Abstract
Background
Cholangiocarcinoma (CCA) is the primary type of bile duct cancer with high morbidity and mortality, particularly in patients with advanced-stage disease. Treatment of CCA remains unsatisfactory due to the lack of sensitive and specific diagnostic tool for early detection as well as effective chemotherapeutics.
Purpose
To investigate cytotoxic interactions between the three major constituents of the rhizomes of Atractylodes lancea (Thunb.) DC., ie, β-eudesmol (BE), atractylodin (AT), and hinesol (HS), against CCA cell line.
Methods
Cytotoxic activities against the human CCA cells CL-6 of the dual (BE:AT, BE:HS, and AT:HS) and triple (BE:AT:HS) combinations were evaluated using MTT assay. The cytotoxic interaction of each dual combination was assessed at five concentration ratios (10:0, 7:3, 5:5, 3:7, and 0:10) using isobologram analysis. For triple combination, the concentration ratio used in the experiment was 1:1.5:2.5 (BE:AT:HS) and analysis of the interaction was performed using polygonogram analysis at the concentrations that inhibit cell growth by 50% and 90%, respectively.
Results
The BE:AT combination produced the additive effect with sum fractional inhibitory concentration of 0.967±0.02 (mean ± SD). The BE:HS and AT:HS combinations produced a synergistic effect with sum fractional inhibitory concentrations of 0.685±0.08 and 0.767±0.09, respectively. The mixture of the three compounds produced synergistic interaction with combination index values of 0.519±0.10 and 0.65±0.17 (mean ± SD) at the concentrations that inhibit cell growth at the 50% and 90% leveled, respectively.
Conclusion
Results obtained would guide further development of Atractylodes lancea (Thunb.) DC. as potential anti-CCA chemotherapeutics concerning the appropriate pharmaceutical dosage form.
Introduction
Cholangiocarcinoma (CCA) is cancer that arises within the bile duct both inside and outside the liver. The cholangiocytes can be transformed into cancer cells by direct activation from carcinogens, or metastasis of other cancers, particularly hepatocellular carcinoma.Citation1,Citation2 It is one of the most challenging types of cancer due to the lack of tools for early diagnosis as well as effective chemotherapeutics. The significant risk factor for CCA in People’s Republic of China, Korea, Japan, and Southeast Asian countries including Thailand is the consumption of improperly cooked cyprinoid fish which contains infective metacercaria of the liver fluke Opisthorchis viverrini, O. felines, and Clonorchis sinensis, together with dimethylnitrosamine (DMN) from fermented meat.Citation3–Citation5 In Thailand, CCA shared approximately 11% of the cases among new cancer cases reported in 2015, with the highest incidence in the Northeastern region (about 60% of all cases).Citation6 The efficacy of existing chemotherapy and radiotherapy are limited only to patients with early-stage disease.Citation7,Citation8 Treatment efficacy of the standard drugs 5-fluorouracil (5-FU), gemcitabine, or combinations of these drugs with cisplatin is unsatisfactory with low survival rate and high metastatic rate.Citation9 Less than 5% of the advanced-stage patients survive for up to 5 years.Citation10,Citation11 Research on discovery and development of new effective alternative drugs is urgently required to control this type of cancer.
Numerous studies have been carried out in efforts to discover effective cancer chemotherapeutic agents from plant sources with low toxicity. Histological evidence support the role of plants as promising sources of anticancer drugs, ie, vincristine, vinblastine, etoposide, teniposide, paclitaxel, vinorelbine, docetaxel, topotecan, and irinotecan.Citation12 With regard to CCA, a number of plants and plant-derived compounds have been investigated for their anti-CCA potentials both in vitro and in animal models. These include triptolide from Tripterygium wilfordii,Citation13 ubiquitous tannic acid,Citation14 and Atractylodes lancea (Thunb.) DC. (AL) and its isolated compounds (β-eudesmol [BE], atractylodin [AT], atractylon, hinesol [HS], etc).Citation15–Citation19 The interest of our research group has been focusing on research and development of AL as a potential chemotherapeutic for CCA. AL is the therapeutic plant growing in tropical and subtropical zones of Asia such as People’s Republic of China, Japan, and Thailand. Its dried rhizome is commonly used in Chinese (“Cang Zhu”), Japanese campo (“So-jutsu”), as well as Thai (“Khod-Kha-Mao”) traditional medicines. In Chinese traditional medicine, AL has been used as an important crude drug for treatment of rheumatic diseases, digestive disorders, night blindness, and influenza.Citation20 The pharmacological activities of AL rhizomes have previously been reviewed.Citation21 Phytochemical investigations have revealed a series of sesquiterpenoids, monoterpenes, polyacetylenes, phenolic acids, and steroids from AL rhizomes.Citation22–Citation29 The major constituents are AT (14%), BE (6%), atractylon (2%), and HE (1%). Results from a series of our study confirm anti-CCA potential and safety profiles of both the crude ethanolic extract and the purified compound BE both in vitro and in animal models.Citation16–Citation19 The present study aimed to investigate the cytotoxic interactions on human CCA between the three major isolated compounds from the AL rhizome, ie, BE, AT, and HS.
