Abstract
Aims
To observe the effect of constructed ultrasound microbubble crosslinked to albium nanoparticles packaged with tissue-type plasminogen activator (tPA) gene plasmid on the in vivo transfection.
Methods
The rabbits were chosen for all experiments. A highly expressive gene plasmid for tPA was constructed and packaged into a prepared nanoparticle with bovine serum albumin (BSA). This albium nanoparticle packaged with tPA gene plasmid was crosslinked to an ultrasound microbubble prepared with BSA and sucrose to form a nano-targeting vector system for tPA gene transfection. The transfection and effective expression of tPA in heart, liver, leg skeletal muscle and the cervical rib were detected with polyclonal antibodies to tPA using immunohistochemical method; the tPA level and D-dimer content of blood were also tested.
Results
The expression of tPA could be seen in the tissues mentioned above, with the increase in blood tPA level and D-dimer content from 0.20 ± 0.05 µg/L and 81.76 ± 9.84 µg/L before the operation, to the higher levels of 0.44 ± 0.05 µg/L and 669.28 ± 97.74 µg/L after transfection.
Conclusion
The nano-targeting vector system for tPA gene was contructed successfully. This provides a new theory and experimental method for the nano-targeted transgene.
Introduction
Long-term or lifelong anticoagulant therapy is involved with transluminal balloon angioplasty and stenting,Citation1 coronary bypass,Citation2 and heart valve replacement,Citation3 which may induce bleeding complications. Tissue-type plasminogen activator (tPa) is the main activator of the fibrinolytic system and is synthesized by the vessel endothelial cells and released into the blood circulation under the stimulation of fibrin. The function of tPa is to make the plasminogen transfer to plasmin, which promotes fibrinolysis.Citation4 Because the cDNA of tPa has been cloned successfully, tPa reverse-transcript virus vector can be constructed and transfected in vitro to the epithelial cells with a high expression of tPa protein.Citation5 It has been demonstrated experimentally that thrombosis and restenosis after coronary stenting or bypass can be prevented with a long-term outcome when transfected epithelial cells are spread on the surface of stents or cover vascular anastomosis.Citation6
Human recombinant tPa has also been used clinically in thrombolytic treatments for acute myocardial infarction, brain embolism, and pulmonary infarctionCitation7 with very good results, but it is expensive for patients. We aim to construct the tPa gene plasmid and to transfect it to the cells of a tissue or an organ, followed by long-term anticoagulation and thrombolysis. In our early study, we constructed a tPa gene plasmid and transfected it to pig cardiocytes using the surgery dacron suture as a gene vector, and successfully prevented thrombosis after the valve replacementCitation8 and anastomostic restenosis after coronary bypass.Citation9 Although the surgery dacron suture-carrying gene has been proved to be safe, convenient, and effective, it is traumatic and unfavorable for patients when used as a drug carrier.
Using polymer-constructed nanoparticles as a carrier for gene transfection is a new method that has been used for the past few years.Citation10 Albumin has been used as a material for nanoparticles because of its high biocompatibility and biodegradability with no immunogenicity or cellular toxicity.Citation11 With a positive charge on their surface when pH value in the reactive system is adjusted to acidity, the albumin nanoparticles can not only absorb the gene DNA with a negative charge by a form of static electricity but also carry them in a form of package.Citation12 The main method for in vivo transfection is to inject an objective gene, such as a plasmid DNA or an adenovirus vector, directly to the targeted tissue or cells.Citation13 This method is difficult clinically because of traumatic occlusion.
