Abstract
Introduction
Bronchodilators are the cornerstone of chronic obstructive pulmonary disease (COPD) therapy and long-acting muscarinic antagonists (LAMAs) as a mono or combination treatment play a pivotal role. Several LAMAs are already available on the market in different formulations, but developing a new compound with a higher M3 receptor selectivity and a lower affinity to M2 receptors to increase the therapeutic effect and minimize the adverse effects is still a goal. Moreover, new formulations could improve adherence to therapy.
Areas Covered
This systematic review assesses investigational long-acting muscarinic antagonist in Phase I and II clinical trials over the last decade. It offers insights on whether LAMAs and/or their new formulations in clinical development can become effective treatments for COPD in the future.
Expert Opinion
Research on LAMA seems to have come to a standstill, the few new molecules under study do not show distinctive characteristics compared to the previous ones. Muscarinic antagonist/β2-agonist (MABAs) appear to be the major innovation currently under investigation, and they could theoretically open new research frontiers on the effect between adrenergic and muscarinic interaction in the same cell.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease characterized by persistent airflow limitation. Exacerbations and respiratory symptoms, which worsen quality of life and increase morbidity and mortality, are common manifestations of the disease.Citation1 In COPD, the dysfunction in autonomic nerve regulation of airway smooth muscle tone plays a pivotal role in sustaining the airflow limitation and it is the primary reversible component.Citation2 Therefore, bronchodilators such as long-acting β2 adrenoceptor agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are the main therapy of COPD in any stage, either in monotherapy or in associations.
LAMAs inhibit the effect of acetylcholine (ACh), the parasympathetic nervous system neurotransmitter, at both the ganglionic transmission and the neuroeffector junction levels. There are five G-protein-coupled receptors (M1– M5), which have distinct anatomical distribution and functions.Citation3 The M3 subtypes are mostly present as postjunctional muscarinic receptors (mAChR) in the airway smooth muscles (ASM) of subsegmental bronchus and are mostly absent in lung parenchyma; their inhibition is responsible for the therapeutic bronchodilation. The M2 subtype receptors are prejunctional mAChRs, and even if they are abundant in the lung, they act as autoreceptors that regulate Ach release.Citation4 Conversely, M2 is the predominant muscarinic receptor subtype in the heart, and the main adverse effects (AEs) of LAMAs, such as tachycardia or prolonged QT, are attributed to the inhibition of these receptors.Citation5 Therefore, therapeutic LAMAs for COPD should have a higher kinetic selectivity for M3 receptors and a low affinity for M2 receptors to minimize cardiological AEs.
LABAs are the other cornerstone of bronchodilation therapy. In moderate to severe COPD, several studies have demonstrated that the combination of a LAMA with a LABA provides a superior bronchodilation effect without increasing AEs.Citation6 The pharmacological mechanism is due to the fact that while LAMAs inhibit the parasympathetic pathway, LABAs stimulate the sympathetic pathway. Since the muscarinic and adrenergic receptors reside on the same ASM cell, the intriguing question is whether a molecule that can act on both receptors at the same time can have an even greater effect than two molecules that act on the same receptors but at different times, or on different cells.Citation7 Pharmaceutical companies have been developing bi-functional (or dual pharmacophore) muscarinic antagonist/β2-agonist (MABA) agents for several years; these agents combine muscarinic antagonism and β2-agonism in a single molecule. This would be a really innovative class of drugs, although with an old approach to the disease.
This review will focus on the state of the development of new LAMAs during the last ten years, illustrating the main pharmacological characteristics and describing the main studies, then it will analyse MABAs, a new class of drugs for the treatment of COPD.
Methods – Search Strategy and Selection Criteria
Literature search was conducted in MEDLINE and Embase databases via Ovid, to identify English-language articles published between January 1, 2010, and August 31, 2020, using the following search terms: Long-acting muscarinic antagonists AND chronic obstructive pulmonary disease. The search was limited to study phase I and Phase II clinical trials. Filters were applied to exclude reviews, letters, and information presented at scientific conferences. Because of the small number of selected articles, the search was expanded to clinicaltrial.gov, clinicaltrialsregister.eu, and jmacct.med.or.jp, identifying phase I, phase II and Phase III studies on LAMAs in COPD that recruited patients from January 1, 2010. Moreover, the name of each compound was independently searched on PubMed to avoid missing data.
New LAMAs – Phase I and Phase II Studies
Over the past decade, several LAMAs have been investigated in phase I and phase II trials. Some of these molecules have been abandoned or used in other diseases, others are still in development and, to date, only one has been approved by the Food and Drug Administration (FDA) (, ).
Table 1 Long-Acting Muscarinic Receptor Antagonists (LAMA) in Clinical Development
Figure 1 Muscarinic receptor antagonists in clinical development for the treatment of COPD. Image of the QAX028ʹ molecule is not available.
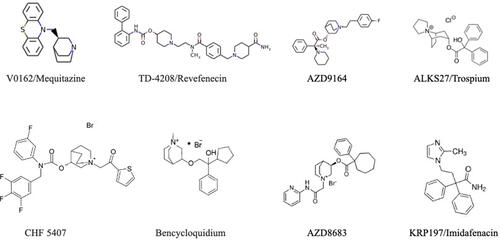
V0162/Mequitazine
V0162 is an anticholinergic compound developed by Pierre Fabre Medicament as an inhalation powder. It is the dextrorotatory enantiomer of mequitazine, an antihistaminic drug commercialized for over 30 years, with one of the highest affinities for muscarinic receptors. The molecular formula of V0162 is 10-[(3R)-1-azabicyclo[2.2.2]oct-3-ylmethyl]-10H-phenothiazine and its molecular weight is 322 g/mol. V0162 has high affinity for M3 mAChRs and displayed rapid off-kinetics with a biphasic time course of binding. V0162 acts as an inverse agonist for M3 mAChR-mediated reporter gene activation, with quite the same efficacy as atropine, ipratropium bromide (IPRA) and tiotropium bromide (TIO), but in contrast with TIO and atropine, V0162’s inhibitory potency was only slightly affected by compound washout.Citation8 Preclinical results showed that V0162 was able to prevent bronchoconstriction induced either by ACh or histamine, and reversed the bronchoconstriction and airway inflammation caused by ovalbumin challenge in sensitized guinea pigs.Citation9 Therapeutic efficacy of V-0162 was investigated in two randomized clinical trials (NCT01348555 and NCT01951222).
