Abstract
Hemophilia B is an X-linked genetic deficiency of coagulation factor IX (FIX) activity associated with recurrent deep tissue and joint bleeding that may lead to long-term disability. FIX replacement therapy using plasma-derived protein or recombinant protein has significantly reduced bleeding and disability from hemophilia B, particularly when used in a prophylactic fashion. Although modern factor replacement has excellent efficacy and safety, barriers to the broader use of prophylaxis remain, including the need for intravenous (IV) access, frequent dosing, variability in individual pharmacokinetics, and cost. To overcome the requirement for frequent factor dosing, novel forms of recombinant FIX have been developed that possess extended terminal half-lives. Two of these products (FIXFc and rIX-FP) represent fusion proteins with the immunoglobulin G1 (IgG1) Fc domain and albumin, respectively, resulting in proteins that are recycled in vivo by the neonatal Fc receptor. The third product has undergone site-specific PEGylation on the activation peptide of FIX, similarly resulting in a long-lived FIX form. Clinical trials in previously treated hemophilia B patients have demonstrated excellent efficacy and confirmed less-frequent dosing requirements for the extended half-life forms. However, gaps in knowledge remain with regard to the risk of inhibitor formation and allergic reactions in previously untreated patient populations, safety in elderly patients with hemophilia, effects on in vivo FIX distribution, and cost-effectiveness. Additional strategies designed to rebalance hemostasis in hemophilia patients include monoclonal-antibody-mediated inhibition of tissue factor pathway inhibitor activity and siRNA-mediated reduction in antithrombin expression by the liver. Both of these approaches are long acting and potentially involve subcutaneous administration of the drug. In this review, we will discuss the biology of FIX, the evolution of FIX replacement therapy, the emerging FIX products possessing extended half-lives, and novel “rebalancing” approaches to hemophilia therapy.
Introduction
Hemophilia B is an X-linked genetic deficiency of coagulation factor IX (FIX) activity, which leads to recurrent and disabling bleeding complications.Citation1 FIX is the zymogen of factor IXa (FIXa), a serine protease critical to amplification of blood coagulation. Numerous mutations in the FIX gene, located on the long arm of chromosome X, are associated with this disorder. In contrast to hemophilia A, FIX deficiency is most commonly caused by single base pair substitutions, resulting in missense, nonsense, or frameshift mutations. Deletions are the second most common gene defect seen in this population.Citation2 The predominance of point mutations, as opposed to the large gene inversions found in hemophilia A, means that a substantial proportion of patients with hemophilia B express some hypofunctioning or nonfunctional protein. The higher prevalence of protein expression in hemophilia B is likely reflected in the lower rates of inhibitor formation (1%–5%) compared to hemophilia A (25%–35%).Citation3,Citation4
Hemophilia B is classified into severe (<1%), moderate (1%–5%), or mild (5%–40%) phenotypes based on the plasma FIX activity of affected individuals.Citation5 The severe phenotype is characterized by spontaneous and recurrent bleeding episodes into joints and muscles, with hemarthroses being the predominant cause of long-term disability.Citation6 The moderate phenotype is characterized by occasional spontaneous bleeds and prolonged bleeding with minor trauma or surgery. Finally, patients with the mild phenotype rarely demonstrate spontaneous bleeding but may have significant bleeding with major trauma or surgery. Aggressive factor replacement is required primarily for patients with moderate and severe hemophilia B phenotypes.
Factor replacement therapy may be provided either “on demand” for symptoms related to bleeding or as “prophylaxis” in which scheduled infusions are undertaken in an attempt to prevent hemorrhage. Primary prophylaxis refers to factor replacement that is started to prevent clinical bleeding episodes in the infant or young child, while secondary prophylaxis refers to replacement therapy that is initiated in response to recurrent bleeding symptoms. Prophylaxis has the potential to change the landscape in hemophilia B by reducing debilitating musculoskeletal complications in patients with severe hemophilia and improving quality of life.Citation7,Citation8 Current clinical research and development efforts are predominantly aimed at manipulating the pharmacokinetic and physiologic properties of FIX to prolong the biological half-life and/or enhance in vivo hemostatic function. Alternative approaches seek to “rebalance” the coagulation response via long-acting agents. Finally, although gene therapy for hemophilia B remains an active area of preclinical and early phase clinical investigation, it is beyond the scope of this review.
Biology of FIX
Biosynthesis, activation, and mechanism of action
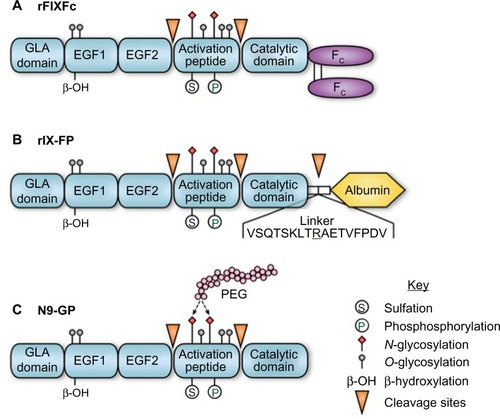
FIX is synthesized by hepatocytes as a 461-amino acid precursor polypeptide that undergoes extensive post-translational modifications including proteolytic removal of the 46-amino acid prepropeptide sequence; vitamin K-dependent γ-carboxylation of selected glutamic acid residues in the N-terminal GLA domain of the mature protein; partial β-hydroxylation of Asp 64; O-linked glycosylation at Ser 61 and 63, Thr 159, 169, 172, and 179; and N-glycosylation (Asn 157 and Asn 167), sulfation (Tyr 155), and phosphorylation (Ser 158) within the activation peptide ().Citation9,Citation10 Zymogen FIX (molecular weight =55,000) is secreted into the circulation and may undergo activation to protease (FIXa) by proteolytic cleavage following Arg 145 and Arg 180, with release of the heavily glycosylated activation peptide. FIXa may be generated by the tissue factor–factor VIIa (TF–FVIIa) complex during the initiation phase or by FXIa during the propagation phase of blood coagulation. In the presence of the cofactor factor VIIIa, calcium, and an appropriate phospholipid surface, FIXa is incorporated into the intrinsic “tenase” (FIXa–FVIIIa) complex.Citation11 Ex vivo modeling of blood coagulation demonstrates that factor X activation by the intrinsic tenase complex is the rate-limiting step for thrombin generation.Citation12,Citation13 Reduced or nonexistent activity of the intrinsic tenase complex in hemophilia A and B results in defective thrombin generation and fibrin clot formation that is associated with delayed clinical bleeding.