Ultrasound contrast agents have been used in diagnostic ultrasound imaging in the past few years.Citation14 It has been demonstrated that ultrasound microbubbles can take the drugs or genes to the targeted tissue and cells for the purpose of treatment or transfection safely and effectively.Citation15 The fundamental principles of ultrasound for targeted treatment are i) cavitation and machinery effects produced by the therapeutic ultrasound, which is another kind of ultrasound for therapeutic purposes that uses deep tissue heating, injure the cell membrane slightly and are reversibly followed by an increase in its permeability; ii) the capillary vessels (≤7 mm) in the ultrasound field are injured and the interspaces between endothelial cells become wide, through which drugs or specific genes can get to the targeted tissue and cells;Citation16 and iii) when the ultrasound microbubbles in the blood circulation pass through the tissue or organs treated with the ultrasound, they are quickly destroyed by a force produced in the ultrasound field, and the drugs or genes carried are released to the tissue or organs for the purpose of treatment or transfection. The systemic toxicity and side effects produced by the drugs and genes significantly decrease because of targeted localization.Citation17
In this study, a highly expressive tPa gene plasmid was constructed and a bovine serum albumin (BSA) nanoultrasound microbubble targeting vector for the tPa gene was made. Rabbit heart, liver, and skeleton muscle in the hind leg and rib were chosen as the targeted tissue for the gene transfection.
Materials and methods
Animals
Twenty-five healthy male New Zealand rabbits, 2.0–2.5 kilogram in weight, were used in an experimental study and provided from Southern Medical University Animal Center, GuangZhou, GuangDong Province, China.
Main reagents and instruments
Chinese hamster ovary (CHO) cell lines, pSecTag2B plasmid, E. Coli JM109, rabbit antihuman tPa antibody, FITC-coupled sheep antirabbit immunoglobulin G antibody, rabbit antisheep polyclonal antibody, immunohistochemical reagents, and BSA were purchased from JingMei Biotech, ShenZhen, GuangDong Province, China. Restriction enzymes HindIII, KpnI, BamHI and XhoI, Vent DNA polymerase, T4 DNA ligase, QIA prep spin miniprep kit, QIA quick gel extraction kit, QIAGEN polymerase chain reaction (PCR) product purification kit, and DNA marker DL 2000 were purchased from New England Biolabs, Hong Kong, China. Perfluoropropane (Halocarbon-218) was supplied from JieRui Co. Ltd, FuShan, GuangDong Province, China. The diagnostic ultrasonic generator is a product of NingBo Scientz Biotech Co. Ltd. The therapeutic ultrasound unit (US-700) was made by ITO Co. Ltd, Tokyo, Japan.
Construction and expression of the pSecTag2B tPa gene
Three EST sequences were obtained from Internet Blast according to the tPa gene sequence. The ID numbers were 6251209, 4861268, and 5190656, respectively. The primers were synthesized as follows: tPa-1F: 5′-CCC AAG CTT ATG GAT GCA ATG AAG AGA GGG-3′, tPa-1R: 5′-GGG GTA CCA CGG TAG GCT GAC CCA TTC-3′, tPa-2F: 5′-GGG GTA CCC ACA GCC TCA CCG AGT CG-3′, tPa-2R: 5′-CGG GAT CCA GCA GGA GCT GAT GAG TAT GCC-3′, tPa-3F: 5′-CGG GAT CCT CTC TGC CGC CCA CTG CT′-3′, and tPa-3R: 5′-CCC TCG AGG CGG TCG CAT GTT GTC AC-3′. As the PCR amplification template, three EST clone strains were abstracted and the three tPa fragments amplified. The pSecTag 2B and three tPa fragments tPa-1, tPa-2, and tPa-3 were digested by HindIII and XhoI, HindIII and KpnI, KpnI and BamHI, and BamHI and XhoI, respectively. These enzymatic products were purified with QIAGEN PCR product purification kit and were linked by T4 DNA ligase at 14°C overnight. The linked products were transfected to E. Coli JM109, and the resistance colony in the aminobenzyl penicillin LB plate culture was chosen. This tPa plasmid was sequenced and transfected to CHO cells by calcium phosphate coprecipitation. The expression of tPa was detected using a rabbit antihuman tPa antibody by indirect immunofluorescence method.
