Abstract
Vascular sarcomas are abnormal proliferations of endothelial cells. They range from benign hemangioma to aggressive angiosarcoma, and are characterized by dysregulated angiogenic signaling. Propranolol is a β-adrenergic receptor inhibitor that has demonstrated clinical efficacy in benign infantile hemangioma, and is now being used experimentally for more aggressive vascular sarcomas and other cancers. In this review, we discuss the use of propranolol in targeting these receptors in vascular tumors and other cancers.
Introduction
Vascular sarcomas are abnormal proliferations of endothelial cells (ECs). They range from benign hemangioma to aggressive angiosarcoma, and are characterized by dys-regulated angiogenic signaling.Citation1 Benign infantile hemangiomas (IHs) are among the most common vascular tumors, with an incidence of approximately 3%.Citation2 The natural history of IH is to first expand during a proliferative phase, and then regress during an involuting phase. Lesions that are symptomatic or otherwise problematic can be treated with topical or systemic agents, including corticosteroids or β-adrenergic receptor inhibitors.Citation3 Propranolol is a nonselective β-adrenergic receptor blocker that has been implicated in several cancers and has had success in treating IH.Citation4
Angiosarcoma is an aggressive cancer of ECs. It can occur anywhere in the body, with the most common sites being cutaneous lesions in the head and neck, breast, and extremities. They can be further subclassified into primary and secondary angiosarcoma, with the latter as a result of chronic lymphedema or radiation exposure. Outcomes for patients with angiosarcoma, even those who present with localized disease, are poor. For patients who develop metastatic disease, median survival is about 1 year.Citation5–Citation7 Primary treatment usually includes a combination of cytotoxic chemotherapy, surgery, and radiation. The advent of drugs targeting angiogenesis pathways were theoretically promising for treating tumors of ECs, but clinical results have been disappointing. Response rates to drugs targeting the VEGF/VEGFR axis range from 9%–20%.Citation1 Combining bevacizumab, an anti-VEGF antibody, with paclitaxel yielded no clinical benefit.Citation8 Drugs targeting other angiogenesis pathways such as the angiopoietin–TIE2 axis have also thus far been unsuccessful.Citation9 The PI3K/AKT/mTOR pathway has also been implicated in both benign and aggressive vascular tumors.Citation10,Citation11
Molecular and genomic characterization has yielded some insights into the drivers of angiosarcoma, but to date no targeted agents have demonstrated a clear benefit for most patients. Some angiosarcomas harbor activating mutations in KDRCitation12 or PLCG1,Citation13 and others have CIC mutations or rearrangementsCitation14 which serve as potential driver events. Secondary angiosarcomas are characterized by MYC and FLT4 amplification.Citation15 Even with this improved understanding as a result of high-throughput sequencing of several cohorts of angiosarcomas, driver events for most cases remain unknown. Recently, focus has sharpened on the β-adrenergic receptors that play a key role in normal EC function and may play a role in supporting angiosarcoma growth. In this review, we will discuss the use of propranolol in targeting these receptors in angiosarcoma and other vascular tumors.
β-adrenergic signaling and cancer
Adrenergic receptors are 7-transmembrane G-protein coupled receptors that consist of α, β, and γ subunit subtypes.Citation16 β-adrenergic receptors play a vital role in several physiologic processes and are key mediators of the physiologic stress response. Drugs have been developed to inhibit the recep tors with varying levels of affinity. Modulators of adrenergic signaling are some of the oldest drugs in clinical use, with clinical benefit particularly for cardiovascular disease and prevention of esophageal varix bleeding in advanced hepatic cirrhosis.
Recently, β-adrenergic signaling is gaining attention as a potential therapeutic target in cancer.Citation17 Several mechanisms by which β-blockers improve outcomes in cancers have been proposed, including both direct anticancer effects and effects on multiple cell types in the cancer microenvironment ().
Figure 1 Adrenergic signaling in the vascular tumor microenvironment.
Abbreviations: Epi, epinephrine; NE, norepinephrine; EC, endothelial cell.