Figure 1 rFIX forms with extended terminal half-lives.
Abbreviations: rFIX, recombinant FIX; FIX, factor IX; EGF, epidermal growth factor; GLA, gamma-carboxylation; PEG, polyethylene glycol.
Tissue distribution
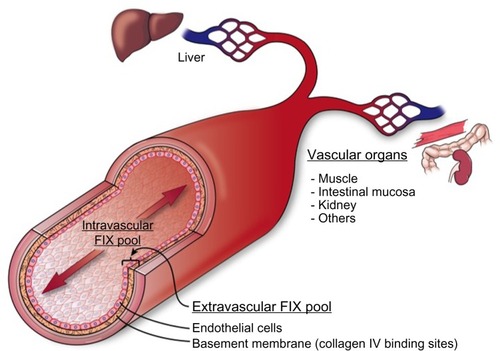
The procofactor factor VIII (280 kDa) circulates in blood primarily bound to von Willebrand factor (500–2,000 kDa), which largely restricts distribution to the intravascular compartment.Citation14 In contrast, FIX is a smaller protein (55 kDa) with access to both intravascular and extravascular compartments. The apparent volume of distribution for FIX is significantly larger than that for plasma, which supports a two-compartment pharmacokinetic modelCitation15 (). A number of clinical observations in hemophilia B patients suggest the presence of a significant noncirculating, extravascular “pool” of FIX, including 1) the rapid initial loss of FIX from the circulation following bolus infusion (enhanced in patients who completely lack FIX antigen), 2) a gradual rise in trough levels with repeated bolus dosing, and 3) a reduced dose requirement over time to maintain 100% levels during continuous infusion of plasma-derived FIX (pdFIX) or recombinant FIX (rFIX).Citation16,Citation17 Similarly, infusion of excess bovine FIX into baboons resulted in a proportional increase in circulating levels of baboon FIX analyzed using a species-specific enzyme-linked immunosorbent assay, suggesting displacement of the host protein from an accessible reservoir.Citation18 Reanalysis of these data suggests that the extravascular component contains at least 3-fold more FIX than that present in the circulation.Citation19 FIX (and FIXa) bind rapidly and reversibly to vascular endothelium and subendothelial extracellular matrix, largely mediated by the interaction of specific residues in the FIX GLA domain with collagen IV, located predominantly in the basement membrane.Citation20,Citation21 FIXa also demonstrates increased affinity for heparin/heparan sulfate relative to other coagulation factors.Citation22 Immunohistochemical staining of both murine and human arteries demonstrate FIX (but not FX) binding on the endothelial surface. Remarkably, ∼80% of injected FIX is sequestered in the liver within 2 minutes post injection in hemophilia B mice, likely accounting for much of the rapid initial clearance.Citation21 The extravascular pool contributed significantly to FIX hemostatic function in the mouse in two experiments. A knock-in mouse model expressing a FIX variant with reduced collagen IV affinity demonstrated delayed hemostasis, despite higher circulating FIX levels relative to wild-type protein.Citation23 Similarly, bolus infusion of a FIX variant with enhanced collagen IV affinity demonstrated a prolonged hemostatic effect in the hemophilia B mouse, persisting days after circulating plasma levels reached <1%.Citation19
Figure 2 Cross section of blood vessel indicating location of extravascular FIX pool.
Abbreviation: FIX, factor IX.
Recombinant FIX
The cellular origin of rFIX impacts posttranslational modifications that may contribute to differences in pharmacokinetic behavior. Recovery of FIX after bolus injection is significantly reduced for rFIX versus pdFIX.Citation24–Citation27 Currently approved formulations of rFIX expressed in Chinese hamster ovary (CHO) cells or HEK 293 cells are fully γ-carboxylated with variable Asp64 β-hydroxylation but demonstrate markedly lower levels of Tyr 155 sulfation and Ser 158 phosphorylation in the activation peptide.Citation28 Similar differences in posttranslational modifications were found for myotubule-synthesized FIX during gene therapy.Citation29 The rFIX from CHO cells and transgenic porcine milk have reduced sialic acid content in the N-glycans of the activation peptide, likely relevant given the demonstrated role of sialylation in glycoprotein clearance.Citation30 Thus, differences in posttranslational modifications are likely responsible for the reduced recovery of rFIX relative to pdFIX, but the specific contribution of individual modifications remain incompletely defined.Citation31
Evolution of hemophilia B therapy
Treatment of hemophilia B became available in the 1960s and 1970s with the development of fresh frozen plasma, followed by intermediate purity preparations such as prothrombin complex concentrates (PCC) or “FIX complex concentrates”.Citation32 However, use of these intermediate purity preparations was complicated by significant infectious and thromboembolic risks. In the early 1980s, it was recognized that blood products for hemophilia A and B, pooled from multiple donors, were contaminated with blood-borne pathogens (particularly human immunodeficiency virus [HIV] and hepatitis B and C). Tragically, these pooled blood products resulted in HIV transmission to ∼50% of all hemophilia patients and up to 90% of severe hemophilia patients.Citation33 Methods for viral screening and inactivation were subsequently developed, which dramatically improved the safety of these products.Citation34 Secondly, intermediate purity PCCs such as Bebulin (Baxter, Deerfield, IL, USA) and Profilnine (Grifols Biologicals Inc., Los Angeles, CA, USA) contain significant amounts of other vitamin K-dependent clotting factors (factors II, VII, and X) and low levels of activated coagulation factors. These products were associated with increased risk of thromboembolic complications, including venous thromboembolism, disseminated intravascular coagulation, and myocardial infarction.Citation35 Highly purified pdFIX preparations were later developed using monoclonal-antibody-based purification that reduced the thromboembolic risks associated with earlier therapies.Citation36 Finally, bioengineering of human FIX in CHO cells was undertaken, with the first purified rFIX product (Benefix, Pfizer, New York, NY, USA) receiving US Food and Drug Administration (FDA) approval in 1997.Citation37
Factor replacement therapy for moderate or severe hemophilia B is based on two major approaches: “on-demand” versus prophylaxis. In the “on-demand” approach, FIX is infused in response to bleeding symptoms to target plasma FIX levels of 60%–80% for major bleeds and 20%–40% for minor bleeds. In the prophylaxis approach, plasma-derived or rFIX is infused 2–3 times per week with the goal of maintaining plasma FIX levels >1%, thereby changing the expected phenotype from severe to moderate hemophilia.Citation38 Prophylactic infusion of replacement factor according to this strategy is superior to on-demand therapy for preventing clinical complications in severe hemophilia patients, particularly disabling hemophilic arthropathy.Citation7,Citation8 In patients with severe hemophilia who are negative for HIV and inhibitors, prophylaxis also reduces the risk of central nervous system hemorrhage by about half.Citation39 Multiple studies suggest that the impact of prophylaxis on quality of life for hemophilia patients is substantial.Citation40 Reduction in hemarthroses leads to less hemophilic arthropathy, fewer orthopedic interventions, and less disability. Despite the clear advantages of prophylaxis, barriers to broader use persist including prominent cost, the need for IV devices with their attending complications, and compliance with frequent factor infusions.Citation41,Citation42 Cost is particularly limiting for the use of prophylaxis in developing countries.Citation43 Practically speaking, prolongation of the dosing interval would enhance compliance and reduce the need for central venous access devices.
