Abstract
Background
Conditioning regimens including total body irradiation (TBI) or cyclophosphamide can mobilize high-mobility group box 1 (HMGB1) to peripheral blood. Additionally, increased plasminogen activator inhibitor (PAI)-1 levels are associated with post-allogeneic hematopoietic stem cell transplantation (aHSCT). However, changes to circulating levels of HMGB1 after aHSCT are poorly understood.
Materials and methods
The study cohort included 289 patients who underwent aHSCT at one of 25 institutions in Japan. We have investigated the relationship between HMGB1 and PAI-1 following aHSCT. A significant increase in HMGB1 levels occurred after conditioning treatment. Additionally, levels of HMGB1 at day 0 were significantly increased in TBI+ patients and cyclophosphamide/TBI patients.
Conclusion
Our data revealed that an increased level of HMGB1 at day 0 following aHSCT correlates with increased PAI-1 after aHSCT, which is consistent with previous reports. Increased HMGB1 at day 0 after a conditioning regimen may play a role in transplantation-associated coagulopathy following aHSCT, because PAI-1 can accelerate procoagulant activity.
Introduction
Allogeneic hematopoietic stem cell transplantation (aHSCT) is a curative treatment for many hematological malignancies. Cyclophosphamide (CY) in combination with either ablative doses of total body irradiation (TBI) or the oral alkylating agent busulfan (Bu) is the most common conditioning regimen for aHSCT.Citation1 However, TBI and CY can induce transplantation-associated coagulopathy (TAC) conditions such as veno-occlusive disease (VOD).Citation2
Several interactions between coagulation-related blood components and the fibrinolytic system are involved in the progression of vascular angiopathy. Notably, plasminogen activator inhibitor (PAI)-1 plays an important role in the pathophysiology of many vascular abnormalities.Citation3 PAI-1 levels fluctuate following aHSCT, and increased PAI-1 levels are associated with post-aHSCT complications.Citation4,Citation5 Therefore, PAI-1 is a potential predictive marker for VOD following aHSCT.Citation6
High-mobility group box 1 (HMGB1) is a nuclear protein which binds nucleosomes and promotes DNA bending.Citation7 HMGB1 plays a crucial role in the cellular response to tissue damage, and is secreted by multiple cell types.Citation8 HMGB1 expression is detectable in various immune and inflammatory diseases,Citation9 and it is sequestered by thrombomodulin in vivo.Citation10 While PAI-1 may play an important role in TAC following aHSCT, a relationship between HMGB1 and PAI-1 following aHSCT has not been previously reported. Here, we have investigated levels of HMGB1 and PAI-1 following aHSCT. To our knowledge, this multi-institutional joint study is the first report of the clinical significance of these biomarkers for aHSCT.
Materials and methods
The study cohort included 289 patients who underwent aHSCT between June 2011 and September 2014 at one of 25 institutions in Japan. The study protocol was approved by Institutional Review Board (IRB) of Kansai Medical University. Written informed consent was obtained from all patients who were registered by faxing documents to Kansai Medical University prior to HSCT. Patients comprised 175 male and 114 female individuals of 7–72 years (median: 45 years). Patient diagnoses were 108 acute myeloid leukemia cases, 66 acute lymphoblastic leukemia cases, 41 myelodysplastic syndrome cases, and 74 other diagnoses. Conditioning with TBI (dose range: 2–12 Gy) was performed in 192 patients, while 97 patients received non-TBI conditioning. Heparin or recombinant thrombomodulin was administered as a preventive therapy for TAC in 236 patients, while 53 patients received no anticoagulant therapy. Donor sources for transplantation were 153 bone marrow cells, 57 peripheral blood stem cells, and 79 cord blood cells. Written informed consent for participation in the study was obtained from all registered patients before HSCT. Clinical and biochemical data were obtained before aHSCT and on days 0, 4, 14, 21, and 28 after aHSCT.
Blood samples from patients were collected into tubes containing sodium citrate or tubes without any anticoagulant, and the blood was allowed to clot at room temperature for a minimum of 1 hour. Serum or citrated plasma was isolated by centrifugation for 20 minutes at 1,000× g at 4°C. Serum was divided into aliquots and frozen at −30°C until use. HMGB1 was measured using the HMGB1 enzyme-linked immunosorbent assay (ELISA) Kit II (Shino-Test Corporation, Kanagawa, Japan). PAI-1 ELISA kits were purchased from BioSource International Inc. (Camarillo, CA, USA). All ELISA kits were used according to the manufacturers’ instructions. As a positive control, recombinant proteins were used in each assay alongside the standard solutions that were provided with the commercial kits. Data are expressed as the means ± standard deviation. Comparisons between groups were made using the Student’s t-test.