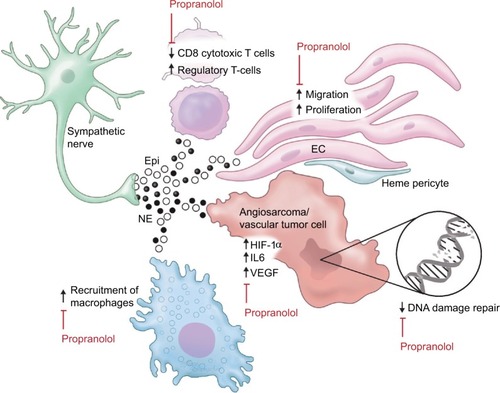
β-adrenergic pathway modulators have direct effects on cancer cells of various subtypes in culture. Stimulation with β-agonists increases cell proliferation in a cAMP-dependent manner in lung adenocarcinoma cells.Citation18 Activation of the β2-adrenergic pathway increases IL-6 production and inactivates the tumor suppressor LKB1 in EGFR mutant lung adenocarcinoma cells, and is a proposed mechanism for resistance to EGFR inhibitors. Indeed β-blocker use was associated with improved benefit from afatinib in the Phase III LUX-Lung3 study.Citation19,Citation20 Similar increases in cancer cell-specific measurements such as proliferation and invasiveness were seen in pancreatic cancer cellsCitation21 and ovarian cancer cells.Citation22 Adrenergic stimulation led to chemoresistance in colon cancer cellsCitation23 and ovarian cancer,Citation24 the latter by stimulating DUSP1. β-blockers are synergistic with cytotoxic chemotherapy against breast cancerCitation25 and neuroblastomaCitation26 cells. Although some of the effect seen in lung cancer seems to be specific for EGFR mutant-containing cells, broader pathways such as DNA damage repair pathways are also regulated in part through β-2 receptors.Citation27
Propranolol is a small molecule nonspecific inhibitor of β-1 and β-2 adrenergic receptors, and is the focus of this review. In addition to the effects on cancer cells themselves, β-receptor inhibition with propranolol decreases proliferation, migration, and differentiation of ECs.Citation28 Propranolol treatment inhibits angiogenesis in EC lines, but has little to no effect on vascular disruption.Citation25 Preclinical studies in cancer models have demonstrated that increased adrenergic signaling through the β-2 receptor results in increased VEGF production in cancer cellsCitation29 and increased tumor vascularization.Citation30 Similarly, the β-agonist isoprenaline stimulates autocrine VEGF signaling in gastric cancer cells and associated ECs via β-2 receptor-mediated signaling.Citation31
Clinical evidence for b-receptor inhibition in cancer
Some of the first retrospective clinical data in support of β-blockers in cancer were seen in breast cancer, where β-blocker use for hypertension is associated with improved cancer-specific survival compared with patients using other types of antihypertensive medications.Citation32 The specificity of β-receptor inhibition has an effect on survival, with a beneficial effect seen in breast cancer patients receiving the nonselective β blocker propranolol but not with the β-1 antagonist atenolol.Citation33 Carvedilol, another nonselective β-blocker, reduces the risk of multiple cancer types with the largest effect seen in upper gastrointestinal and lung cancers in a large study from Asia.Citation34 Additional studies show benefit of β-blocker use in patients with colorectalCitation35 and pancreaticCitation36 cancer. A prospective nonrandomized study of propranolol in the adjuvant setting for resected melanoma found an 80% reduction in melanoma recurrence.Citation37 Overall, prospective clinical evidence supporting a role for propranolol in cancer treatment or prevention is limited. A summary of the largest existing clinical studies describing the impact of β-blockers on cancer incidence and outcomes is provided in .