Highly purified pdFIX
Highly purified pdFIX products are isolated from plasma or PCC by monoclonal-antibody-based affinity chromatography, which dramatically reduces contaminating vitamin K-dependent clotting factors (II, VII, and X).Citation44 Thus, infusion of highly purified pdFIX in patients with hemophilia B is not associated with elevated in vivo markers of coagulation activation that are commonly seen with PCC products.Citation45 Rigorous purification and viral inactivation processes, including immunoaffinity chromatography, dry heating, solvent/detergent treatment, sodium thiocyanate incubation, and membrane ultrafiltration have markedly reduced infectious risks. Indeed, there have been no documented transmissions of hepatitis B virus, hepatitis C virus, or HIV since the introduction of effective virus inactivation procedures.Citation46 Rare reports describe contamination with infectious prions and parvovirus transmission, but the clinical implications of these findings remain unclear.Citation47,Citation48 In general, plasma-derived factor remains a very safe option for replacement therapy for most patients worldwide.Citation49 Available high-purity pdFIX products in the USA include AlphaNine (Grifols) and MonoNine (CSL Behring, King of Prussia, PA, USA) (). In clinical studies, these products have demonstrated excellent hemostatic efficacy (∼90%) and safety profiles in the prophylaxis, on-demand, and surgical settings.Citation44,Citation50–Citation53 The estimated plasma half-life for highly purified pdFIX preparations is 16–19 hours,Citation26,Citation54,Citation55 although other estimates are closer to 30 hours,Citation56 likely due to methodological differences. Dosing 2–3 times weekly is appropriate for prophylaxis, although significant variation between individuals is observed.Citation56,Citation57
Table 1 Therapeutic approaches to hemophilia B
Recombinant FIX
The human FIX gene was identified and cloned in early 1980s,Citation58 followed by insertion of the human FIX cDNA sequence into the CHO cell line and expression of rFIX in 1982.Citation59 rFIX is purified by a four-step chromatographic process, including nanofiltration for viral reduction. Comparison of pharmacokinetics in patients with hemophilia B demonstrates that the recombinant protein has consistently lower (∼30%–50%) in vivo recovery (peak levels) with a similar terminal half-life (17–19 hours) to pdFIX following bolus injection.Citation26,Citation54 Treatment of 56 previously treated patients (PTP) with rFIX demonstrated excellent hemostatic efficacy, with 80.9% of hemorrhages resolving with one dose, the majority of hemorrhages (61%) on prophylaxis occurring >72 hours after the last dose, and 27 surgical procedures in 20 patients demonstrating good or excellent hemostasis. Toxicity was limited to the development of a transient inhibitor in one patient, but no evidence of thromboembolism or viral transmission was observed.Citation60 Similarly, treatment of 63 previously untreated patients (PUP) with rFIX also demonstrated excellent hemostatic efficacy, with 75% of hemorrhages resolving with one dose, 91% of prophylaxis responses in 32 patients rated as “excellent”, and 30 procedures on 23 patients with good or excellent hemostasis. Five patients demonstrated allergic reactions, and two of them demonstrated inhibitors (3%). No thrombotic or viral transmission events were observed.Citation61 A French study on patients with moderate-to-severe hemophilia did not show any difference in quality of life between rFIX and pdFIX, although rFIX consumption was higher by a factor of 1.40. As expected, increased cost was associated with prophylaxis compared to on-demand therapy.Citation62
Modified rFIX products with prolonged terminal half-lives
Clinical practice guidelines recommend that the goal of prophylaxis in hemophilia B is to maintain plasma FIX levels >1%,Citation5 which has led to strategies designed to prolong the terminal plasma half-life of FIX. These products, which increase the feasibility of once-weekly FIX administration, have been recently approved or are in late clinical development stages ().
rFIXFc fusion protein
The Fc domain of the IgG molecule binds to the neonatal Fc receptor (FcRn), which is present on many adult cells, including endothelial cells, monocytes/macrophages, and epithelial cells.Citation63 Following internalization by the endothelial cell, proteins that bind to the FcRn receptor in the acidified endosome are protected from lysosomal sorting and degradation, allowing recycling back to the cell surface with pH-dependent release into the circulation. Recycling via the FcRn is the mechanism responsible for the extended in vivo half-life of IgG, albumin, and therapeutic IgG1-Fc fusion proteins.Citation63 Similarly, direct fusion (no linker region) of a monomeric Fc domain from human IgG1 to the carboxyl terminus of human FIX (rFIXFc) results in a long-acting rFIX (). rFIXFc demonstrates a 3- to 4-fold prolongation of terminal half-life relative to rFIX in multiple species (mouse, rat, dog, and monkey). Posttranslational modifications of rFIXFc expressed in HEK293 cells appear similar to rFIX expressed in CHO cells (Benefix, Pfizer). On a molar basis, the specific activity of rFIXFc is ∼50% that of conventional rFIX, and the precise impact of the Fc domain on factor X activation kinetics was not reported.Citation28
A Phase I–II clinical study of rFIXFc in 14 patients with hemophilia B demonstrated in vivo recovery ∼20% higher than rFIX and confirmed an ∼3-fold prolongation of terminal half-life.Citation64 rFIXFc was subsequently examined in a Phase III study of 123 PTPs (median age: 30 years) divided into four treatment groups: weekly dose-adjusted prophylaxis, interval-adjusted prophylaxis, on-demand therapy, and perioperative treatment. As expected, prophylaxis groups demonstrated significantly lower median annualized bleeding rates of 3.1% and 2.4%, respectively, compared to 18.6% for on-demand therapy. The vast majority (90.4%) of bleeding episodes resolved with one injection. Hemostasis was rated as good or excellent in 14 major surgeries performed in 12 subjects. No inhibitors or anaphylactic reactions were observed, and the only thrombotic episode was an obstructive clot in the urinary collection system of a patient with previous hematuria. The terminal half-life for rFIXFc was 82 hours, compared to 33 hours for rFIX (Benefix).Citation65 A post hoc analysis comparing patients who received prophylaxis for >6 months on this study with their prestudy prophylaxis suggested that rFIXFc markedly reduced infusion frequency and FIX consumption with fewer bleeding episodes.Citation66 In 2014, rFIXFc (Alprolix, Biogen, Cambridge, MA, USA) was approved by the FDA for prophylaxis and treatment of bleeding episodes in individuals with hemophilia B, in both routine and perioperative settings. An ongoing clinical study (NCT02234310) will evaluate the safety and efficacy of rFIXFc in the PUP population with severe hemophilia B.
