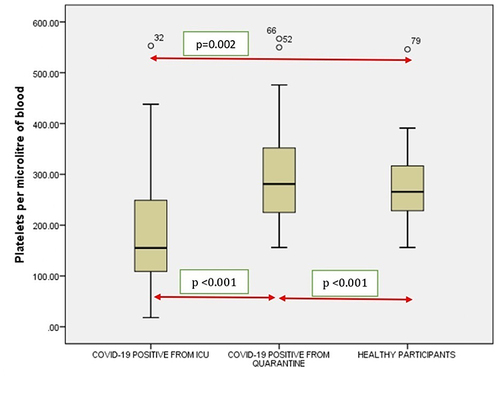
It was noticed that no significant correlation existed between COVID-19 cases from quarantine with healthy participants in WBC () count (p = 0.154), Hgb () (p = 0.108,) (), (), Lymphocyte count () (p = 0.831), Monocyte count () (p = 0.476), Eosinophil count () (p = 0.041), Basophil count () (p = 0.648). However, significant correlations occurred between platelets () and neutrophil count () (p<0.001).
Discussion
The virus responsible for COVID-19 is mutating so rapidly that we are unable to understand the different speculations that its effects are having on the human body. Nonetheless, the haematological and biochemical parameters are still to be elucidated perfectly, so that future generations can control the mutated virus in the human body. Therefore, we abridged a comparative scrutiny of haematological attributes of 38 ICU COVID-19 patients, 29 quarantined COVID-19 patients and 20 controls.
Leukocytosis, low RBCs (compared to quarantined subjects and controls), low haemoglobin (compared to quarantined subjects and controls), decreased platelets (compared to quarantined subjects and controls), neutrophilic leukocytosis, lymphocytopenia were some of the prominent features of our study. In Chongqing (China), the common symptoms that were observed were cough, fatigue, fever and expectoration; these symptoms were also observed in many other studies.Citation30–33 But some major symptoms like myalgia, fever, malaise and chills were also reportedCitation34 and common symptoms like chest pain and dyspnoea were also reported.Citation35
The haematological disorders observed in critically ill COVID-19 patients are complex and multifactorial. In this study of 38 individuals with COVID-19 hospitalized in the ICU of Aseer Hospital, it was seen that significant correlation was seen between the RBC, Hb, Neutrophil count and Lymphocyte count (p < 0.001) of ICU patients with COVID-19 and healthy controls. It was noticed that abnormal hematologic parameters (including lymphocytopenia, neutrophilia and eosinopenia) and declining kidney functions were correlated with a greater peril of alarming hospital itinerary. Many previous researches have depicted that approximately 5% of SARS-CoV-2 patients become grievously sick, evolving organ malfunctions and ultimately failure.Citation36 Recently, a study has depicted irreversible damage caused to the erythrocyte proteome by COVID-19.Citation37 Reactive oxygen species plays a vital role in leading to the damage of the RBCs, memorialize the neutrophil activation. SARS-CoV-2 attacking facilitates iron removal from the heme prosthetic group, which leads to the loss of functional haemoglobin.Citation38,Citation39 The authors found that oxidative stress connected with COVID-19 damages essential proteins in erythrocytes, including those that influence membrane structure and the ability to transport and deliver oxygen. Because mature erythrocytes cannot synthesize new proteins to replace damaged ones and the average lifespan of erythrocytes is 120 days, the authors hypothesize that the circulation of irreversibly damaged erythrocytes with impaired function could contribute to the long-term effects of COVID-19.Citation25 The mechanism behind the lymphopenia in ICU patients might be due to the direct attack by the virus in the lymphocytes or the mechanism might be one of immune-mediated apoptosis of lymphocytes.Citation40–42 The research shows that the median age of the ICU patients was 55.5 years while the median age of the quarantined patients was 25 years; this is same as some previous studies which showed that the median age of the patients who were in quarantined with COVID-19 was lower than the ICU cohort.Citation43,Citation44 It is a renowned fact that the aged people greater number of limited organ function, comorbidities, biological aging, impaired immune system and more serious complications, which were pointed out by earlier studies on aged people afflicted with COVID-19Citation45,Citation46 hence it is of utmost importance that clinicians should treat them as a high-risk group.