Table 1 Clinical evaluation of β-blockers in multiple cancer subtypes
Treatment of IH
The antiangiogenic properties of propranolol have led it to be used in vascular tumors. Indeed, propranolol has seen perhaps its greatest success in oncology in IH. There is significant controversy surrounding the cell of origin, with evidence that there is a hemangioma stem cell (HemSC) which induces proliferative changes in adjacent cells in the microenvironment.Citation38 Despite the tendency of these tumors to first proliferate and then regress in characteristic phases, some IHs are problematic and require treatment.Citation3 The proliferating phase of IH is characterized by VEGF-A production which stimulates hemangioma endothelial cell (HemEC) proliferation.Citation39 Indeed, patients with IH have high increased circulating levels of VEGF-A.Citation40,Citation41 At the receptor level, VEGFR1 expression levels in hemangiomas are lower than those in normal ECs,Citation42–Citation45 consistent with its accepted role as a VEGF-A trap counteracting the stimulatory effects of VEGF-A ligand binding to VEGFR2. Decreased VEGFR1 expression levels in HemECs results in increased VEGFR2 signaling,Citation43 and VEGFR2 knockdown in HemECs decreases cell viability and increased apoptosis, whereas VEGFR2 overexpression has the opposite effect.Citation46 Proliferating IH has a relatively high expression of Ang2 and low expression of Ang1,Citation47,Citation48 as do hemangioma-derived pericytes;Citation49 however, hemangioma-derived cell lines demonstrate increased migration and survival in response to Ang1, but not Ang2, highlighting the complicated roles these 2 ligands play.Citation48
The tumor microenvironment also plays a critical role in hemangioma formation. Jagged1 expression on ECs and cell–cell contact between HemECs and HemSCs is required for HemSC differentiation into pericyte.Citation44 Hemangioma-derived pericytes do not stabilize developing blood vessels as would be expected with physiologic pericytes.Citation49 Jagged1 and Notch4 expression levels in proliferating IH are 6.5-fold and 3.2-fold higher, respectively, than those in placenta vessel control.Citation47 Notch effector proteins HEY1, HEYL, and HES1 are highly expressed in HemSCs, whereas HEY2 is highly expressed in HemECs alone.Citation50 Interestingly Notch1, Notch4, and Jagged1 have increased expression in involuting hemangioma ECs, and it was concluded that the involution was at least partially caused by the cells’ differentiation into a more determined EC phenotype as a result of increased Notch signaling.Citation51
Treatment with the β-blocker propranolol at a dose of 2 mg per kg of body weight per day leads to regression of cutaneous IH lesionsCitation4 as well as potentially more life-threatening infantile hepatic hemangiomasCitation52 and subglottic hemangiomas.Citation53 Conversely, the use of β-2 sympathomimetic tocolytics doubled the rate of IH in preterm infants from 11% to 22% in the group studied.Citation54 Although the mechanism remains unclear, 1 proposed mechanism by which propranolol may induce regression is by reducing the expression of HIF-1α, which in turn decreases HIF-1α-mediated signaling through the VEGF and STAT3 pathways.Citation55 Moreover, propranolol may be targeting Hempericytes.Citation49 Interestingly, GLUT1-positive cells derived from proliferating hemangiomas exhibit stem-like properties, and their growth is inhibited by mTOR inhibition but not by propranolol.Citation56 mTOR and HIF-1a contribute to hemangioma proliferation via an autocrine VEGF signaling loop.Citation57 Interestingly, treatment with propranolol leads to similar gene expression changes in IH and normal ECs, suggesting that the regression seen with propranolol is multifactorial, involving drug effect on multiple cell types in the microenvironment.Citation58
Propranolol in intermediate-grade vascular sarcomas and angiosarcoma
Given their clinical success in IH, β-blockers have been studied in models of other vascular tumors. β-receptors 1, 2, and 3 are present on hemangioendothelioma and angiosarcoma by immunohistochemistry, and treatment with high doses of propranolol causes apoptosis and is synergistic with cytotoxic chemotherapy in hemangioendothelioma and angiosarcoma cell lines.Citation59 Compared with hemangiomas, fewer of the aggressive tumors express β-2 and β-3 receptors, with about 40% of the 44 angiosarcomas in 1 series staining strongly for β-2 receptor and variable staining across the various types of hemangioendothelioma.Citation60 mRNA expression profiling of transformed mouse ECs that behave like angiosarcoma cells revealed a broad array of differentially expressed genes after treatment with propranolol.Citation61
The role of β-2 receptor signaling in both adaptive and innate immunityCitation62 also makes propranolol appealing for treatment of angiosarcoma. B- and T-lymphocytes express the β-2 adrenergic receptor and are responsive to β-agonists.Citation63 Chronic β-adrenergic receptor signaling suppresses CD8+ cytotoxic T-cells, thus reducing T-cell responses to immune checkpoint inhibitors.Citation64 Lymphocyte egress from lymph nodes and interferon transcription is regulated by sympathetic innervation and norepinephrine.Citation65,Citation66 The presence of infiltrating CD8+ T-cells correlates with survival in angiosarcoma patients.Citation67 Furthermore, β-adrenergic signaling affects myeloid cells in the micro-environment by regulating secretion of IL-6 and IL-8.Citation68,Citation69 Macrophage recruitment to tumors may be increased by beta adrenergic mediated secretion of chemotactic molecules by tumor cells.Citation70 IL-6 production in angiosarcoma tumor cells increases the number of tumor promoting macrophages.Citation71 Small reports aimed at targeting tumor-associated macrophages in angiosarcoma were promising,Citation72,Citation73 suggesting that the anti-inflammatory effect of propranolol will be beneficial in angiosarcoma.