Preparation of the BSA nanoparticles loaded with tPa gene plasmid
The preparation of BSA nanoparticles loaded with tPa gene plasmid was carried out according to the methods published by Arnedo et alCitation10 and Zhang et alCitation18 with some improvements. Briefly, 2 mg tPa plasmid DNA was firstly incubated with 10 mL albumin aqueous solution (1% w/v; pH 5.5). Then, the aqueous phase was desolvated with ethanol dropwise (ethanol:water = 2:1). The coacervates were hardened with 30 µL glutaraldehyde (concentration: 0.5%, w/v) for 2 hours. After ethanol was eliminated by evaporation, the nanoparticles were purified by centrifugation at 17,000 rpm for 30 minutes to eliminate free albumin and excess crosslinking agent. The purified nanoparticles by centrifugation were resuspended in pure water and dispersed with ultrasound generator (180 W, 20 kHz, for 30 sec) and stored at 4°C for further use. Some of the nanoparticles were taken for determination of particle size, morphological observation, surface Zeta electric potential, envelopment rate, and electrophoresis for gel retardation.
Preparation of the ultrasonic microbubbles
The preparation of albumin ultrasonic microbubbles was carried out according to the method reported by Du et alCitation19 with some improvements. Ten milliliters of BSA (5%, w/v) with sucrose (final concentration: 10%, w/v) was prepared in 50 mL plastic centrifuge tubes and saturated with oxygen and perfluoropropane (flow rate: 6 mL/min) by turning for 10 minutes and dispersed with ultrasonic generator (180 W, 20 kHz, for 1 min). All procedures were carried out under sterile conditions. The prepared microbubbles were stored at 4°C for further use.
The linkage of the nanopaticles to the microbubbles
The nanopaticles packaged with tPa gene plasmid (containing 1 mg plasmid DNA) were mixed with 5 mL microbubbles (containing 1.2 × 109/mL) at room temperature. Ten microliters of 50% glutaraldehyde (final concentration: 0.1%, w/v) was added to the mixture prepared as stated previously for incubation for 2 hours at 4°C. This linked product was centrifugated at 200 rpm for 1 minute, the floatage was taken and washed with 0.9% sodium chloride under the centrifugation at 200 rpm three times, and the supernatant was stored at 4°C for ultrasound targeted transfection.
Targeted transfection
New Zealand rabbits were used in all experiments. All surgical procedures were carried out on the animals under general anesthesia (25 mg/kg sodium pentobarbital). The heart and liver were observed with two-dimensional diagnostic ultrasound. Soon after injecting 5 mL, the linked ultrasonic microbubbles from the rabbit ear margin vein and a strong resonance of the ultrasound signal were clearly seen. All experimental animals were randomly divided into two groups, the experimental group and the control group. The experimental group (n = 1) was divided into three subgroups, heart (n = 6), liver (n = 6), and skeleton (n = 4). Those organs and the tissue were treated with therapeutic ultrasound (1 MHz, 1.5 w/cm2, 30 min, according to Ling et alCitation20 with some improvements) after injection of the ultrasound microbubbles. The control groups (n = 9) were also divided into three subgroups, signal microbubbles (n = 3) (no ultrasound treatment was operated after the microbubble injection), nanogene (n = 3) (the nanoparticles with tPa gene plasmid without the microbubble linkage were injected into the animals with the treatment of the ultrasound), and blank control (n = 3) (only physiologic saline injection and ultrasound treatment). The whole experiment was observed for 4 weeks after the operation.
Pathology and immunohistochemical stain
All animals were sacrificed after the observation finished. The hearts, livers, targeted muscles, and ribs with ultrasound exposure were taken for pathology and immunohistochemical staining in which a polyclonal antibody to tPa (dilution: 1:100) was used with the indirect immunohistochemical method for tPa targeted expression. Lungs and kidneys were taken for study on targeting specificity and systemic toxicity of the transfected tPa gene. The blood was taken for D-dimer with the immunoturbidimetric method and tPa concentration with the enzyme-linked immunosorbent assay method following the manufacturer’s instruction.