Methods
Cell culture and test compounds
The CCA cell line CL-6 was kindly provided by Associate Professor Dr Adisak Wongkajornsilp, Department of Pharmacology, Faculty of Medicine (Siriraj Hospital), Mahidol University. The experimental use of the CL-6 cell was approved by Thammasat University Biosafety Committee (008/2561). The cell was cultured in Roswell Park Memorial Institute 1640 medium (Gibco Co. Ltd., Grand Island, NY, USA). The culture medium was supplemented with 10% (v/v) heated fetal bovine serum and 100 IU/mL of antibiotic–antimycotic solution (Gibco Co. Ltd.). All cells were maintained at 37°C in 5% CO2 atmosphere and 95% humidity (HERACELL 150i, Thermo Scientific, Waltham, MA, USA).
The bioactive constituents of AL rhizome under investigation, ie, BE, AT, HS, and 5-FU were purchased from Wako (Wako Ltd., Osaka, Japan). The stock solution of each compound was prepared in 50% ethanol at the concentration of 5 mg/mL, and the working solution was prepared freshly before use.
Cytotoxic assay
The cytotoxic effects of dual and triple combinations of BE, AT, and HS on CL-6 were evaluated using MTT assay (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. The cells (0.75×104 cells/well) were seeded into each well of a 96-well microtiter plate and incubated at 37°C under 5%CO2 atmosphere for 24 hours. Cell confluence was examined under the light microscope.
Analysis of the cytotoxic activity of dual combinations
The cytotoxic interaction of each dual combination, ie, BE-AT, BE-HS, or AT-HS was evaluated at the five concentration ratios (10:0, 7:3, 5:5, 3:7, and 0:10), with a serial dilution for each combination pair. The highest concentration used was 200 µg/mL (899 µM BE, 1098 µM AT, and 899 µM HS). Following incubation at 37°C for 48 hours, 20 µL of the MTT reagent was added into each well and further incubated for an additional 4 hours. The cell suspension was carefully removed, and dimethyl sulfoxide (Ameresco, Solon, OH, USA) was added, and the absorbance was measured at 565 nm within 15 minutes. 5-FU was used as a reference compound for comparison of cytotoxic activity. The experiment was performed at least three times, each time in triplicate. The concentration that inhibits cell growth by 50% (IC50) of each compound (from the starting ratios of 10:0 and 0:10) and was estimated using Calcusyn™ (Biosoft, Cambridge, UK). The fractional inhibitory concentration (FIC) index of each combination pair (representing combination scores) and the sum FIC of five distinctive ratios was calculated as the ratio of IC50 of the combination and that of each compound alone. The isobologram of each combination interaction was generated from the average sum FIC index (sum of the IC50 of each combination pair divided by IC50 of each single compound). Sum FIC values indicate the types of cytotoxic interactions: synergism if sum FIC <1, addition if sum FIC =1, and antagonism if sum FIC >1.