Unfortunately much of what is known clinically about propranolol and its utility in treating angiosarcoma specifically are based on case reports. In 1 patient, serial biopsy before and 1 week after initiation of propranolol 40 mg twice a day resulted in a decrease in proliferative index of the tumor assessed by Ki67 staining from around 30% of positive cells to around 20%, causing a reduction in proliferation of 34%.Citation74 As this was a single case report, and so this difference may be accounted for by sampling variance, and any determination of clinical benefit is confounded by the addition of cytotoxic chemotherapy and radiation in this patient’s treatment course.
Most reports combine propranolol with cytotoxic chemotherapy. In a preclinical model of transformed ECs, propranolol was synergistic with vinblastine, but not the chemotherapeutic agents more commonly used for angiosarcoma, such as doxorubicin or paclitaxel.Citation75 Several patients treated with propranolol combined with metronomic vinblastine and methotrexate derived clinical benefit from this combination, though distinguishing the potential contribution of propranolol in this combination is impossible based on the described studies.Citation75,Citation76
Metronomic chemotherapy has been shown to have anti-angiogenic effects and can improve the anticancer effects of cyclophosphamide in some settings.Citation77 Due to the anti-angiogenic effects of low-dose metronomic chemotherapy, this strategy presented a promising method for treating angiosarcoma. Treatment with metronomic trofosfamide, pioglitazone, and rofecoxib led to clinical responses in 3 of 5 angiosarcoma patients in 1 small series.Citation78 Due its better toxicity profile, metronomic chemotherapy with cyclophosphamide was used in elderly patients with angiosarcoma with evidence of efficacy.Citation79 Case reports combining propranolol combined with metronomic cyclophosphamide in angiosarcoma suggest promising results that warrant further investigation.Citation80,Citation81 The optimal dose of propranolol for angiosarcoma is not currently known. A dose finding study is currently underway in France investigating increasing dosing of pro-pranolol combined with a stable dose of cyclophosphamide (NCT02732678).
Conclusion and future directions
The prospect of utilizing propranolol in angiosarcoma is a promising one, with hints of benefit in preclinical work and small case series and reports. However, in spite of the excitement describing the revolutionary potential of propranolol in angiosarcoma, the current role for propranolol remains an open question. Confounding factors in all of the published reports, combined with the fact that all of these studies are small case series or reports, limit the ability to make conclusive recommendations. Prospective studies with larger numbers of patients are needed. One potential study design would incorporate propranolol in the adjuvant setting to investigate a specific benefit from β-2 inhibition without the confounding impact of coadministered chemotherapy. Alternatively, incorporating propranolol into currently used systemic regimens may be preferred, but with the rarity of angiosarcoma and lack of consensus on initial management particularly for localized disease this may be difficult. Thankfully, propranolol is a relatively cheap and well-studied drug for other indications. A prospective, multicenter randomized trial should be feasible and would be able to answer once and for all if we should be including propranolol in our angiosarcoma treatment schema.
Disclosure
MJW is funded by the Conquer Cancer Foundation–ASCO Young Investigator Award and QuadW Foundation–AACR Fellowship for Clinical/Translational Sarcoma Research. The authors report no other conflicts of interest in this work.
