Abstract
Teng-Long-Bu-Zhong-Tang (TLBZT) is a Chinese herbal formula for colorectal carcinoma treatment. TLBZT effectively induces cell senescence in colorectal carcinoma, accompanied by p21 upregulation. In this study, we further explored the role of p21 in TLBZT-induced cell senescence, as well as the mechanism by which TLBZT upregulates p21. Specific knockdown of p21 expression by small interfering RNA significantly attenuated TLBZT-induced cell senescence in human colorectal carcinoma LS174T cells. Silencing of p53 by small interfering RNA did not affect TLBZT-induced p21 upregulation. Meanwhile, TLBZT inhibited histone deacetylase activity. Furthermore, TLBZT increased acetylation levels of histone H3 and H4, enhancing their binding to the p21 promoter. These data suggested that TLBZT induces cell senescence in LS174T cells through a mechanism involving p21 upregulation via histone H3 and H4 acetylation. This study provides new insights into the application of TLBZT for colorectal carcinoma treatment.
Introduction
Colorectal cancer is the third most frequently diagnosed cancer in males and the second in females worldwide.Citation1 The overall survival of patients with early-stage colorectal carcinoma has improved in the past decades. However, treatment outcomes of metastatic colorectal cancer are less than satisfactory, even though targeted therapeutic drugs are currently used.Citation2,Citation3 Ethnomedicine constitutes another resource for the treatment of colorectal carcinoma. Most ethnic drugs are nontoxic with low cost, and have been used for thousands of years. There are abundant clinical experiences which are a resource for the development of new drugs for colorectal carcinoma treatment.
In China, Traditional Chinese Medicine (TCM) plays an important role in colorectal cancer treatment. TCM effectively enhances the curative effects of chemotherapy while reducing its toxic side effects, palliates clinical syndromes, prevents recurrence and metastasis, improves the quality of life and immune function, and prolongs survival in colorectal cancer.Citation4,Citation5 Cancer patients are treated with multiple Chinese herbs based on the syndromes (Zheng) and diseases present in individual patients, indicating that TCM is a form of personalized treatment. Most Chinese herbs are medicated in a prescription and applied as decoction.
Based on the TCM principles and clinical practice, we have established a herbal formula for colorectal cancer treatment, namely, Teng-Long-Bu-Zhong-Tang (TLBZT). TLBZT effectively induces cell senescence, accompanied with p21 upregulation and reduced retinoblastoma protein (RB) phosphorylation in colorectal carcinoma.Citation6,Citation7 However, the relationship between TLBZT-induced cell senescence and p21 upregulation remains unclear. This study assessed whether TLBZT induction of cell senescence is dependent on p21 and explored the potential mechanism by which TLBZT upregulates p21.
Materials and methods
Chemicals and reagents
Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum were obtained from Hyclone (Logan, UT, USA). Antibodies against acetylated histone H3 (AcH3) and H4 (AcH4), histone H3 and H4, p21, p53, and β-actin were obtained from Cell Signaling Technology (Danvers, MA, USA). Cellular Senescence Detection Kit was purchased from Cell Biolabs (San Diego, CA, USA). Histone deacetylase (HDAC) activity assay kit and Trichostatin A (TSA) were from BioVision (San Francisco, CA, USA). Chromatin immunoprecipitation (ChIP) assay kit was obtained from Beyotime Institute of Biotechnology (Jiangshu, People’s Republic of China). Small interfering RNAs (siRNAs) targeting p21 and p53, and control siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Lipofectamine™ 2000 was from Thermo Fisher Scientific (Waltham, MA, USA). Sodium valproate (SVP) was purchased from Sigma-Aldrich (St Louis, MO, USA).
TLBZT extraction
The main herbs in TLBZT formula (Chinese patent ZL200910197565.2) are roots of Actinidia chinensis (Teng-Li-Geng, 30 g), Solanum nigrum (Long-Kui, 15 g), Duchesnea indica (She-Mei, 15 g), Atractylodes macrocephala (Bai-Zhu, 9 g), Coix seed (Yi-Yi-Ren, 30 g), and Viscum coloratum (Hu-Ji-Sheng, 15 g). All these herbs were from Longhua Hospital according to the original proportions, and decocted twice with eight-fold volume of distilled water for 1 h. The resulting decoction was filtered and centrifuged twice at 12,000 rpm for 30 min to remove insoluble ingredients. The supernatants were mixed with an equal volume of ethanol and incubated at 4°C overnight, and centrifuged at 12,000 rpm for 30 min. The resulting supernatants were lyophilized, weighed, dissolved in DMEM, and adjusted to 400 mg/mL. Finally, the preparation was sequentially passed through 0.45 and 0.22 μm filters, for sterilization. The gas chromatography–mass spectrometry profile of TLBZT has been described previously.Citation7
Cell culture
Human colorectal carcinoma LS174T cells were obtained from Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. LS174T cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a humidified environment containing 5% CO2.