We calculated the cutoff value for the HMGB1 level at day 0 using receiver operating characteristic curve analysis. This is because HMGB1 levels at day 0 were significantly elevated compared with levels before beginning the conditioning regimen (). Finally, patients were divided into two subgroups based on their HMGB1 levels at day 0 after aHSCT. Group A was defined as patients showing a >1.5-fold increase in plasma HMGB1 levels after aHSCT relative to levels before beginning the conditioning regimen. Group B was defined as those with a <1.5-fold increase in plasma HMGB1. Differences between PAI-1 levels in Groups A and B were evaluated by one-way analysis of variance followed by Dunnett’s test. All statistical analyses were performed using StatFlex (version 6) software (Artec Inc., Osaka, Japan), and P<0.05 indicated a statistically significant difference.
Table 1 Levels of HMGB1 and PAI-1 before and after aHSCT
Results
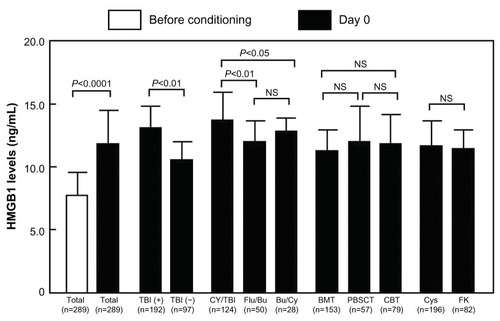
shows the levels of HMGB1 and PAI-1 before and after aHSCT. A significant increase in HMGB1 levels occurred after conditioning treatment, with peak levels observed at day 0 (). PAI-1 levels were significantly increased at days 14, 21, and 28 after aHSCT ().
depicts the elevation of HMGB1 levels at day 0 after aHSCT. The levels of HMGB1 at day 0 were significantly increased in TBI+ patients and CY/TBI patients (TBI+ vs TBI−, 12.7±2.3 vs 10.8±1.4 ng/mL, P<0.01; CY/TBI vs fludarabine/Bu, 13.3±2.6 vs 12.2±1.1 ng/mL, P<0.01; CY/TBI vs Bu/CY, 13.3±2.6 vs 12.6±0.8 ng/mL, P<0.05; ). Classification of patients by graft source or graft versus host disease prophylaxis did not reveal significant differences between patient groups ().
Figure 1 The comparison of HMGB1 levels.
Abbreviations: TBI, total body irradiation; CY, cyclophosphamide; Flu, fludarabine; Bu, busulfan; BMT, bone marrow transplantation; PBSCT, peripheral blood stem cell transplantation; CBT, cord blood transplantation; Cys, cyclosporine; FK, tacrolimus; NS, not significant; HMGB1, high-mobility group box 1; SD, standard deviation.
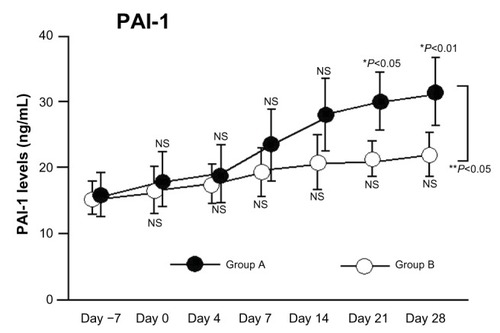
We divided patients into two subgroups based on HMGB1 levels after aHSCT. Group A (increased HMGB1) had significantly increased plasma PAI-1 levels relative to baseline. Following aHSCT, PAI-1 levels were significantly higher in Group A compared with Group B (two-factor analysis of variance, P<0.05; ).
Figure 2 Changes in PAI-1 levels following aHSCT conditioning regimens with and without significant elevation in HMGB1.
Abbreviations: PAI-1, plasminogen activator inhibitor-1; aHSCT, allogeneic hematopoietic stem cell transplantation; HMGB1, high-mobility group box 1; NS, not significant.
Discussion
An increased risk of developing TAC is associated with the inclusion of TBI in the conditioning regimen for aHSCT.Citation1 We identified increased levels of HMGB1 at day 0 in patients treated with conditioning regimens that included TBI or CY. This suggests that HMGB1 may be involved in the development of TAC following conditioning for aHSCT. Cutler et alCitation6 reported that increased vascular endothelial cell dysfunction is a predictive indicator of VOD. Although the etiology of VOD remains unclear, we suspect that increased levels of proinflammatory cytokines – such as HMGB1 – contribute to VOD. HMGB1 is implicated in the pathophysiology of a variety of inflammatory and immunological diseases.Citation9,Citation10 Several previous reports have examined the role of HMGB1 in aHSCT.Citation11–Citation13 Tagami et alCitation11 reported that HMGB1 may play a role during the mobilization of stem cells from the bone marrow into the systemic circulation. Additionally, Yujiri et alCitation12 suggested that HMGB1 may be a useful marker of acute graft versus host disease. Furthermore, Kornblit et alCitation13 proposed that an inherited variation in HMGB1 is associated with outcome after aHSCT. However, these previous reports did not examine changes in the level of HMGB1 after aHSCT. Our study details changes in HMGB1 levels postconditioning for aHSCT in a large patient cohort. Our results suggest that increased HMGB1 levels following myeloablative conditioning influence outcome after aHSCT.