References
- WagnerMJRaviVMenterDGSoodAKEndothelial cell malignancies: new insights from the laboratory and clinicNPJ Precis Oncol2017111129872699
- DickisonPChristouEWargonOA prospective study of infantile hemangiomas with a focus on incidence and risk factorsPediatr Dermatol201128666366921995808
- ChenTSEichenfieldLFFriedlanderSFInfantile hemangiomas: an update on pathogenesis and therapyPediatrics201313119910823266916
- Leaute-LabrezeCDumas de la RoqueEHubicheTBoraleviFThamboJBTaïebAPropranolol for severe hemangiomas of infancyN Engl J Med2008358242649265118550886
- D’AngeloSPMunhozRRKukDOutcomes of systemic therapy for patients with metastatic angiosarcomaOncology201589420521426043723
- FuryMGAntonescuCRVan ZeeKJBrennanMFMakiRGA 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapyCancer J200511324124716053668
- FayetteJMartinEPiperno-NeumannSAngiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 casesAnn Oncol200718122030203617974557
- Ray-CoquardILDomontJTresch-BruneelEPaclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trialJ Clin Oncol201533252797280226215950
- D’AngeloSPMahoneyMRVan TineBAAlliance A091103 a phase II study of the angiopoietin 1 and 2 peptibody trebananib for the treatment of angiosarcomaCancer Chemother Pharmacol201575362963825672915
- PerryBBanyardJMcLaughlinERAKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivoArch Dermatol2007143450450617438183
- GreenbergerSYuanSWalshLARapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangiomaJ Invest Dermatol2011131122467247621938011
- AntonescuCRYoshidaAGuoTKDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitorsCancer Res200969187175717919723655
- BehjatiSTarpeyPSSheldonHRecurrent PTPRB and PLCG1 mutations in angiosarcomaNat Genet201446437637924633157
- HuangSCZhangLSungYSRecurrent CIC gene abnormalities in angiosarcomas: a molecular study of 120 cases with concurrent investigation of PLCG1, KDR, MYC, and FLT4 gene alterationsAm J Surg Pathol201640564565526735859
- GuoTZhangLChangNESingerSMakiRGAntonescuCRConsistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesionsGenes Chromosomes Cancer2011501253320949568
- HeplerJRGilmanAGG proteinsTrends Biochem Sci199217103833871455506
- ColeSWSoodAKMolecular pathways: β-adrenergic signaling in cancerClin Cancer Res20121851201120622186256
- ParkPGMerrymanJOrloffMSchullerHMβ-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: implications for individuals with preexisting chronic lung diseaseCancer Res19955516350435087627955
- NilssonMBSunHDiaoLStress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with β-blockersSci Transl Med20179415 pii: eaa4307
- SequistLVYangJCYamamotoNPhase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutationsJ Clin Oncol201331273327333423816960
- HuangXYWangHCYuanZHuangJZhengQNorepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via β-adrenergic receptor-dependent activation of P38/MAPK pathwayHepatogastroenterology20125911588989322020907
- SoodAKBhattyRKamatAAStress hormone-mediated invasion of ovarian cancer cellsClin Cancer Res200612236937516428474
- YaoHDuanZWangMAwonugaAORappoleeDXieYAdrenaline induces chemoresistance in HT-29 colon adenocarcinoma cellsCancer Genet Cytogenet20091902818719380024
- KangYNagarajaASArmaiz-PenaGNAdrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancerClin Cancer Res20162271713172426581245
- PasquierECiccoliniJCarreMPropranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatmentOncotarget201121079780922006582
- PasquierEStreetJPouchyCβ-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastomaBr J Cancer2013108122485249423695022
- HaraMRKovacsJJWhalenEJA stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1Nature2011477736434935321857681
- LamySLachambreMPLord-DufourSBeliveauRPropranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cellsVascul Pharmacol2010535–620020820732454
- LutgendorfSKColeSCostanzoEStress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell linesClin Cancer Res200691245144521
- ThakerPHHanLYKamatAAChronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinomaNat Med200612893994416862152
- LuYXuQZuoYIsoprenaline/β2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesisBMC Cancer201717187529262812
- PoweDGVossMJZänkerKSβ-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survivalOncotarget20101762863821317458