Differential count (Neutrophil, eosinophil, etc) showed some remarkable differences from the normal in both ICU and COVID-19 positive cases, which also correlated with previous studies. All these add to the remarkable distinction, which can be pursued in future studies.Citation47–49
An essential pathogenetic mechanism in COVID-19 which leads to kidney and lung injury is endothelial injury.Citation50–52 Comparison of haematological findings showed that the RBC, Hb, Neutrophil count and Lymphocyte count (p < 0.001) of ICU patients had significant differences with healthy controls. Materializing evidence hints that neutrophil, and the imbalance between neutrophil extracellular trap (NET) which plays a key role in the pathophysiology of coagulopathy, inflammation, organ damage, and also immunothrombosis, mainly is responsible for the characteristics of severe cases of COVID-19.Citation53 This also mostly matches earlier research done on patients with COVID-19.Citation54,Citation55 Damage to the endothelium leads to recruitment of perivascular T-cells and distorts the alveolar capillary barricade in the lungs.Citation56 Acutely damaged endothelium in COVID-19 is correlated with structurally distorted capillaries and further evidence of compensatory neovascularisation.Citation57 This lower than is desirable endothelial barricade provokes oedema of the lungs, and subsequent proteinuria, which are commonly seen in patients afflicted with severe kidney and lung diseases.Citation58,Citation59 As abundant NETs are found in the circulation and in kidney and lung tissues of patients afflicted with COVID-19,Citation60 their stockpiling delineates a key provocation to generate renal and pulmonary microvascular thrombosis, which ultimately leads to COVID-19-related organ failure.Citation61,Citation62 A nonspecific inflammatory marker, the PLR, implies concurrent interaction between platelet count and lymphocyte count, reflects aggregation, as well as inflammatory pathways. In many cases, it has been found to be elevated in response to many acute as well as chronic proinflammatory conditions. Our study had elevated PLR in ICU, compared to healthy controlsCitation25,Citation63–65 and has reported a strong correlation between elevated PLR and mortality in Covid-19 patients like some other studies.
Limitations of the Study
We have to admit that the study has many limitations. First, retrospective cross-sectional research furnishes no features and details on a cause to effect relationship. Secondly, the findings have been hampered by the limited number of haematological parameters studied. Another drawback that we faced was that we were not able to perfectly match the study participants as required. Also, some more parameters were not done, and we were not able to include them in our study. Since this was a retrospective cohort study, it was impossible to follow timing of blood count. Also, another drawback was the number of patients in ICU who died; we were unable to get the data due to technical issues. Further exploration of these markers is required for utilization for risk stratification in patients afflicted with COVID-19. By doing research in a larger number of COVID-19 cases combined with COVID-19 autopsy information will surely help us to get more accurate information on the clinical characteristics and their contribution in managing the disease. This will additionally assist in formulating a vigorous scheme to dominate and attenuate the COVID-19 pandemic at large.
Conclusion
We have seen in this research that characteristics of ICU patients, including age, leukocyte count, platelet count, RBC count and differential leukocyte count may be noteworthy futuristic for developing managing plans for critically ill SARS-COV-2 afflicted patients. These alterations in the said parameters have shown that patients having severe COVID-19 disease could be treated beforehand with the said alterations, and thus these may also serve as a possible biomarker for those people who needs hospitalization and ICU care. Also, close monitoring of platelets should be done to prevent clotting in ICU patients. Also, it was seen that PLR determination was useful in COVID-19 for determination of the health peril. However, further evaluation of these parameters is needed to be utilized for peril stratification purposes. COVID-19 is very unpredictable, since it is mutating constantly in defined periods, which leads to variety in the clinical symptoms and signs. This is the main reason that high-risk patients need constant and strict monitoring and timely supportive measures and adequate treatment.
Data Sharing Statement
The datasets used in this study are available from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in relation to this work.
Acknowledgment
The authors are much indebted to the Institute of Research and Consulting Studies at King Khalid University for the financial support of this research (grant number # 17-46-S-2020).