FIX-albumin fusion protein
The FIX-albumin (rIX-FP) fusion protein represents an alternative approach to prolongation of rFIX half-life that similarly takes advantage of recycling via the FcRn. Albumin interacts with the FcRn at a site independent of the IgG Fc domain but has similar pH-dependent binding affinity that allows recycling back to the circulation, resulting in a serum half-life of ∼20 days.Citation67 In rIX-FP, the albumin moiety has been fused to the C-terminus of FIX via a cleavable linker containing a FIX activation site. The design of this fusion protein allows proteolytic release of the FIXa molecule from albumin upon activation by TF–FVIIa or factor XIa (). The molar-specific activity of this fusion protein expressed in CHO cells is 20- to 30-fold higher than the fusion protein expressed with noncleavable linkers.Citation68 In rats and rabbits, rIX-FP demonstrated a 1.6- to 1.7-fold increased recovery and ∼4-fold increase in terminal half-life relative to rFIX, along with demonstrated efficacy in a tail-tip bleeding model in hemophilia B mice.Citation68 Similar prolongation of therapeutic levels was observed in dogs with hemophilia B and non-hemophilic cynomolgus monkeys.Citation69
In a multicenter, dose-escalation Phase I trial of 25 PTPs (mean age: 35 years), rIX-FP demonstrated a 44% increase in recovery and ∼5-fold increase in half-life (mean: 92 hours) relative to rFIX, with no inhibitors or hypersensitivity events reported.Citation70 A second Phase I–II study of 17 PTPs (mean age: 26 years) demonstrated similar pharmacokinetic results and included an 11-month safety and efficacy evaluation. During the extension study, 13 patients received weekly prophylaxis and four patients were treated on demand. Seven patients on prophylaxis were treated for 14 spontaneous bleeds during the prophylaxis period, while six had no bleeds, with a median annualized spontaneous bleeding rate (AsBR) of 1.13. For both on-demand and prophylaxis patients, 95% bleeding episodes were treated with single infusion and no inhibitors, antibodies to rIX-FP, or allergic reactions were detected.Citation71 Preliminary results of multinational, Phase II–III studies evaluating the safety and efficacy of rIX-FP in PTP (adults and children) were reported at the 2015 International Society of Thrombosis and Haemostasis biannual meeting. These studies included 27 PTPs <12 years of age treated for 12 months with weekly prophylaxis or on demand. No inhibitors or antibodies to rIX-FP were reported, and 97% bleeding episodes were treated with one or two infusions.Citation72 The adult study (age 12–61 years) enrolled 63 patients treated on demand (N=23) or with a 7-day prophylaxis interval (N=40) for 6 months, after which on-demand patients were switched to 7-day prophylaxis, and the therapy of selected prophylaxis patients was extended to 10- or 14-day intervals. As expected, a marked reduction in bleeding episodes was observed for prophylaxis (median AsBR: 0.00) versus on-demand. Additionally, 21 prophylaxis patients extended their treatment interval to 14 days, with no significant increase in bleeding and 50% reduction in factor consumption relative to historical use. Finally, ∼94% of bleeding episodes resolved with one factor injection, and no serious adverse events were observed, including development of inhibitors or antibodies to rIX-FP.Citation73 A surgical substudy reported 12 major operations in ten patients treated with preoperative rIX-FP, and the hemostatic response was characterized as good or excellent in all cases with 2–7 infusions during the 14-day postoperative period.Citation74 The Biologics License Application for rIX-FP was accepted for review by the FDA in February 2015. An ongoing clinical study (NCT02053792) will address the safety and efficacy of rIX-FP in the PUP population.
GlycoPEGylated rFIX
Covalent modification of therapeutic proteins with polyethyelene glycol (PEG) chains is an established approach to prolonging protein half-life and in vivo activity.Citation75 N9-GP (nonacog β-pegol) is a rFIX expressed in CHO cells and has been modified by site-directed glycoPEGylation with a 40-kDa PEG molecule on one (95%) or both (5%) N-glycans within the activation peptide (). Upon proteolytic the activation of FIX, the activation peptide containing the PEG moiety is removed, leaving native rFIXa. The kinetics of FIX activation by FXIa is identical to unmodified protein, while the activation by TF–FVIIa demonstrates a modest increase in Km for FIX. The specific activity of N9-GP is equivalent to plasma-derived or native rFIX. Preclinical studies of N9-GP in pigs and dogs with hemophilia B demonstrated a 2-fold increase in recovery and an ∼5- to 7-fold increase in half-life relative to unmodified rFIX, along with demonstrated efficacy in a tail-tip bleeding model in hemophilia B mice.Citation76
A Phase I trial of N9-GP in 16 PTPs with hemophilia B demonstrated markedly enhanced recovery and a half-life (93 hours) that was ∼5-times longer than nonpegylated rFIX. No inhibitors were detected, but one patient experienced a hypersensitivity reaction.Citation77 A multinational, randomized, single-blind Phase III trial of the safety and efficacy of N9-GP was conducted in 74 PTPs (mean age: 31 years) with hemophilia B. Patients chose between on-demand (N=15) and prophylaxis (N=59) therapy, with the latter group randomized between two different weekly doses. Pharmacokinetic analysis confirmed previous observations in the Phase I trial. Overall estimated success rate of achieving hemostasis was 92%, with best results in prophylaxis group. Median AsBR was 0.97 and <0.01 for 10 or 40 U/kg weekly prophylaxis, respectively, and 11.1 for on-demand therapy. No inhibitors, hypersensitivity reactions, or thromboembolic complications were reported.Citation78 An extension of this trial (NCT01395810) and a study in patients undergoing major surgery (NCT01386528) have been completed but not reported. Additional trials are ongoing to evaluate N9-GP in both pediatric PTP (NCT01467427) and PUP (NCT02141074) populations.