Statistical analysis
The results are expressed in x ± s. The Student’s t-test was used for statistics, with a statistically significant difference of P < 0.05.
Results
Construction of pSecTag2B-tPa and gene expression
The results of the indirect immunofluorescence in the transferred CHO cells are shown in . The strong positive reaction of the fluorescence in the gene-transferred CHO cell is present (: a1). It is negative in the control group (: a2).
Figure 1 a1) A strong positive reaction with tissue-type plasminogen activator (tPa) antibody was present in Chinese hamster ovary cells transfected with tPa plasmid (immunofluorescence 400×). a2) A negative reaction with tPa antibody in the control group (immunofluorescence 400×). b) The results of gel electrophoresis. c) Microbubbles under 400× microscope.
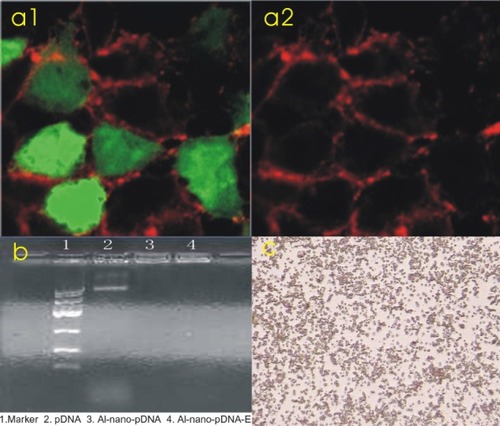
Analytic results for albumin nano-tPa gene plasmid
Particle size analysis showed that the grain size ranged from 49.10 nm to 152.40 nm and was 132 nm, on average, with very even distribution. The polydispersity was 0.33. The surface Zeta potential ranged from +31.32 to +41.42 mV. The enveloping rate was calculated as 73.58% according to the following formula: (Wg − Wf)/Wg × 100%, where Wg is the initial plasmid DNA added, and Wf is the amount of plasmid DNA determined in the supernatants obtained during the purification steps with analysis by ultraviolet spectrophotometer. All of the analytic results met the needs of the experimental study.
Gel retardation and DNase I protection
shows the result of 0.9% agarose gel electrophoresis, from which we can see that the plasmid DNA packaged by the nanoparticles with or without DNase I digestion could not move in the electric field and was retarded in the initial well (DNA:albumin was 1:100). The plasmid DNA nonpackaged with the nanoparticles was digested by the DNase I, and the moving strap in the gel electrophoresis could not be seen, indicating that the nanoparticles could protect the plasmid DNA from DNase I digestion.
Morphological and physical features of ultrasound microbubbles
The size of the prepared microbubbles was 2–5 micrometer in diameter and cystic with very good dispersity, which could fit the vascular ultrasound visualization (). The 10% sucrose–albumin linked to the nanoparticles was heat resistant (40°C, 30 min) and could be stored at 4°C for 30 days without morphological change.
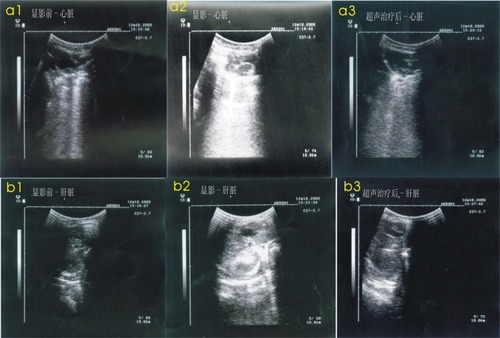
Ultrasound imaging and targeted transfection
shows the changes to the ultrasound images before and after the ultrasound treatment. After venous injection of the microbubbles with tPa gene plasmid, the ultrasound visualization of the heart (: a1, a2, a3) and liver (: b1, b2, b3) obviously increased compared with preinjection and decreased to the normal image following the ultrasound treatment. There was no change in ultrasound visualization in the nanogene and blank control groups.