siRNA treatment
For siRNA transfection, LS174T cells were cultured in six-well plates to 60% confluency, and 80 pmol of specific or control siRNA was introduced into the cells using Lipofectamine™ 2000, according to the manufacturer’s recommendations.Citation8 After 24 h of transfection, the cells were treated with TLBZT and subjected to further assays.
Cell senescence detection
siRNA-transfected or nontransfected LS174T cells (1.5×103) were seeded in 96-well plates and treated with different doses of TLBZT and SVP or TSA for 5 days. Cell senescence was detected with a commercial kit. Briefly, the cells were lysed at 4°C for 5 minutes and centrifuged at 12,000 rpm for 10 minutes at 4°C. Cell lysates were collected and incubated with fluorometric SA-β-Gal substrate at 37°C, protected from light, for 2 h. Fifty microliters of the reaction mixture was transferred to a 96-well plate, stopped by adding 200 μL of stop solution, and read with a fluorescence plate reader at 360 nm (excitation)/465 nm (emission). The results were expressed as fold of control.
Western blot
Western blot was performed as described previously.Citation8,Citation9 Briefly, the collected cells were lysed, and total protein was separated by 8%–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The membrane was then blocked with 5% non-fat milk, washed, and probed with the indicated antibodies. Blots were washed and incubated with IRDye 700- and IRDye 800-conjugated secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA, USA), and visualized on Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
HDAC activity detection
TLBZT-treated or untreated LS174T cells were collected and assessed for HDAC activity with a specific kit, according to the manufacturer’s instructions. Briefly, the cells were lysed at 4°C for 5 minutes and centrifuged at 12,000 rpm for 10 minutes at 4°C. Cell lysates were collected and incubated with HDAC colorimetric substrate at 37°C for 1 h. The reaction mixture was read in a plate reader at 405 nm. The results were expressed as fold of control.
ChIP-quantitative polymerase chain reaction
ChIP was performed with a commercial assay kit using antibodies against AcH3 and AcH4, or control IgG, according to the manufacturer’s manual. Purified DNA was used as a template for quantitative polymerase chain reaction (qPCR) amplification using p21 promoter-specific primers.Citation10 The results were expressed as % of input: % input=2−(Ct[ChIP]–Ct[input])×100%.
Statistical analysis
Results are mean ± standard deviation (SD) from at least two independent triplicate experiments. Differences between control and TLBZT treatments were analyzed by one-way analysis of variance. Differences were considered significant at P<0.05.
Results
TLBZT-induced cell senescence is dependent on p21
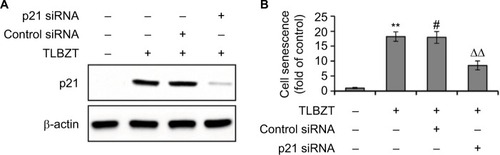
We have previously demonstrated that TLBZT-induced cell senescence in LS174T cells is accompanied by p21 upregulation.Citation6,Citation7 In this study, we further assessed whether TLBZT-induced cell senescence is associated with p21. As shown in , p21 knockdown by specific siRNA significantly abrogated TLBZT-induced cell senescence. These observations suggested TLBZT-induced cell senescence is dependent on p21.
Figure 1 Role of p21 in TLBZT-induced cell senescence.
Abbreviations: siRNA, small interfering RNA; TLBZT, Teng-Long-Bu-Zhong-Tang.