- BarronTIConnollyRMSharpLBennettKVisvanathanKβ blockers and breast cancer mortality: a population-based studyJ Clin Oncol201129192635264421632503
- LinCSLinWSLinCLKaoCHCarvedilol use is associated with reduced cancer risk: a nationwide population-based cohort studyInt J Cardiol201518491325705003
- JansenLHoffmeisterMArndtVChang-ClaudeJBrennerHStage-specific associations between β blocker use and prognosis after colorectal cancerCancer201412081178118624415516
- BegMSGuptaASherDImpact of concurrent medication use on pancreatic cancer survival-SEER-medicare analysisAm J Clin Oncol Epub2017110
- De GiorgiVGrazziniMBenemeiSPropranolol for off-label treatment of patients with melanoma: results from a cohort studyJAMA Oncol201742e172908
- KhanZABoscoloEPicardAMultipotential stem cells recapitulate human infantile hemangioma in immunodeficient miceJ Clin Invest200811872592259918535669
- GreenbergerSBoscoloEAdiniIMullikenJBBischoffJCorticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cellsNew Engl J Med2010362111005101320237346
- ZhangLLinXWangWCirculating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patientsPlast Reconstr Surg2005116120020415988268
- KleinmanMEGreivesMRChurginSSHypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangiomaArterioscler Thromb Vasc Biol2007272664267017872454
- JiYChenSLiKLiLXuCXiangBSignaling pathways in the development of infantile hemangiomaJ Hematol Oncol201471324479731
- JinninMMediciDParkLSuppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangiomaNat Med200814111236124618931684
- BoscoloEMullikenJBBischoffJVEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangiomaAm J Pathol201117952266227721945324
- PicardABoscoloEKhanZAIGF-2 and FLT-1/VEGF-R1 mRNA levels reveal distinctions and similarities between congenital and common infantile hemangiomaPediatr Res200863326326718287964
- OuJMYuZYQiuMKKnockdown of VEGFR2 inhibits proliferation and induces apoptosis in hemangioma-derived endothelial cellsEur J Histochem2014581226324704994
- CalicchioMLCollinsTKozakewichHPIdentification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profilingAm J Pathol200917451638164919349369
- YuYVarugheseJBrownLFMullikenJBBischoffJIncreased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cellsAm J Pathol200115962271228011733376
- BoscoloEMullikenJBBischoffJPericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1Arterioscler Thromb Vasc Biol201333350150923288163
- AdepojuOWongAKitajewskiAExpression of HES and HEY genes in infantile hemangiomasVasc Cell2013319
- WuJKAdepojuODe SilvaDA switch in Notch gene expression parallels stem cell to endothelial transition in infantile hemangiomaAngiogenesis2010131152320069356
- Mazereeuw-HautierJHoegerPHBenlahrechSEfficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosisJ Pediatr2010157234034220488455
- DenoyelleFLeboulangerNEnjolrasOHarrisRRogerGGarabedianENRole of propranolol in the therapeutic strategy of infantile laryngotracheal hemangiomaInt J Pediatr Otorhinolaryngol20097381168117219481268
- MayerMMinichmayrAKlementFTocolysis with the β-2-sympathomimetic hexoprenaline increases occurrence of infantile haemangioma in preterm infantsArch Dis Child Fetal Neonatal Ed2013982F108F11122611112
- LiPGuoZGaoYPanWPropranolol represses infantile hemangioma cell growth through the β2-adrenergic receptor in a HIF-1α-dependent mannerOncol Rep20153363099310725872592
- HuangLNakayamaHKlagsbrunMMullikenJBBischoffJGlucose transporter 1-positive endothelial cells in infantile hemangioma exhibit features of facultative stem cellsStem Cells201533113314525187207
- MediciDOlsenBRRapamycin inhibits proliferation of hemangioma endothelial cells by reducing HIF-1-dependent expression of VEGFPLoS One201278e4291322900063
- StilesJAmayaCPhamRPropranolol treatment of infantile hemangioma endothelial cells: a molecular analysisExp Ther Med20124459460423170111
- StilesJMAmayaCRainsSTargeting of β adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcomaPLoS One201383e6002123555867
- ChisholmKMChangKWTruongMTKwokSWestRBHeerema-McKenneyAEβ-Adrenergic receptor expression in vascular tumorsMod Pathol2012251446145122743651
- ZhouSLiuPJiangWZhangHIdentification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysisOncol Lett20171364267427528588707
- PadroCJSandersVMNeuroendocrine regulation of inflammationSemin Immunol201426535736824486056