Alteration in rFIX function by mutagenesis
Preclinical data exist for targeted mutagenesis of human FIX designed to enhance coagulant activity or in vivo therapeutic properties. These approaches include incorporation of the FIX Padua mutation or combined mutations to enhance coagulant activity,Citation79 introduction of additional N-glycosylation sites to enhance pharmacokinetics and subcutaneous (SC) absorption,Citation80 and disruption of antithrombin- and heparin-mediated regulation to enhance in vivo protease activity.Citation81 Although the overall risk of inhibitors is lower in hemophilia B (see “Inhibitor formation and anaphylaxis” section), variant FIX sequences will require careful analysis for potential inhibitor formation.Citation82
Gaps in knowledge
Although the potential advantages of rFIX proteins with extended half-lives for prophylaxis in hemophilia B are clear, there remain significant gaps in our knowledge with regard to the risk of inhibitor formation, safety in older hemophilic populations, and impact on the in vivo distribution of FIX.
Inhibitor formation and anaphylaxis
The incidence of inhibitors in hemophilia B (1%–5%) is significantly less than that in hemophilia A (25%–35%).Citation4,Citation83,Citation84 This difference in inhibitor prevalence likely reflects the predominance of missense mutations in severe hemophilia B, as opposed to the large gene inversions associated with severe hemophilia A.Citation85 The former mutations are much more likely to be associated with sufficient protein expression to confer immune tolerance, while the latter completely lacks protein expression, resulting in a substantially higher inhibitor risk. Consistent with this notion, the risk of inhibitor formation in hemophilia B is greatest in those patients with large gene deletions.Citation86 This risk is highest in the PUP population during the first 5–6 years of life, occurring at a median of 11 exposure days to FIX product.Citation87 Long-term safety and efficacy studies of rFIX demonstrate ∼3% rate of inhibitor formation, consistent with rates seen with plasma-derived products.Citation61 The Phase II–III studies for all three extended half-life products were performed in PTP populations with substantial previous FIX exposure and exclusion of patients with evidence of inhibitors.Citation65,Citation73,Citation78 Clearly, the true incidence of inhibitor formation with these products will not be known until studies in PUP populations are completed. Patients with hemophilia B are uniquely at risk (compared to hemophilia A) for allergic reactions to factor infusion. These reactions occur in 1%–3% cases, with comparable incidence for both pdFIX and rFIX products.Citation88 Allergic reactions are commonly associated with inhibitors and may actually precede inhibitor development.Citation85 The exact mechanism for the allergic reactions remains unknown, but they are postulated to involve extravascular IgE-mediated responses or codeletion of adjacent immune-response loci with large FIX gene deletions.Citation86 The true incidence of allergic reactions with the extended half-life agents will likewise not be known until clinical trials are completed in PUP populations.
Safety
As the dramatic impact of HIV infection on the hemophilia population fades, increasing life expectancy means that the thromboembolic risk associated with an aging hemophilic population may yield additional safety concerns. On the basis of the data reported in an Italian registry in 2009, it is estimated that approximately 15% of hemophiliacs are aged 45 years or above, and approximately 2% are older than 65 years.Citation89 Mean subject age in recent clinical trials for the extended half-life agents range from 26 to 35 years of age, with additional exclusions for comorbid conditions.Citation65,Citation73,Citation78 As additional risks may need to be considered in the older population, it will be important to include elderly patients with hemophilia B in future trials to the extent possible. For example, activation of rFIXFc (or rIX-FP with incomplete linker cleavage) may generate long-acting rFIXa forms that could potentially elevate thromboembolic risk in an older population. Likewise, although PEGylated protein therapies have a wide therapeutic index and substantial track record in the clinic, the life-long nature of hemophilia treatment raises concern about potential toxicity due to chronic PEG exposure.Citation90 Conversely, PEGylated proteins tend to be less immunogenic than the unmodified protein, which could lead to a reduced rate of inhibitor formation.Citation75
The role of extravascular FIX
The contribution of the extravascular pool to the hemostatic function of FIX in mouse models suggests that the access of modified rFIX forms to collagen IV binding sites in the sub-endothelium may be therapeutically relevant ().Citation19,Citation23 Tritium-labeled rIX-FP and rFIX demonstrate similar overall tissue distribution, with early enhancement in liver levels and the expected prolonged half-life for the fusion protein in nonhemophilic rats.Citation91 However, no direct analysis of binding to endothelium or tissue distribution in hemophilia B animals is available. N9-GP demonstrates 20-fold reduced affinity for binding to an endothelial monolayer relative to unPEGylated rFIX, suggesting that the PEG moiety may reduce access to, or affinity for, collagen IV binding sites.Citation76 We are not aware of any published analysis of endothelial binding or in vivo tissue distribution for rFIXFc. It is not known whether reduced access or binding to extravascular sites by extended half-life forms impacts duration of the hemostatic effect or relative risk of bleeding at low trough levels compared to conventional FIX. However, the hemostatic contribution of extravascular FIX suggests that plasma FIX activity levels may not represent an ideal surrogate for therapeutic efficacy.
Cost-effectiveness of factor replacement
A major impetus behind the development of extended half-life FIX products was to enhance the feasibility of prophylaxis in hemophilia B. Thus, the contribution of these therapies will be judged, in part, on their ability to impact the cost-effectiveness of prophylaxis. Prophylaxis is clearly more expensive up-front than on-demand replacement therapy, although savings due to reduced joint bleeds and related complications potentially makes prophylaxis ultimately more cost-effective.Citation92 Determination of indirect cost savings (hospitalizations, disability, and productivity) related to prophylaxis is both complex and critical to this cost-effectiveness analysis. Limitations in resources remain a major obstacle to prophylaxis in hemophilia, particularly in developing countries. At least, the costs associated with the extended half-life products will need to be justified by proportional reduction in factor use due to less frequent dosing for prophylaxis and surgical procedures.
Novel approaches
Although prophylactic factor replacement results in significant improvements in hemophilia outcomes, the need for vascular access and compliance with frequent infusions remain major challenges. Several long-acting strategies attempt to rebalance hemostasis in hemophilia by “inhibiting the inhibitor”. These strategies have the potential advantages of being effective in the presence of anti-FIX inhibitors and not requiring IV access.