Additional information
Funding
References
- Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054. doi:10.1016/j.ijantimicag.2020.106054
- Worldometers. COVID live update; 2021.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi:10.1001/jama.2020.2648
- Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Critical Care. 2020;24(1):1–5. doi:10.1186/s13054-020-02911-9
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi:10.1001/jama.2020.6775
- Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi:10.1001/jama.2020.7202
- Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. New Engl J Med. 2020;382(21):2012–2022. doi:10.1056/NEJMoa2004500
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi:10.1001/jama.2020.4326
- Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi:10.1164/rccm.202003-0736LE
- Zhang D, Guo R, Lei L, et al. COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2020. doi:10.1101/2020.03.24.20042655
- Foldes D, Hinton R, Arami S, Bain BJ. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19). Am J Hematol. 2020;95(7):861–862. doi:10.1002/ajh.25834
- Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID −19 infection. Am J Hematol. 2020;95:999–1000. doi:10.1002/ajh.25793
- Zini G, Bellesi S, Ramundo F, d’Onofrio G. Morphological anomalies of circulating blood cells in COVID −19. Am J Hematol. 2020;95:870–872. doi:10.1002/ajh.25824
- Kaur G, Sandeep F, Olayinka O, Gupta G. Morphologic changes in circulating blood cells of COVID-19 patients. Cureus. 2021;13(2):e13416.
- Nazarullah A, Liang C, Villarreal A, Higgins RA, Mais DD. Peripheral blood examination findings in SARS-CoV-2 infection. Am J Clin Pathol. 2020;154(3):319–329. doi:10.1093/ajcp/aqaa108
- Klok F, Kruip M, Van der Meer N, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi:10.1016/j.thromres.2020.04.041
- Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–2279. doi:10.1093/eurheartj/ehaa1103
- Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106(5):551–561. doi:10.1016/S0092-8674(01)00477-9
- Assinger A, Kral JB, Yaiw KC, et al. Human Cytomegalovirus–platelet interaction triggers toll-like receptor 2–dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol. 2014;34(4):801–809. doi:10.1161/ATVBAHA.114.303287
- Guo L, Feng K, Wang Y, et al. Critical role of CXCL4 in the lung pathogenesis of influenza (H1N1) respiratory infection. Mucosal Immunol. 2017;10(6):1529–1541. doi:10.1038/mi.2017.1
- Chaipan C, Soilleux EJ, Simpson P, et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80(18):8951–8960.
- Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood, J Am Soc Hematol. 2015;126(3):378–385.
- Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):1–22. doi:10.1186/s13045-020-00954-7
- Man MA, Rajnoveanu R-M, Motoc NS, et al. Neutrophil-to-lymphocyte ratio, platelets-to-lymphocyte ratio, and eosinophils correlation with high-resolution computer tomography severity score in COVID-19 patients. PLoS One. 2021;16(6):e0252599. doi:10.1371/journal.pone.0252599
- Simadibrata DM, Pandhita BAW, Ananta ME, Tango T. Platelet-to-lymphocyte ratio, a novel biomarker to predict the severity of COVID-19 patients: a systematic review and meta-analysis. J Intensive Care Soc. 2020;23:20–26.
- Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv. 2020. doi:10.1101/2020.02.10.20021584
- Kosikowska U, Rybojad P, Stępień–Pyśniak D, Żbikowska A, Malm A. Changes in the prevalence and biofilm formation of Haemophilus influenzae and Haemophilus parainfluenzae from the respiratory microbiota of patients with sarcoidosis. BMC Infect Dis. 2016;16(1):1–13. doi:10.1186/s12879-016-1793-7
- Tian S, Liu H, Liao M, et al. Analysis of Mortality in Patients with COVID-19: Clinical and Laboratory Parameters. Open Forum Infectious Diseases. Oxford University Press US; 2020.