Analysis of the cytotoxic activity of triple combinations
The concentration ratio used for evaluation of cytotoxic activity of the triple combination (BE:AT:HS) was based on the previously reported average IC50 value of each compound from our laboratory (20.1, 31.0, and 52.3 µM for BE, AT and HS, respectively).Citation15,Citation16 The estimated combination ratio of BE:AT:HS used in the experiment was 1:1.5:2.5. The experiment was performed as previously described for the dual combinations (three independent experiments, triplicate each). The combination index (CI) value at the IC50 or IC90 was determined as follow: (IC50 [or IC90] of BE in the triple combination divided by IC50 [or IC90] of BE alone) + (IC50 [or IC90] of AT in the triple combination divided by IC50 [or IC90] of AT alone) + (IC50 [or IC90] of HS in the triple combination divided by IC50 [or IC90] of compound HS alone). For analysis of combination interaction, the polygonogram was generated using CompuSyn™ (ComboSyn Inc., Paramus, NJ, USA). Criteria for classification of the types of cytotoxic interaction are as follows: very strong synergism if CI <0.1, strong synergism if CI =0.1–0.3, synergism if CI =0.3–0.7, moderate synergism if CI =0.7–0.85, slight synergism if CI =0.85–0.90, nearly additive if CI =0.9–1.10, slight antagonism if CI =1.10–1.20, moderate antagonism if CI =1.20–1.45, antagonism if CI =1.45–3.3, strong antagonism if CI =3.3–10, and very strong antagonism if CI >10.Citation30,Citation31
Results
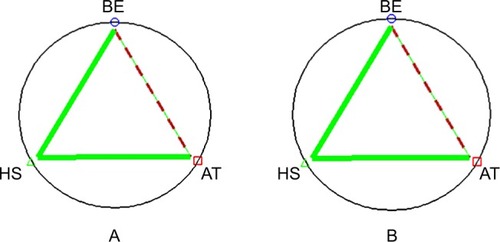
The IC50 values (mean ± SD) of BE, AT, HS and 5-FU were 21.5±2.12, 24±1.98, 91.81±8.0, and 122±2.08 µM, respectively. For dual combinations, the sum FIC indexes (mean ± SD) indicative of types of drug interaction were 0.967±0.02 (BE:AT), 0.685±0.08 (BE:HS), and 0.767±0.09 (AT:HS), suggesting additive, synergistic, and synergistic interaction, respectively. The IC50 of BE, AT, and HS alone were 21.5±2.12, 24±1.98, and 91.81±8.0 µM, respectively. The IC50 of the reference drug 5-FU was 90±7.9 µM. The isobolograms of FICs of the three dual combinations are illustrated in (). For triple combinations, the CI (mean ± SD) values at the IC50 and IC90 levels were 0.519±0.10 and 0.65±0.17, indicating synergistic and moderately synergistic interaction, respectively ().
Figure 1 Isobolograms of the sum FIC values (mean ± SD) of the dual combinations of the three compounds isolated from AL and types of interactions: (A) BE and AT combination (additive effect with sum FIC of 0.967±0.02); (B) AT and HS (synergistic effect with sum FIC of 0.685±0.08); and (C) BE and HS (synergistic effect with sum FIC of 0.767±0.09).
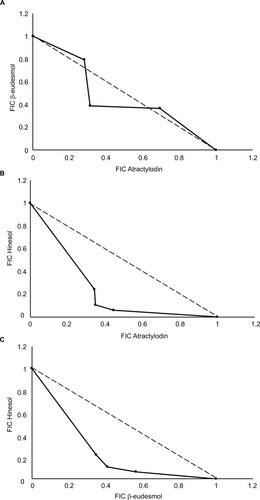
Figure 2 Polygonograms of the sum CI values (mean ± SD) of the triple combinations of BE:AT:HS (concentration ratio =1:1.5:2.5) at (A) IC50 and (B) IC90. The solid lines represent synergism; dashed lines represent nearly additive effect).
Discussion
Traditional herbal products are heterogeneous in nature. It is likely that the existence of various components from the plant parts used could act synergistically or at least additively and provide therapeutic advantages over the use of a single active constituent isolated from the plant with regard to both clinical efficacy and tolerability. This supports the traditional uses of herbal medicines as concoction for most diseases or pathological conditions. The study was the first that confirmed the cytotoxic synergistic interaction of the three major compounds from AL rhizome, ie, β-eudesmol (BE), atractylodin (AT), and hinesol (HS), on the human CCA cell line CL-6. Based on the IC50 value, BE or AT alone exerted its cytotoxic potency of about four times that of the reference drug 5-FU, while the potency of cytotoxic activity of HS alone was similar to 5-FU. Interestingly, the dual and triple combinations containing the HS component (BE:HS, AT:HS, BE:AT:HS) produced synergistic interaction. On the other hand, the BE:AT combination produced only additive interaction despite relatively higher amounts of both compounds compared to BE:HS or AT:HS combination (6%, 14%, and 1% for BE, AT and HS, respectively), and in addition, relatively weak cytotoxic activity of HS (IC50: 21.5, 24.0 and 91.8 µM for BE, AT, and HS, respectively). Results of this in vitro study confirmed that of the previous in vivo studies in the CCA-xenografted nude mouse model which demonstrated potent activity of the AL extract and BE at tolerable dose levels.Citation16–Citation18 It is noted however that, with the relatively low amounts of all the three constituents in the AL extract, its anti-CCA activity observed in the in vivo CCA-xenografted mouse and O. viverrini/dimethylnitrosamine-induced CCA