Figure 2 a1) A normal heart ultrasound image before injection of the microbubbles. a2) An increased heart ultrasound image after injection of microbubbles. a3) A heart ultrasound image after the treatment to return it to normal. b1) A normal liver ultrasound image before injection of the microbubbles. b2) An increased liver ultrasonic image after injection of the microbubbles. b3) A liver ultrasound image after the treatment to return it to normal.
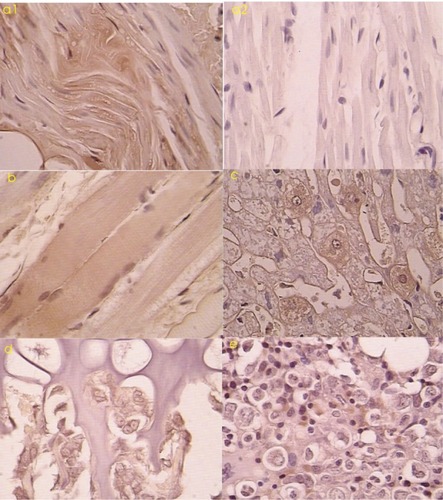
Histopathology and tPa gene expression
Routine pathology showed that myocardial cells, liver cells, and skeletal muscle cells exposed to the ultrasound treatment were larger than those normal cells with the rich cytoplasm. Immunohistochemical analysis was performed on all tissue samples. TPA-positive cells were scattered in the tissue with exposure to the ultrasound treatment. These positive cells included myocardial cells (: a1, a2), skeletal muscle cells (: b), and liver cells (: c). In the liver, these positive cells were mainly distributed near the portal area with a radiating arrangement. In skeleton and cartilage tissue, some chondrocytes in the germinal layer (: d), myeloid cells, and some interstitial cells were tPa positive (: e).
Figure 3 a1) Tissue-type plasminogen activator (tPa)-positive myocardium treated with ultrasound microbubbles (immunohistochemical stain 200×). a2) tPa-negative myocardium treated without ultrasound microbubbles (immunohistochemical stain 200×). b) tPa-positive skeleton muscles after transfection (immunohistochemical stain 200×) c) tPa-positive liver cells after transfection (immunohistochemical stain 400×). d) tPa-positive chondrocytes in the cartilage germinal layer after transfection (immunohistochemical stain 400×). e) tPa-positive myeloid cells and interstitial cells in medulla ossium after transfection (immunohistochemical stain 400×).
Contents of blood D-dimer and tPa
shows the changes in the blood content of D-dimer and tPa before and after the targeted transgene. We could see that the blood contents of D-dimer and tPa increased significantly 4 weeks after the targeted transgene, which indicates a higher fibrinolysis.
Table 1 Blood content of D-dimer and tissue-type plasminogen activator (tPA) before and after the targeting transgene (µg/L, x ± s)
Discussion
We have demonstrated in several experimental studies that the constructed tPa plasmid used in the present study carried by surgery dacron suture or chitosan nanospheres could be transfected in vivo to cardioocytes and skeleton muscle cells with a high expression of tPa protein. But all these methods were traumatic and might be suitable only for experimental or surgical use. Albumin chosen as nanomaterial not only has some benefits as mentioned previously but also was easy to link to the protein ultrasound microbubbles for the purpose of targeting. There are two different methods for nanoparticles to carry genes. One of them is the single-step method, in which a gene is added to the albumin solution before the nanoparticles are prepared, and the gene DNA is encapsulated in the matrix of the nanoparticles. This method has some advantages because of the higher concentration of the drug, delayed and controllable release, enzyme resistance, and a longer time to transfect the gene. Another method is the two-step method, in which the albumin nanoparticles are prepared before the gene is added, and the pH value of the solution is adjusted to be acidic, which makes a more positive charge on the surface of the nanoparticles and links easily to the gene DNA with the negative charge. The nanoparticles made this way are equal in size, and the particle diameter is changed easily but without the advantages mentioned previously.Citation21 In this study, the one-step method was carried out to prepare the albumin nanoparticles packaged with the tPa gene plasmid, which met the needs of the study because of its suitable size (132.0 nm in diameter), good dispersity, high encapsulation rate (73.58%), and effective DNase I protection according to the results of gel electrophoresis.