Anti-tissue factor pathway inhibitor therapy
Inhibition of the TF–FVIIa complex by tissue factor pathway inhibitor (TFPI) shuts down the extrinsic pathway following initiation of coagulation, making thrombin generation in the propagation phase dependent on the FIXa–FVIIIa complex (defective in hemophilia). Antagonizing TFPI-mediated inhibition via inhibitory antibodies allows TF–FVIIa activity to persist, resulting in continued thrombin generation via the extrinsic pathway that may, in part, compensate for defective factor X activation by the FIXa–FVIIIa complex in hemophilia. Concizumab, a monoclonal antibody against the kunitz-2 inhibitor domain of TFPI, improves clot formation in hemophilic blood and plasma and substantially reduces cuticle bleeding in a rabbit hemophilia model.Citation93 In a Phase I trial involving 28 healthy volunteers and 24 hemophilia patients (21 hemophilia A and 3 hemophilia B) without inhibitors, both IV and SC concizumab was well tolerated, demonstrating nonlinear pharmacokinetics with dose-dependent, target-mediated clearance. Concizumab demonstrated dose-dependent increases in D-dimer and prothrombin fragment 1+2 in both healthy volunteers and hemophilia patients, and antibody was detectable for up to 42 days at the highest doses tested.Citation94 Additional humanized antibodies against TFPI are currently under development.
siRNA-mediated antithrombin gene knockdown
Reduction in antithrombin activity can restore normal levels of thrombin generation in hemophilic (A or B) plasma or whole blood. ALN-AT3 is an siRNA targeting a conserved region of the antithrombin (SERPINC1) transcript that has been conjugated with Gal-Nac to facilitate uptake by the asia-loglyoprotein receptor in the liver. ALN-AT3 demonstrated dose-dependent reduction in antithrombin protein levels (up to >90%) in both mice and nonhuman primates, correcting clotting and bleeding phenotypes in the hemophilia A mouse. Similarly, ALN-AT3 rectified thrombin generation in nonhuman primates with antibody-induced hemophilia A.Citation95 An ongoing Phase I trial has reported preliminary results in four healthy volunteers and 12 hemophiliacs (A and B) on ascending weekly dose levels, indicating that ALN-AT3 was generally well tolerated with no significant thromboembolic events. A maximal 86% reduction in plasma antithrombin was achieved with normalization of patient thrombin generation. A third arm will investigate the effects and tolerability of monthly dosing.Citation96
Conclusion
Progress in the management of moderate-to-severe hemophilia B has been made possible by the availability of purified plasma-derived and rFIX with substantially reduced risks of infectious and thromboembolic complications. Further, the superior clinical outcomes associated with prophylaxis versus on-demand approaches to factor replacement have been broadly recognized. Current development efforts in hemophilia B therapy are focused on overcoming the barriers to broader use of prophylaxis, including requirement for IV access, compliance with frequent infusions, poor recovery of rFIX, and cost. Modified rFIX proteins with extended half-lives (FIXFc, rIX-FP, and N9-GP) may have a substantial impact on the feasibility of prophylaxis via significant prolongation of dosing intervals and potential for higher troughs that may have lifestyle implications for the active patient with hemophilia B. Unanswered questions regarding the extended half-life forms include the risk of inhibitor formation and allergic reactions in PUP populations, safety in elderly populations, and the potential therapeutic implications of altered in vivo distribution. Alternative approaches attempt to rebalance coagulation in hemophilia by inhibiting TFPI activity or antithrombin expression to normalize thrombin generation. These latter approaches represent long acting and potentially SC approaches to hemophilia therapy that address many of the barriers to prophylactic therapy. These novel, long-acting approaches to hemophilia B therapy have the potential to markedly reduce common complications such as hemophilic arthropathy, enhance the ability to lead a physically active lifestyle, and improve the quality of life for patients with moderate-to-severe hemophilia B.
Acknowledgments
This work was supported, in part, by funding from the University of Wisconsin Institute for Clinical and Translational Research (JPS) and the Pfizer US Hemophilia ASPIRE Program (JPS).
Disclosure
JPS has received previous research funding from the Bayer Hemophilia Awards Program and the Novo Nordisk Access to Insight Program. He has current research funding from a Pfizer US Hemophilia ASPIRE Hemophilia Grant. The authors report no other conflicts of interest in this work.
References
- SrivastavaABrewerAKMauser-BunschotenEPGuidelines for the management of hemophiliaHaemophilia2013191e1e4722776238
- BowenDJHaemophilia A and haemophilia B: molecular insightsMol Pathol200255212714411950963
- GomezKKlamrothRMahlanguJMancusoMEMingotMEOzeloMCKey issues in inhibitor management in patients with haemophiliaBlood Transfus201412Suppl 1s319s32924333092
- CollinsPWPalmerBPChalmersEAFactor VIII brand and the incidence of factor VIII inhibitors in previously untreated UK children with severe hemophilia A, 2000–2011Blood2014124233389339725339360
- WhiteGC2ndRosendaalFAledortLMLusherJMRothschildCIngerslevJDefinitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and HaemostasisThromb Haemost200185356011307831
- AcharyaSSExploration of the pathogenesis of haemophilic joint arthropathy: understanding implications for optimal clinical managementBr J Haematol20121561132322050780
- Manco-JohnsonMJUpdate on treatment regimens: prophylaxis versus on-demand therapySemin Hematol2003403 Suppl 33914690062
- Manco-JohnsonMJAbshireTCShapiroADProphylaxis versus episodic treatment to prevent joint disease in boys with severe hemophiliaN Engl J Med2007357653554417687129
- FurieBFurieBCMolecular and cellular biology of blood coagulationN Engl J Med1992326128008061538724
- KaufmanRJPost-translational modifications required for coagulation factor secretion and functionThromb Haemost1998796106810799657426
- MannKGBiochemistry and physiology of blood coagulationThromb Haemost199982216517410605701
- HoffmanMMonroeDMOliverJARobertsHRFactors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulationBlood1995865179418017655009
- RandMDLockJBvan’t VeerCGaffneyDPMannKGBlood clotting in minimally altered whole bloodBlood1996889343234458896408
- BjörkmanSBerntorpEPharmacokinetics of coagulation factors: clinical relevance for patients with haemophiliaClin Pharmacokinet2001401181583211735604