PAI-1 is expressed by many cells, including endothelial cells, vascular smooth muscle cells, and platelets. In addition, PAI-1 expression can be regulated by many factors, including cytokines, oxidative stress, and cellular signaling molecules. Moreover, some previous reports have shown that all patients with transplantation-related complications – especially those with thrombotic complications – had significantly increased mean and maximal levels of PAI-1 over the observational period following aHSCT.Citation5,Citation6 Thus, PAI-1 is a known potential predictive marker for TAC following aHSCT. In this study, increased HMGB1 levels were associated with a significant increase in plasma PAI-1 levels relative to baseline. Additionally, PAI-1 levels in patients with increased HMGB1 were significantly higher than those in patients without increased HMGB1. These results suggest that increased PAI-1 levels could activate endothelial cells via HMGB1 following aHSCT. However, the development of TAC after aHSCT depends on multiple factors. Therefore, determination of whether PAI-1/HMGB1 levels are associated with TAC after aHSCT requires further validation.
We have demonstrated that a significant increase in HMGB1 level can follow aHSCT-conditioning treatment, with peak levels present at day 0. Additionally, HMGB1 levels correlate with PAI-1 levels after aHSCT. This increased HMGB1 expression may promote PAI-1-related TAC following aHSCT. Further studies employing larger patient cohorts are needed to confirm whether changes in the levels of these markers contribute to TAC following aHSCT.
Acknowledgments
The authors thank Mitsuko Ueda and Dr Michiomi Shimizu for technical assistance, and thank Drs Yoshio Saburi (Oita Hospital), Masanori Matsumoto (Nara Medical University), Nobiyoshi Arima (Kitano Hospital), Shigeru Chiba (Tsukuba University), Yoichi Ishida (Iwate University), and Kenichi Sawada (Akita University) for collected data. This work was partly supported by a grant from the Japan Foundation of Cardiovascular Research and by a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan.
Disclosure
SN has received research funding from Asahi Kasei Farma. The authors report no other conflicts of interest in this work.
References
- BredesonCLeRademacherJKatoKProspective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantationBlood20131223871387824081656
- CantoniNGerullSHeimDOrder of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCTBone Marrow Transplant20114634434920548339
- KuniyasuATokunagaMYamamotoTOxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression through reactive oxygen species generation and ERK1/2 activation in 3T3-L1 adipocytesBiochim Biophys Acta2011181115316221146630
- SalatCHollerEKolbHJPlasminogen activator inhibitor-1 confirms diagnosis of hepatic veno-occlusive disease in patients with hyperbilirubinemia after bone marrow transplantationBlood199789218421889058743
- PihuschMWegnerHGoehringPDiagnosis of hepatic veno-occlusive disease by plasminogen activator inhibitor-1 plasma antigen levels: a prospective analysis in 350 allogeneic hematopoietic stem cell recipientsTransplantation2005801376138216340778
- CutlerCKimHTAyanianSPrediction of veno-acclusive disease using biomarkers of endothelial injuryBiol Blood Marrow Transplant2010161180118520184961
- SeongSYMatzingerPHydrophobicity; an ancient damage-associated molecular pattern that initiates innate immune responsesNat Rev Immunol2004446947815173835
- MatsuokaNItohTWataraiHHigh-mobility group box 1 is involved in the initial events of early loss of transplanted islets in miceJ Clin Invest201012073574320124731
- KangRChenRZhangQHMGB1 in health and diseaseMol Aspects Med201440111625010388
- AbeyamaKSternDMItoYThe N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanismJ Clin Invest20051151267127415841214
- TagamiKYujiriTTanimuraAElevation of serum high-mobility group box 1 protein during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilisationBr J Haematol200613556756917054673
- YujiriTTagamiKTanakaYIncreased serum levels of high-mobility group box 1 protein in patients who developed acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantationEur J Haematol20108536636720659154
- KornblitBMasmasTPetersonSLAssociation of HMGB1 polymorphisms with outcome after allogeneic hematopoietic cell transplantationBiol Blood Marrow Transplant20101623925219819342