hamster models was shown to be higher than the isolated compound BE alone.Citation17–Citation19 Significant inhibitory effects of AL extract on CCA tumor growth (2% of untreated control) were observed at the dose levels of 1,000, 3,000 and 5,000 mg/kg body weight, while the inhibitory effect of only 70% was found in the 5-FU treated mice.Citation17 The survival time of mice was about 3–4 times more prolonged following all dose levels of AL extract compared with 5-FU and untreated control. More importantly, the extract also produced potent inhibitory effects on lung metastasis. The AL extract at 5,000, 3,000, 1,000 mg/kg body weight and 5-FU and untreated control, respectively, resulted in 95%, 75%, 30%, 50%, and 10% inhibition of lung metastasis. Comparable anti-CCA activity and inhibitory activity on lung metastasis were also observed with BE.Citation19 In the hamster model, the promising anti-CCA activity of AL was observed at all dose levels, particularly at the highest oral dose level of 5,000 mg/kg body weight for 30 days. The median survival time was significantly prolonged (about two times) in mice treated with the extract at all dose levels compared with 5-FU treated and untreated control groups.Citation19 Altogether, results suggest that the three major compounds in the AL rhizomes play an important role in the anti-CCA activity of AL. The study supports synergistic interaction of the active ingredients observed in all animal models.Citation16–Citation19 Other constituents including atractylon (2% yield) as well as other constituents may also interact additively or synergistically with these compounds to exert promising anti-CCA activity of the AL extract. It is noted however that results of the in vitro and in vivo studies, particularly with different CCA cell types in various CCA cells, may be different due to complex environment. CCA encompasses at least three pathological conditions, ie, intrahepatic, hilar, and distal CCA, which respond differently to chemotherapeutic drugs. The CL-6 cell line used in the study was obtained from patients with intrahepatic CCA, which is the most common CCA type in Southeast Asia. Nevertheless, studies in other types of CCA are encouraged for further development of AL for CCA. The potential anticancer and antiangiogenesis properties of AL extract and these major constituents, particularly BE, have been well demonstrated in various types of animal and human cancers, these include murine blastoma cells, HeLa (human cervical cells), SGC-7901 (human gastric cancer cells), BEL-7402 (human liver cancer cells), H33, S180, HL-60 leukemic cells, and gastric cancer.Citation32–Citation36 For CCA, the cytotoxic activity, anticlonogenic activity, antioxidative activity, and inhibitory activity on cell invasion and angiogenesis of the crude ethanolic extract of AL have previously been demonstrated by our group.Citation16
Oral pharmaceutical formulation of the standardized crude extract rather than each isolated compound should be considered for further development of AL as promising anti-CCA chemotherapeutics. The multi-ingredient characteristics of the plant extract would be expected to optimize therapy regarding both efficacy (synergistic anti-CCA activity) and tolerability (buffering effect). Apart from therapeutic benefit, this approach offers an advantage over the isolated compounds concerning both time consumption and resource consumption during the development process. The strategy of drug development based on multiple targets of action using systems pharmacology, rather than a single molecular target, would be a more suitable approach for investigation of mechanisms of action of herbal medicines.
Conclusion
The results obtained would guide further development of Atractylodes lancea (Thumb.) DC. as potential anti-CCA chemotherapeutics concerning the appropriate pharmaceutical dosage form.
Acknowledgments
The study was supported by Thammasat University, Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma of Thammasat University; National Research Council of Thailand (NRCT) and National Research University Project of Thailand (NRU); and the Office of Higher Education Commission of Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
- RenshawKMalignant neoplasms of the extrahepatic biliary ductsAnn Surg192276220522117864681
- LazaridisKNGoresGJCholangiocarcinomaGastroenterology200512861655166715887157
- SripaBKaewkesSSithithawornPLiver fluke induces cholangiocarcinomaPLoS Med200747e20117622191
- Leyva-IlladesDMcmillinMQuinnMDemorrowSCholangiocarcinoma pathogenesis: Role of the tumor microenvironmentTransl Gastrointest Cancer201211718023002431
- RizviSGoresGJMolecular Pathogenesis of CholangiocarcinomaDig Dis201432556456925034289