TLBZT-induced p21 expression is independent of p53
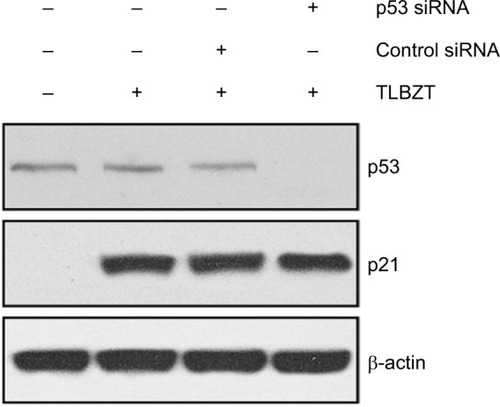
In a previous study, we found that p53 expression did not change upon TLBZT treatment.Citation6 Therefore, we also determined whether TLBZT-related p21 upregulation is associated with p53. As shown in , p53 was expressed in LS174T cells at low levels. Consistent with previous findings, TLBZT did not alter p53 expression. Knockdown of p53 expression by specific siRNA did not affect p21 expression. These findings demonstrated that TLBZT induced p21 expression in a p53-independent manner.
Figure 2 Role of p53 in TLBZT-induced p21 expression.
Abbreviations: siRNA, small interfering RNA; TLBZT, Teng-Long-Bu-Zhong-Tang.
TLBZT inhibits HDAC activity
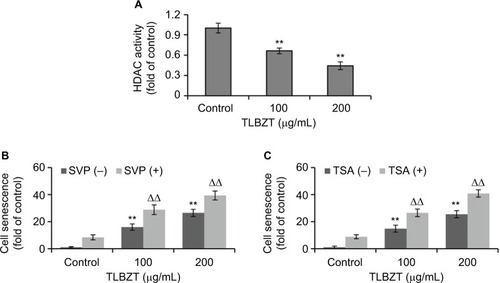
It has been reported that HDAC inhibition could upregulate p21 and induce cell senescence.Citation11,Citation12 We, therefore, assessed the effects of TLBZT on HDAC activity. Interestingly, TLBZT inhibited HDAC activity in a dose-dependent manner (). In addition, inhibition of HDAC activity by SVP and TSA enhanced the effects of TLBZT on LS174T cell senescence ( and ). These findings suggested that HDAC inhibition contributed to TLBZT-induced cell senescence in LS174T cells.
Figure 3 Effects of TLBZT on HDAC activity.
Abbreviations: HDAC, histone deacetylase; SVP, sodium valproate; TLBZT, Teng-Long-Bu-Zhong-Tang; TSA, trichostatin A.
TLBZT induces histone H3 and H4 acetylation
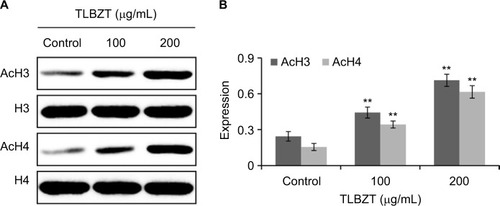
HDACs regulate gene transcription by deacetylating the α-acetyl lysine residue within the NH2-terminal tail of core histones, including histone H3 and H4. The effects of TLBZT on histone H3 and H4 acetylation in LS174T cells were detected by Western blot. As shown in , TLBZT increased the acetylation of histone H3 and H4 in a dose-dependent manner.
Figure 4 Effects of TLBZT on H3 and H4 acetylation.
Abbreviations: AcH3, acetylated histone H3; AcH4, acetylated histone H4; H3, histone H3; H4, histone H4; TLBZT, Teng-Long-Bu-Zhong-Tang.
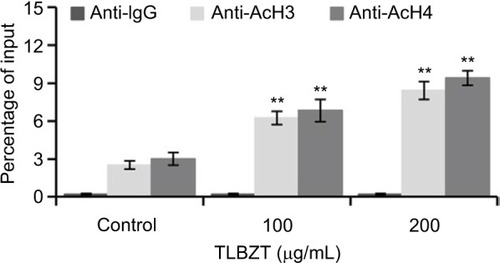
TLBZT upregulates histone H3 and H4 acetylation at the p21 promoter region
ChIP assays and qPCR were performed to detect AcH3 and H4 at the p21 promoter region by using specific antibodies and primers. As shown in , qPCR revealed a significant increase of p21 promoter fragments in ChIP-precipitated DNA from TLBZT-treated LS174T cells. These observations suggested increased acetylation of histone H3 and H4 at the p21 promoter region upon TLBZT treatment.
Figure 5 Effects of TLBZT on histone H3 and H4 acetylation at the p21 promoter region.
Abbreviations: AcH3, acetylated histone H3; AcH4, acetylated histone H4; ChIP, chromatin immunoprecipitation; qPCR, quantitative polymerase chain reaction; TLBZT, Teng-Long-Bu-Zhong-Tang.