Determining how to get the albumin nano-tPa gene to the targeted tissue after being injected into the blood is the main purpose in this study. Albumin can be crosslinked to another protein by glutaraldehyde. Thus, when albumin nanoparticles are crosslinked to the fragment of an antibody, they can c onnect to the specific ligand on the surface of the cells in a tissue for the purpose of targeting.Citation22 Albumin has been used as a material for preparation of ultrasonic microbubbles because of its innocuousness and easy preparation.Citation23 We prepared the albumin ultrasound microbubbles and linked them to the constructed albumin nano-tPa plasmid successfully using glutaraldehyde at a final concentration of 0.1% (w/v) because the microbubbles could be denaturated and destroyed easily at a higher concentration (1%, w/v) of glutaraldehyde and could not be crosslinked with the nanoparticles in a lower concentration (0.05%, w/v) of glutaraldehyde.
Some researchers found that the prepared microbubbles could be reinforced when the albumin was mixed with some sucrose (40%, w/v) before the microbubbles were prepared. Half-life was predominantly prolonged from 16 days without sucrose to 6 months with sucrose.Citation19 In our system, we found that when 40% sucrose was used, the solution prepared significantly increased in viscosity and difficulty for ultrasound, which could raise the temperature of the solution and make the albumin degenerate. Also, a high concentration of sucrose is not acceptable for patients to inject into their bodies. Therefore, 10% sucrose was chosen for preparation of the microbubbles with a half-1ife of 30 days and excellent stability. Perfluoropropane is often used for the core of microbubbles, and their stability in blood increased when some oxygen was added before the perfluoropropane was given. This obtains a better imaging effect.Citation19 In this study, oxygen equilibrium before saturated perfluoropropane was used for preparation of the microbubbles.
The microbubbles carried with albumin nano-tPa plasmid could not escape from blood in the circulation because of their size (2–5 µm in diameter) and could take the tPa gene to the tissue and cells for transfection under the guidance of ultrasound, according to the mechanism of ultrasound-targeted transfection. The rabbit hearts, livers, and skeleton muscle tissue of the legs and ribs on the chest was chosen as the target for transfection in this experimental study. The expression of tPa by those cells was detected using immunohistochemical stain 4 weeks after transfection. The expressive intensity of tPa antigen in liver cells, myeloid cells, and germinal cells of the rib cartilages was stronger than that of cardiac muscle cells and skeleton muscle cells. This might be due to the fast growth of the cells in liver and rib cartilage for regeneration to be easily infected, but in the heart and skeleton muscle, the cells grew at resting stage to be hard to be infected.Citation24 TPA expression after the targeted transfection was followed by an increase in blood tPa content and D-dimer level, which reflected the increase in the anticoagulative activity of tPa in the body. For unspecific transfection and systemic toxicity, lung and kidney tissue was also taken for pathological and immunohistochemical observations. Without the ultrasound treatment, this tissue, including the heart, liver, skeleton muscle, and rib tissue, was negatively expressive for tPa antigen and morphologically normal. These results indicated that the transfection of the albumin nano-tPa gene plasmid carried by the ultrasound microbubbles was target specific.
Our initial study indicated that targeted transfection and the increase in expression of albumin nano-tPa gene in specific tissue could be mediated by microbubble rupture triggered by ultrasound. This provides a new and effective method for in vivo transgene and gene therapy for human diseases. However, some problems with gene transfection, such as effectiveness, stability, and manipulability, are not yet solved.
Acknowledgments
This work was supported by National High Technology Research and Development Program 863 (Project: No. 2007AA021809, No. 2007AA021803, No. 2007AA021904).
Disclosure
The authors report no conflicts of interest in this work.