- Song J-W, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11(1):1–10. doi:10.1038/s41467-020-17240-2
- Yang H, Xu Y, Li Z, Yan L, Wang J, Liao P. The clinical implication of dynamic hematological parameters in COVID-19: a retrospective study in Chongqing, China. Int J Gen Med. 2021;14:4073. doi:10.2147/IJGM.S321292
- Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi:10.1002/jmv.25783
- Li R, Tian J, Yang F, et al. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. doi:10.1016/j.jcv.2020.104363
- Liu C-L, Lu Y-T, Peng M-J, et al. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest. 2004;126(2):509–517. doi:10.1378/chest.126.2.509
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. doi:10.1093/cid/ciu359
- Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. New Engl J Med. 2020;383(25):2451–2460. doi:10.1056/NEJMcp2009575
- Sheng L, Wang X, Tang N, Meng F, Huang L, Li D. Clinical characteristics of moderate and severe cases with COVID-19 in Wuhan, China: a retrospective study. Clin Exp Med. 2021;21(1):35–39. doi:10.1007/s10238-020-00662-z
- Thomas T, Stefanoni D, Dzieciatkowska M, et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J Proteome Res. 2020;19(11):4455–4469. doi:10.1021/acs.jproteome.0c00606
- Russo A, Tellone E, Barreca D, Ficarra S, Laganà G. Implication of COVID-19 on erythrocytes functionality: red blood cell biochemical implications and morpho-functional aspects. Int J Mol Sci. 2022;23(4):2171. doi:10.3390/ijms23042171
- Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–387. doi:10.1016/j.arcmed.2020.04.019
- Rabaan AA, Al-Ahmed SH, Haque S, et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174–184.
- Panesar N. What caused lymphopenia in SARS and how reliable is the lymphokine status in glucocorticoid-treated patients? Med Hypotheses. 2008;71(2):298–301. doi:10.1016/j.mehy.2008.03.019
- Chu H, Zhou J, Wong BH-Y, et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213(6):904–914. doi:10.1093/infdis/jiv380
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi:10.1093/cid/ciaa248
- Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926–929. doi:10.1111/jgs.16472
- Kang S-J, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(2):154. doi:10.3947/ic.2020.52.2.154
- Asghar MS, Khan NA, Haider Kazmi SJ, et al. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J Community Hospital Int Med Perspect. 2020;10(6):514–520. doi:10.1080/20009666.2020.1816276
- Huang J, Cheng A, Lin S, Zhu Y, Chen G. Individualized prediction nomograms for disease progression in mild COVID‐19. J Med Virol. 2020;92(10):2074–2080. doi:10.1002/jmv.25969
- Qian G-Q, Yang N-B, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM Int J Med. 2020;113(7):474–481. doi:10.1093/qjmed/hcaa089
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi:10.1016/j.kint.2020.05.006
- Dupont A, Rauch A, Staessens S, et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41(5):1760–1773. doi:10.1161/ATVBAHA.120.315595
- Ackermann M, Anders H-J, Bilyy R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;28(11):3125–3139. doi:10.1038/s41418-021-00805-z
- Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi:10.1001/jamainternmed.2020.2033
- Shang Y, Liu T, Wei Y, et al. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020;24:100426. doi:10.1016/j.eclinm.2020.100426
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
- Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. doi:10.1038/nm.2843
- Garsen M, Rops AL, Rabelink TJ, Berden JH, van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transpl. 2014;29(1):49–55. doi:10.1093/ndt/gft410
- Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176–1189. doi:10.1161/CIRCULATIONAHA.120.048488
- Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–6157. doi:10.1172/JCI141374
- Middleton EA, He X-Y, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi:10.1182/blood.2020007008
- Dolhnikoff M, Duarte-Neto AN, De Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thrombosis Haemostasis. 2020;18(6):1517–1519. doi:10.1111/jth.14844
- Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi:10.1016/j.ebiom.2020.102925
- Mousavi SA, Rad S, Rostami T, et al. Hematologic predictors of mortality in hospitalized patients with COVID-19: a comparative study. Hematology. 2020;25(1):383–388. doi:10.1080/16078454.2020.1833435
- Seyit M, Avci E, Nar R, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. 2021;40:110–114. doi:10.1016/j.ajem.2020.11.058
- Sarkar S, Kannan S, Khanna P, Singh AK. Role of platelet‐to‐lymphocyte count ratio (PLR), as a prognostic indicator in COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2022;94(1):211–221. doi:10.1002/jmv.27297