Discussion
Cell senescence is a state of irreversible cell cycle arrest caused by a variety of stimuli. Senescent cells maintain some metabolic activity, but can no longer proliferate, even when stimulated with mitogens. Cell senescence plays an important role in tumorigenesis suppression, and is considered one of the useful mechanisms for anticancer therapy.Citation13–Citation15 Chemotherapeutic agents, such as cisplatin, doxorubicin, and camptothecin, have been reported to inhibit growth of cancer cells by inducing cell senescence.Citation16–Citation18 Natural products such as curcumin, resveratrol, and β-asarone have demonstrated cell senescence–inducing effects in cancer cells.Citation19–Citation21
Cell senescence is closely related to the activation of p21/pRB/E2F signaling.Citation22,Citation23 P21, also known as cyclin-dependent kinase inhibitor 1A, CDK-interacting protein 1, or wild-type p53-activated fragment 1, can inhibit a variety of cyclin/CDK complexes and induce the hypophosphorylation or dephosphorylation of protein RB.Citation24–Citation26 Hypophosphorylated pRB binds to E2F and prevents it from activating target genes essential in the cell cycle, which may lead to cell cycle arrest. In this study, p21 knockdown significantly abrogated TLBZT-induced cell senescence in LS174T cells, suggesting TLBZT-induced cell senescence is dependent on p21.
Transcription of p21 can be activated both in p53-dependent and -independent manners.Citation26,Citation27 The classical p53–p21 pathway is usually activated by DNA damage in response to genotoxic agents and other stimuli. Based on the nature of DNA damage, p53 may selectively discriminate between promoters in inducing target genes, thereby regulating their expression and promoting apoptosis, senescence, or autophagy.Citation28,Citation29 DNA-damaging drugs may induce p53–p21-dependent cell senescence in cancer cells.Citation30–Citation32 Unlike cytotoxic chemotherapeutic drugs, the herbs in TLBZT are nontoxic. As demonstrated previously, p53 expression does not change in LS174T cells upon TLBZT treatment.Citation6 Meanwhile, siRNA knockdown of p53 did not affect TLBZT-induced p21 expression, suggesting that TLBZT upregulated p21 expression in a p53-independent manner.
In addition to p53 regulation, other mechanisms contribute to p21 upregulation, for example, BRCA1, E2F1, E2F3, and HDAC inhibitors.Citation33 HDAC inhibitors release HDAC1 from the Sp1 site and promote acetylation of histone H3 and H4 at the p21 promoter region, easing the access of transcription factors to induce p21 expression.Citation26 Sodium butyrate (NaBu), a well-known inhibitor of class I and II HDACs, upregulates p21 and induces cell senescence in various cancer cells.Citation11,Citation34,Citation35 In this study, TLBZT inhibited HDAC activity; HDAC inhibitors, SVP and TSA, enhanced the effects of TLBZT on LS174T cell senescence. TLBZT also increased histone H3 and H4 acetylation at the p21 promoter region. These findings suggested that TLBZT upregulates p21 expression through histone acetylation.