- National Cancer Institute of ThailandHospital-based cancer registry annual reportBangkokNational Cancer Institute of Thailand2015
- NakeebAPittHARadiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinomaHPB20057427828218333207
- Ramírez-MerinoNAixSPCortés-FunesHChemotherapy for cholangiocarcinoma: An updateWorld J Gastrointest Oncol20135717117623919111
- BridgewaterJGallePRKhanSAGuidelines for the diagnosis and management of intrahepatic cholangiocarcinomaJ Hepatol20146061268128924681130
- CiomborKKGoffLWAdvances in the management of biliary tract cancersClin Adv Hematol Oncol2013111283423416860
- RogersJELawLNguyenVDSecond-line systemic treatment for advanced cholangiocarcinomaJ Gastrointest Oncol201456371413
- DholwaniKKSalujaAKGuptaARShahDRA review on plant-derived natural products and their analogs with anti-tumor activityIndian J Pharmacol2008402495821279166
- TengchaisriTChawengkirttikulRRachaphaewNReutrakulVSangsuwanRSirisinhaSAntitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamstersCancer Lett1998133216917510072166
- NausPJHensonRBleekerGWehbeHMengFPatelTTannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathwaysJ Hepatol200746222222917069924
- MahavorasirikulWViyanantVChaijaroenkulWItharatANa-BangchangKCytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitroBMC Complement Altern Med2010105520920194
- PlengsuriyakarnTViyanantVEursitthichaiVCytotoxicity, toxicity, and anticancer activity of Zingiber officinale Roscoe against cholangiocarcinomaAsian Pac J Cancer Prev20121394597460623167387
- PlengsuriyakarnTViyanantVEursitthichaiVAnticancer activities against cholangiocarcinoma, toxicity and pharmacological activities of Thai medicinal plants in animal modelsBMC Complement Altern Med2012122322448640
- PlengsuriyakarnTKarbwangJNa-BangchangKAnticancer activity using positron emission tomography-computed tomography and pharmacokinetics of β-eudesmol in human cholangiocarcinoma xenografted nude mouse modelClin Exp Pharmacol Physiol201542329330425545782
- PlengsuriyakarnTMatsudaNKarbwangJViyanantVHirayamaKNa-BangchangKAnticancer Activity of Atractylodes lancea (Thunb.) DC in a Hamster Model and Application of PET-CT for Early Detection and Monitoring Progression of CholangiocarcinomaAsian Pacific Journal of Cancer Prevention201516156279628426434829
- OuyangZZhangLZhaoMWangPWeiYFangJIdentification and quantification of sesquiterpenes and polyacetylenes in Atractylodes lancea from various geographical origins using GC-MS analysisRevista Brasileira de Farmacognosia2012225957963
- Na-BangchangKPlengsuriyakarnTKarbwangJResearch and Development of Atractylodes lancea (Thunb) DC. as a Promising Candidate for Cholangiocarcinoma ChemotherapeuticsEvid Based Complement Alternat Med20172017592923411629348769
- ChenZ-LCaoW-YZhouG-XWichtlMA sesquiterpene lactam from Artractylodes macrocephalaPhytochemistry1997454765767
- KanoYKomatsuK-IchiSaitoK-IchiBandoHSakuraiTA new polyacetylene compound from Atractylodes rhizomeChem Pharm Bull1989371193194
- YaharaSHigashiTIwakiKStudies on the constituents of Atractylodes lanceaChem Pharm Bull1989371129953000
- ReschMSteigelAChenZLBauerR5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lanceaJ Nat Prod19986133473509544564
- ZhangYXuSLinYSbXLinCGastrointestinal inhibitory effects of sesquiterpene lactones from Atractylodes macrocephalaZhong Yao Cai1999221263664012571906
- DingH-YWuY-CLincH-CPhytochemical and Pharmacological Studies on Chinese ChangzhuJ Chin Chem Soc2000473561566
- ReschMHeilmannJSteigelABauerRFurther phenols and poly-acetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activityPlanta Med200167543744211488458
- KitajimaJKamoshitaAIshikawaTGlycosides of Atractylodes lanceaChem Pharm Bull200351667367812808245
- ChouTCMartinNCompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 ValuesParamus, NJComboSyn Inc.2005
- ChouTCTheoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studiesPharmacol Rev200658362168116968952
- TsunekiHMaELKobayashiSAntiangiogenic activity of beta-eudesmol in vitro and in vivoEur J Pharmacol20055122-310511515840394
- MaELLiYCTsunekiHElMYcLBeta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferationJ Asian Nat Prod Res2008101-215916718253884
- MazzioEASolimanKFIn vitro screening of tumoricidal properties of international medicinal herbs: part IIPhytother Res201024121813182420564497
- ZhaoJHSunJXGaoPFast-track surgery versus traditional perioperative care in laparoscopic colorectal cancer surgery: a meta-analysisBMC Cancer20141460725148902
- MasudaYKadokuraTIshiiMTakadaKKitajimaJHinesol, a compound isolated from the essential oils of Atractylodes lancea rhizome, inhibits cell growth and induces apoptosis in human leukemia HL-60 cellsJ Nat Med201569333233925833731