References
- ValgimigliMMinarelliMAntiplatelet and antithrombotic treatment after primary percutaneous coronary intervention: balancing safety and efficacyAm Heart J20101606 SupplS36S4121147290
- MenownIBContemporary management of coronary heart diseaseJR Coll Physicians Edinb20104014447
- EmeryRWKroghCCMcAdamsSLong-term follow up of patients undergoing reoperative surgery with aortic or mitral valve replacement using a St. Jude Medical prosthesisJ Heart Valve Dis201019447348420845896
- WuZJYangSFZhengSSConstruction and biological activities of human tPA eukaryotic expression plasmidHepatobiliary Pancreat Dis Int20043451151515567735
- PodrazikRMWhitehillTAEkhteraeDHigh-level expression of recombinant human tPA in cultivated canine endothelial cells under varying conditions of retroviral gene transferAnn Surg199221644464521417194
- EkhteraeDStanleyJCRetroviral vector-mediated transfer and expression of human tissue plasminigen activator gene in human endothelial and vascular smooth muscle cellsJ Vasc Surg19952169539627776475
- AlbersGWAmarencoPEastonJDAntithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic TherapyChest20041263 Suppl483S512S15383482
- JiSYJiJYangXHLingWPExperimental study on construction of recombinant tPa gene plasmid to prevent the thrombosis after mechanical valve replacementSouth China Journal of Cardiovascular Diseases2006122132135
- JiJZhangHWZhangZJLingWPTransfection of tissue-type plasminogen activator gene to prevent vascular anatomotic restenosis after coronary bypassChina Journal of Modern Medicine2007171416831686
- ArnedoAIracheJMMerodioMEspuelas MillánMSAlbumin nanoparticles improved the stability nuclear accumulation and anticytomegaloviral activity of a phosphodiester oligonucleotideJ Control Release20049421722714684285
- AynieIVauthierCChacunHSpongelike alginate nanoparticles as a new potential system for the delivery of antisense oligonucleotidesAntisense Nucleic Acid Drug Dev1999930131210435755
- LangerKBalthasarSVogelVOptimization of the preparation process for human serum albumin (HSA) nanoparticlesInt J Pharm200325716918012711172
- JiSYJiJYangXHLingWPStudy on construction of nano tPA plasmid to prevent thrombosis after mechanical valve replacement in dogsChina Journal of Modern Medicine2009191929192923
- WangZXWangZGTreatment of ultrasound contrast agents for gene or drug deliveryChin J Interv Imaging200634304308
- PorterTRXieFTherapeutic ultrasound for gene deliveryEchocardiography200118434935311415508
- ZhaoYZLuCRecent advances in the applications of ultrasonic microbubbles as gene delivery systemsActa Pharmaceutica Sinica200742212713117518038
- LaoYXiuJCProgress of ultrasound microbubble contrast agent with gene and drug targeting therapyJ Med Imaging2007171213951361
- ZhangYDGuoYLiuWDAlbumin nanoparticles used as gene carrierChin J Exp Surg2004211214561458
- DuYFWanMXZhaoWStudy on the preparation of a new sugar albumin microbubble ultrasound contrast agentActa Pharmaceutica Sinica20013611859862
- LingZYWangZRanHTExperiment study on ultrasound-mediated microbubble destruction deliver VEGF gene to ischemic myocardium of ratsChinese Ultrasound Med2002187502504
- ArnedoAEspuelasSIracheJMAlbumin nanoparticles as carriers for a phosphodiester oligonucleotideInt J Pharm2002244597212204565
- LiuXBCaiMYPreparation and immunological characterization of mitoxantrone-loaded immuno-nanoparticles against human hepatomChinese Journal of Immunology200016526222652622269
- WangKMaGHPreparation and characterization of albumin nanospheres as drug carrierChinese Journal of Process Engineering200442156159
- OuDBZhengQSEmbryonic stem cell differentiation towards cardiomyocytes and its application in cardiac tissue engineeringJournal of Clinical Rehabilitative Tissue Engineering Research2008123467196722