- PochetRDelespesseGβ-Adrenoreceptors display different efficiency on lymphocyte subpopulationsBiochem Pharmacol19833210165116556305367
- NissenMDSloanEKMattarolloSRβ-adrenergic signaling impairs antitumor CD8(+) T-cell responses to B-cell lymphoma immunotherapyCancer Immunol Res2018619810929146881
- KohmAPSandersVMNorepinephrine and β 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivoPharmacol Rev200153448752511734616
- NakaiAHayanoYFurutaFNodaMSuzukiKControl of lymphocyte egress from lymph nodes through β2-adrenergic receptorsJ Exp Med20142112583259825422496
- D’AngeloSPShoushtariANAgaramNPPrevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironmentHum Pathol201546335736525540867
- NilssonMBArmaiz-PenaGTakahashiRStress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanismJ Biol Chem200728241299192992617716980
- ShahzadMMArevaloJMArmaiz-PenaGNStress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasisJ Biol Chem201028546354623547020826776
- Armaiz-PenaGNGonzalez-VillasanaVNagarajaASAdrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growthOncotarget2015664266427325738355
- YangJKantrowSSaiJINK4a/ARF [corrected] inactivation with activation of the NF-κB/IL-6 pathway is sufficient to drive the development and growth of angiosarcomaCancer Res201272184682469522836752
- IshibashiMFujimuraTHashimotoASuccessful treatment of MMP-9-expressing angiosarcoma with low-dose docetaxel and bisphosphonateCase Rep Dermatol201245922308113
- FujimuraTKambayashiYFurudateSKakizakiAAibaSImmunomodulatory effect of bisphosphonate risedronate sodium on CD163+ arginase 1+ M2 macrophages: the development of a possible supportive therapy for angiosarcomaClin Dev Immunol2013201332541224489574
- ChowWAmayaCNRainsSChowMDickersonEBBryanBAGrowth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockadeJAMA Dermatol2015151111226122926375166
- PasquierEAndréNStreetJEffective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside studyEBioMedicine20166879527211551
- BanavaliSPasquierEAndreNTargeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcomaEcancermedicalscience2015949925624880
- BrowderTButterfieldCEKrälingBMAntiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancerCancer Res20006071878188610766175
- VogtTHafnerCBrossKAntiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumorsCancer200398102251225614601096
- MirODomontJCioffiAFeasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcomaEur J Cancer201147451551921251814
- DaguzeJSaint-JeanMPeuvrelLVisceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranololJAAD Case Rep20162649749928004027
- DaguzeJSaint-JeanMDrenoBLarge nose angiosarcoma treated effectively with oral cyclophosphamide combined with propranololJ Eur Acad Dermatol Venereol2018322e52e5428833588
- Melhem-BertrandtAChavez-MacgregorMLeiXβ-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancerJ Clin Oncol201129192645265221632501
- BotteriEMunzoneERotmenszNTherapeutic effect of β-blockers in triple-negative breast cancer postmenopausal womenBreast Cancer Res Treat201314056757523912960
- De GiorgiVGrazziniMGandiniSTreatment with β-blockers and reduced disease progression in patients with thick melanomaArch Intern Med2011171877978121518948
- De GiorgiVGandiniSGrazziniMBenemeiSMarchionniNGeppettiPEffect of β-blockers and other antihypertensive drugs on the risk of melanoma recurrence and deathMayo Clin Proc2013881196120324182700
- LemeshowSSørensenHTPhillipsGβ-blockers and survival among Danish patients with malignant melanoma: a population-based cohort studyCancer Epidemiol Biomarkers Prev2011202273227921933972
- JohannesdottirSASchmidtMPhillipsGUse of β-blockers and mortality following ovarian cancer diagnosis: a population-based cohort studyBMC Cancer2013138523433478
- WatkinsJLThakerPHNickAMClinical impact of selective and nonselective β-blockers on survival in patients with ovarian cancerCancer2015121193444345126301456
- HuangTPooleEMEliassenAHHypertension, use of antihypertensive medications, and risk of epithelial ovarian cancerInt J Cancer2016139229129926934358
- GrytliHHFagerlandMWFossaSDTaskenKAHaheimLLUse of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapyProstate201373325026022821802
- GrytliHHFagerlandMWFossaSDTaskenKAAssociation between use of β-blockers and prostate cancer-specific survival: a cohort study of 3,561 prostate cancer patients with high-risk or metastatic diseaseEur Urol201465363564123351721