- BjörkmanSCarlssonMBerntorpEPharmacokinetics of factor IX in patients with haemophilia B. Methodological aspects and physiological interpretationEur J Clin Pharmacol19944643253327957517
- BrietEThree Problems of Hemophilia BLeiden, The NetherlandsUniversity of Leiden1977
- UprichardJAdamidouDGoddardNJMannHAYeeTTFactor IX replacement to cover total knee replacement surgery in haemophilia B: a single-centre experience, 2000–2010Haemophilia2012181464921545378
- SternDMKnitterGKisielWNawrothPPIn vivo evidence of intravascular binding sites for coagulation factor IXBr J Haematol19876622272323496917
- FengDStaffordKABrozeGJStaffordDWEvidence of clinically significant extravascular stores of factor IXJ Thromb Haemost201311122176217824112220
- CheungWFvan den BornJKühnKKjellénLHudsonBGStaffordDWIdentification of the endothelial cell binding site for factor IXProc Natl Acad Sci U S A1996932011068110738855310
- GuiTLinHFJinDYCirculating and binding characteristics of wild-type factor IX and certain Gla domain mutants in vivoBlood2002100115315812070021
- BajajSPRapaportSIProdanosCA simplified procedure for purification of human prothrombin, factor IX and factor XPrep Biochem19811143974127312833
- GuiTRehemanANiHAbnormal hemostasis in a knock-in mouse carrying a variant of factor IX with impaired binding to collagen type IVJ Thromb Haemost20097111843185119583826
- WhiteGShapiroARagniMClinical evaluation of recombinant factor IXSemin Hematol1998352 Suppl 233389565165
- WhiteGC2ndShapiroADKurczynskiEMKimHCBergmanGEVariability of in vivo recovery of factor IX after infusion of monoclonal antibody purified factor IX concentrates in patients with hemophilia B. The Mononine Study GroupThromb Haemost19957357797847482403
- EwensteinBMJoistJHShapiroADPharmacokinetic analysis of plasma-derived and recombinant F IX concentrates in previously treated patients with moderate or severe hemophilia BTransfusion200242219019711896334
- PoonMCLillicrapDHensmanCCardRScullyMFRecombinant factor IX recovery and inhibitor safety: a Canadian post-licensure surveillance studyThromb Haemost200287343143511916075
- PetersRTLowSCKamphausGDProlonged activity of factor IX as a monomeric Fc fusion proteinBlood2010115102057206420056791
- ArrudaVRHagstromJNDeitchJPosttranslational modifications of recombinant myotube-synthesized human factor IXBlood200197113013811133752
- GilGCVelanderWHVan CottKEAnalysis of the N-glycans of recombinant human factor IX purified from transgenic pig milkGlycobiology200818752653918456721
- WhiteGC2ndBeebeANielsenBRecombinant factor IXThromb Haemost19977812612659198163
- MenacheDProthrombin complex concentrates: clinical useAnn N Y Acad Sci19813707477566791551
- EvattBLThe tragic history of AIDS in the hemophilia population, 1982–1984J Thromb Haemost20064112295230116972935
- MannucciPMClinical evaluation of viral safety of coagulation factor VIII and IX concentratesVox Sang19936441972038517048
- KeyNSNegrierCCoagulation factor concentrates: past, present, and futureLancet2007370958543944817679021
- LimentaniSAGowellKPDeitcherSRHigh-purity factor IX concentrates for treatment of hemophilia B: relative purity and thrombogenic potentialActa Haematol199594Suppl 112177571989
- PipeSWRecombinant clotting factorsThromb Haemost200899584085018449413
- NilssonIMBerntorpELöfqvistTPetterssonHTwenty-five years’ experience of prophylactic treatment in severe haemophilia A and BJ Intern Med1992232125321640190
- WitmerCPresleyRKulkarniRSoucieJMMannoCSRaffiniLAssociations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United StatesBr J Haematol2011152221121621114482
- OladapoAOEpsteinJDWilliamsEItoDGringeriAValentinoLAHealth-related quality of life assessment in haemophilia patients on prophylaxis therapy: a systematic review of results from prospective clinical trialsHaemophilia201521e344e35826390060
- CoppolaATagliaferriADi CapuaMFranchiniMProphylaxis in children with hemophilia: evidence-based achievements, old and new challengesSemin Thromb Hemost2012381799422314606
- BerntorpEJoint outcomes in patients with haemophilia: the importance of adherence to preventive regimensHaemophilia20091561219122719659939
- ChandyMManagement of hemophilia with minimal factor replacement in developing countries: role of ancillary therapySemin Thromb Hemost200531550150616276457
- KimHCMcMillanCWWhiteGCBergmanGEHortonMWSaidiPPurified factor IX using monoclonal immunoaffinity technique: clinical trials in hemophilia B and comparison to prothrombin complex concentratesBlood19927935685751531035
- HamptonKKPrestonFELoweGDWalkerIDSampsonBReduced coagulation activation following infusion of a highly purified factor IX concentrate compared to a prothrombin complex concentrateBr J Haematol19938422792848398831
- TaborEThe epidemiology of virus transmission by plasma derivatives: clinical studies verifying the lack of transmission of hepatitis B and C viruses and HIV type 1Transfusion19993911–121160116810604241
- BrownPWillRGBradleyRAsherDMDetwilerLBovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: background, evolution, and current concernsEmerg Infect Dis20017161611266289
- Bartolomei CorsiOAzziAMorfiniMFanciRRossi FerriniPHuman parvovirus infection in haemophiliacs first infused with treated clotting factor concentratesJ Med Virol19882521651702839609
- MannucciPMThe safety of plasma-derived versus recombinant concentratesOccasional Papers2004514
- LissitchkovTMatysiakMZavilskaKA clinical study assessing the pharmacokinetics, efficacy and safety of AlphaNine®, a high-purity factor IX concentrate, in patients with severe haemophilia BHaemophilia201117459059621299747
- WhiteGC2ndSafety and recovery of Mononine in multiple-dose, high-dose regimensActa Haematol199594Suppl 15357 discussion 57–587571996
- MénartCPetitPYAttaliOMassignonDDechavanneMNégrierCEfficacy and safety of continuous infusion of Mononine during five surgical procedures in three hemophilic patientsAm J Hematol19985821101169625577
- HootsWKLeissingerCStablerSContinuous intravenous infusion of a plasma-derived factor IX concentrate (Mononine) in haemophilia BHaemophilia20039216417212614367
- AlameluJBevanDSorensenBRangarajanSPharmacokinetic and pharmacodynamic properties of plasma-derived vs recombinant factor IX in patients with hemophilia B: a prospective crossover studyJ Thromb Haemost201412122044204825315324
- Mauser-BunschotenEPKleine BuddeILopaciukSAn ultrapure plasma-derived monoclonal antibody-purified factor IX concentrate (Nonafact(R)), results of phase III and IV clinical studiesHaemophilia201117343944521362109
- BjörkmanSAhlénVPopulation pharmacokinetics of plasma-derived factor IX in adult patients with haemophilia B: implications for dosing in prophylaxisEur J Clin Pharmacol201268696997722281721