Conclusion
In summary, the current findings demonstrated that TLBZT induces cell senescence in LS174T cells in a mechanism dependent on p21 upregulation via histone H3 and H4 acetylation. This study provides new insights into the application of TLBZT for colorectal carcinoma treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- SpiliopoulouPArkenauHTRationally designed treatment for metastatic colorectal cancer: current drug development strategiesWorld J Gastroenterol20142030102881029525132745
- StricklerJHHurwitzHIPalliative treatment of metastatic colorectal cancer: what is the optimal approach?Curr Oncol Rep201416136324293074
- DengSHuBAnHMTraditional Chinese Medicinal syndromes and treatment in colorectal cancerJ Cancer Ther201236888897
- DengSHuBShenKPPathogenesis and treatment of colorectal cancer in Traditional Chinese MedicineWorld Sci Technol Mod Trad Chin Med Mater Med201214418581862 Chinese
- HuBAnHMShenKPDuQSenescence-inducing effects of Chinese herbal medicine Tenglong Buzhong Decoction on human colon carcinoma LS-174-T cells and the mechanismJ Chin Integr Med201081110481052 Chinese
- DengSHuBAnHMTeng-Long-Bu-Zhong-Tang, a Chinese herbal formula, enhances anticancer effects of 5-Fluorouracil in CT26 colon carcinomaBMC Complement Altern Med201313112823758730
- HuBDuQDengSLigustrum lucidum Ait. fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21Oncol Rep20143231037104225017491
- HuBAnHMShenKPLiver Yin deficiency tonifying herbal extract induces apoptosis and cell senescence in Bel-7402 human hepatocarcinoma cellsExp Ther Med201231808622969849
- NohJHJungKHKimJKAberrant regulation of HDAC2 mediates proliferation of hepatocellular carcinoma cells by deregulating expression of G1/S cell cycle proteinsPLoS One2011611e2810322132221
- AnHMXueYFShenYLDuQHuBSodium valproate induces cell senescence in human hepatocarcinoma cellsMolecules20131812149351494724304587
- RomanovVSAbramovaMVSvetlikovaSBp21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrateCell Cycle20109193945395520935470
- CairneyCJBilslandAEEvansTRCancer cell senescence: a new frontier in drug developmentDrug Discov Today2012175–626927622314100
- AcostaJCGilJSenescence: a new weapon for cancer therapyTrends Cell Biol201222421121922245068
- NardellaCClohessyJGAlimontiAPandolfiPPPro-senescence therapy for cancer treatmentNat Rev Cancer201111750351121701512
- FangKChiuCCLiCHChangYTHwangHTCisplatin-induced senescence and growth inhibition in human non-small cell lung cancer cells with ectopic transfer of p16INK4aOncol Res2007161047948818196872
- JacksonJGPereira-SmithOMPrimary and compensatory roles for RB family members at cell cycle gene promoters that are deacetylated and downregulated in doxorubicin-induced senescence of breast cancer cellsMol Cell Biol20062672501251016537896
- HanZWeiWDunawaySRole of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecinJ Biol Chem200227719171541716011877436
- MosieniakGSliwinskaMAPrzybylskaDCurcumin-treated cancer cells show mitotic disturbances leading to growth arrest and induction of senescence phenotypeInt J Biochem Cell Biol20162274334326916504
- FangYDeMarcoVGNichollMBResveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosisCancer Sci201210361090109822417066
- LiuLWangJShiLβ-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expressionPhytomedicine201320651252023357361
- RoninsonIBTumor cell senescence in cancer treatmentCancer Res200363112705271512782571
- DimriGPWhat has senescence got to do with cancerCancer Cell20057650551215950900
- Flores-RozasHKelmanZDeanFBCdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzymeProc Natl Acad Sci USA19949118865586597915843
- el-DeiryWSTokinoTVelculescuVEWAF1, a potential mediator of p53 tumor suppressionCell19937548178258242752
- OckerMSchneider-StockRHistone deacetylase inhibitors: signalling towards p21cip1/waf1Int J Biochem Cell Biol2007397–81367137417412634
- WarfelNAEl-DeiryWSp21WAF1 and tumourigenesis: 20 years afterCurr Opin Oncol2013251525823159848
- ChiantoreMVVannucchiSManginoGSenescence and cell death pathways and their role in cancer therapeutic outcomeCurr Med Chem200916328730019149578
- HillRBodzakEBloughMDLeePWp53 Binding to the p21 promoter is dependent on the nature of DNA damageCell Cycle20087162535254318719376
- NinomiyaYCuiXYasudaTArsenite induces premature senescence via p53/p21 pathway as a result of DNA damage in human malignant glioblastoma cellsBMB Rep2014471057558024499675
- QuKLinTWeiJCisplatin induces cell cycle arrest and senescence via upregulating P53 and P21 expression in HepG2 cellsNan Fang Yi Ke Da Xue Xue Bao201333912531259 Chinese24067199
- te PoeleRHOkorokovALJardineLCummingsJJoelSPDNA damage is able to induce senescence in tumor cells in vitro and in vivoCancer Res20026261876188311912168
- AbbasTDuttaAp21 in cancer: intricate networks and multiple activitiesNat Rev Cancer20099640041419440234
- LorenzVHessenkemperWRödigerJKyrylenkoSKraftFBaniahmadASodium butyrate induces cellular senescence in neuroblastoma and prostate cancer cellsHorm Mol Biol Clin Investig201171265272
- EdmondVBrambillaCBrambillaEGazzeriSEyminBSRSF2 is required for sodium butyrate-mediated p21(WAF1) induction and premature senescence in human lung carcinoma cell linesCell Cycle201110121968197721555914