- National Hemophilia Foundation no. 179MASAC recommendation concerning prophylaxis (regular administration of clotting factor concentrate to prevent bleeding)2007
- ChooKHGouldKGReesDJBrownleeGGMolecular cloning of the gene for human anti-haemophilic factor IXNature198229958791781806287289
- KaufmanRJWasleyLCFurieBCFurieBShoemakerCBExpression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cellsJ Biol Chem198626121962296283733688
- RothDAKesslerCMPasiKJHuman recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentratesBlood200198133600360611739163
- ShapiroADDi PaolaJCohenAThe safety and efficacy of recombinant human blood coagulation factor IX in previously untreated patients with severe or moderately severe hemophilia BBlood2005105251852515383463
- PolackBCalvezTChambostHEQOFIX: a combined economic and quality-of-life study of hemophilia B treatments in FranceTransfusion20155571787179725652955
- KontermannREStrategies for extended serum half-life of protein therapeuticsCurr Opin Biotechnol201122686887621862310
- ShapiroADRagniMVValentinoLARecombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patientsBlood2012119366667222110246
- PowellJSPasiKJRagniMVPhase 3 study of recombinant factor IX Fc fusion protein in hemophilia BN Engl J Med2013369242313232324304002
- PowellJShapiroARagniMSwitching to recombinant factor IX Fc fusion protein prophylaxis results in fewer infusions, decreased factor IX consumption and lower bleeding ratesBr J Haematol2015168111312325209873
- ChaudhuryCBrooksCLCarterDCRobinsonJMAndersonCLAlbumin binding to FcRn: distinct from the FcRn-IgG interactionBiochemistry200645154983499016605266
- MetznerHJWeimerTKronthalerULangWSchulteSGenetic fusion to albumin improves the pharmacokinetic properties of factor IXThromb Haemost2009102463464419806248
- NolteMWNicholsTCMueller-CohrsJImproved kinetics of rIX-FP, a recombinant fusion protein linking factor IX with albumin, in cynomolgus monkeys and hemophilia B dogsJ Thromb Haemost20121081591159922726310
- SantagostinoEPROLONG-9FP clinical development program – phase I results of recombinant fusion protein linking coagulation factor IX with recombinant albumin (rIX-FP)Thromb Res2013131Suppl 2S7S1023537724
- MartinowitzULissitchkovTLubetskyAResults of a phase I/II open-label, safety and efficacy trial of coagulation factor IX (recombinant), albumin fusion protein in haemophilia B patientsHaemophilia201521678479025990590
- KenetGChambostHMaleCEfficacy, pharmacokinetics (PK) and safety results of a phase 3 clinical study of recombinant fusion protein linking coagulation factor IX with albumin (RIX-FP) in previously treated children with hemophilia BPaper presented at: International Society of Thrombosis and Haemostasis Meeting, 2015Toronto, Canada
- SantagostinoEMartinowitzULissitchkovTEfficacy and safety results of a phase 3 pivotal clinical study of recombinant fusion protein linking coagulation factor IX with albumin (RIX-FP) in previously treated patients with hemophilia BPaper presented at: International Society of Thrombosis and Haemostasis Meeting2015Toronto, Canada
- NegrierCLepatanLMLubetskyAEfficacy and safety of recombinant fusion protein linking coagulation factor IX with albumin (RIX-FP) in previously treated patients with hemophilia B undergoing a surgical procedurePaper presented at: International Society of Thrombosis and Haemostasis MeetingJune 20–25, 2015Toronto, Canada
- CalicetiPVeroneseFMPharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugatesAdv Drug Deliv Rev200355101261127714499706
- ØstergaardHBjelkeJRHansenLProlonged half-life and preserved enzymatic properties of factor IX selectively PEGylated on native N-glycans in the activation peptideBlood201111882333234121700771
- NegrierCKnobeKTiedeAGiangrandePMøssJEnhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia BBlood2011118102695270121555744
- CollinsPWYoungGKnobeKRecombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trialBlood2014124263880388625261199
- KaoCYYangSJTaoMHJengYMYuISLinSWIncorporation of the factor IX Padua mutation into FIX-Triple improves clotting activity in vitro and in vivoThromb Haemost2013110224425623676890
- BrooksARSimDGritzanUGlycoengineered factor IX variants with improved pharmacokinetics and subcutaneous efficacyJ Thromb Haemost20131191699170623692404
- WestmarkPRTanratanaPSheehanJPSelective disruption of heparin and antithrombin-mediated regulation of human factor IXJ Thromb Haemost20151361053106325851619
- SainiSHamasaki-KatagiriNPandeyGSGenetic determinants of immunogenicity to factor IX during the treatment of haemophilia BHaemophilia201521221021825470321
- PuetzJSoucieJMKemptonCLMonahanPEHemophilia Treatment Center NetworkIPrevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection databaseHaemophilia2014201253123855900
- KatzJPrevalence of FIX Inhibitors among patients with hemophilia B: results of large scale North America surveyHaemophilia199622831
- DiMicheleDInhibitor development in haemophilia B: an orphan disease in need of attentionBr J Haematol2007138330531517614818
- WarrierIEwensteinBMKoerperMAFactor IX inhibitors and anaphylaxis in hemophilia BJ Pediatr Hematol Oncol199719123279065715
- EhrenforthSKreuzWScharrerIIncidence of development of factor VIII and factor IX inhibitors in haemophiliacsLancet199233987935945981347102
- RechtMPollmannHTagliaferriAMussoRJancoRNeumanWRA retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia BHaemophilia201117349449921518148
- SiboniSMMannucciPMGringeriAHealth status and quality of life of elderly persons with severe hemophilia born before the advent of modern replacement therapyJ Thromb Haemost20097578078619220727
- WebsterRDidierEHarrisPPEGylated proteins: evaluation of their safety in the absence of definitive metabolism studiesDrug Metab Dispos200735191617020954
- HerzogEHarrisSHensonCBiodistribution of the recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in ratsThromb Res2014133590090724680550
- GaterAThomsonTAStrandberg-LarsenMHaemophilia B: impact on patients and economic burden of diseaseThromb Haemost2011106339840421833450
- HildenILauritzenBSørensenBBHemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia modelBlood2012119245871587822563084
- ChowdaryPLethagenSFriedrichUSafety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trialJ Thromb Haemost201513574375425641556
- SehgalABarrosSIvanciuLAn RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophiliaNat Med201521549249725849132
- PasiKJGeorgievPMantTA subcutaneously administered investigational RNAi therapeutic (ALN-AT3) targeting antithrombin for treatment of hemophilia: interim weekly and monthly dosing results in patients with hemophilia A or BBlood2015126551