In a phase I/II combined study, a dose-escalation study assessing safety in healthy volunteers was followed by a proof-of-concept study in COPD patients, to test the efficacy and safety of V0162.
In 66 healthy subjects, the maximum dose without any AEs was 2400 μg. In 20 COPD subjects, 1600 μg of V0162 increased Forced Expiratory Volume in 1 second (FEV1) by 148 ± 137 mL, compared to 36 ± 151 mL with the placebo (p = 0.003), and reduced hyperinflation. There also was a non-statistically significant reduction of dyspnoea. No serious AEs were reported and the most common AEs were productive and non-productive cough, dyspnoea and pruritus, suggesting that the slight plasmatic exposure observed could support the good safety profile.Citation9 A randomized clinical trial to assess the bronchodilator properties of V0162 during 8 days in adult patients with asthma usually treated with Inhaled Corticosteroid (ICS) and LABA has been registered, but no data is available and no further clinical development trials on this molecule are known.
TD-4208/Revefenecin
Revefenacin/TD-4208 (REV) inhalation solution is a once-daily nebulized LAMA developed by Theravance Biopharma and Mylan, which received its first global approval in the USA on November 9th, 2018, as maintenance treatment of patients with COPD.Citation10 The approved dosage of REV inhalation solution is 175 µg once daily, delivered via a standard jet nebulizer connected to an air compressor.
REV is a potent lung-selective LAMA that competitively and reversibly binds to the M3 receptors in the airway smooth muscle, thereby inhibiting bronchoconstriction and increasing bronchodilation, and also displays kinetic functional selectivity for M3 compared to M2 receptors, with dissociation half-life significantly longer for M3 than for M2 receptors.Citation11 A dose-dependent bronchoprotection against ACh- or methacholine-induced bronchoconstriction has been observed in anesthetized dogs and rats that received single or repeated dosing (once daily for 7 days) of inhaled REV.Citation12
As for REV pharmacokinetics (PK), peak plasma concentrations (Cmax) of the drug and its active metabolite occur 14–41 min after nebulization, and the absolute bioavailability of orally administered REV is very low (<3%). Steady state was reached within 7 days of repeated inhaled administration of REV, and mean steady-state volume of distribution following intravenous administration in healthy volunteers was 218 L. In vitro human plasma protein binding for REV and its active metabolite, respectively, averaged 71% and 42%, while their elimination half-life after once-daily dosing of inhaled REV in COPD patients was 22–70 h. In the NCT01704404 phase II trial, REV demonstrated a rapid onset and sustained duration of bronchodilator effect over 24 h following once-daily single or multiple nebulized-dose administration in patients with COPD. A population PK analysis indicated that systemic exposure of REV and its active metabolite is not significantly affected by age, gender, smoking status or weight,Citation13 increased modestly in subjects with severe renal impairment but was similar between subjects with moderate hepatic impairment and normal hepatic function.Citation14
Cardiovascular safety of REV for nebulization has been assessed in randomized clinical trials.Citation15–Citation17 In 48 healthy subjects that received a therapeutic (175 µg) or supratherapeutic (700 µg) dose of inhaled REV no clinically meaningful negative effects were observed (NCT02578082) and in two 12-week phase III efficacy trials and in a 52-week phase III safety trial in patients with moderate to very severe COPD, long-term use of inhaled REV had no clinically relevant effect on QTc interval compared to placebo.Citation16
In two phase II trials on COPD patients (NCT01704404 and NCT02109172), REV administered in a single or multiple nebulized-dose showed a rapid onset and sustained duration of bronchodilator effect over 24 h following once-daily administration, with a PK profile that is commensurate with low systemic exposure.Citation18
In a phase II multiple-dose trial (NCT02040792), 355 COPD patients were randomized in a double-blind, placebo-controlled parallel group study and once-daily treatments (44, 88, 175 or 350 μg REV or matching placebo) were administered through a standard jet nebulizer for 28 days. REV (88, 175 and 350 μg) significantly improved day 28 trough FEV1 over placebo; 44 μg produced a sub-therapeutic response while the 350 μg dose did not demonstrate additional efficacy over the 175 μg REV. REV was generally well tolerated, with minimal reports of systemic anti-cholinergic effects.Citation19 This data suggests that the doses of 88 μg and 175 μg of REV are appropriate for use in longer-term safety and efficacy trials.
In two phase III studies (NCT02459080 and NCT02512510), REV showed significantly greater improvements in FEV1 versus placebo among COPD patients, furthermore, there was a greater number of St. George’s Respiratory Questionnaire and transition dyspnea index responders.Citation20
In a randomized, parallel-group, 52-week trial (NCT02518139), 1055 participants with moderate to very severe COPD received REV 88 μg or 175 μg in a double-blind manner, or open-label active control TIO. REV was generally well tolerated over 52 weeks of treatment, and had a safety profile that supported its use as a long term once-daily nebulized bronchodilator for the treatment of COPD.Citation20
In the NCT03573817 phase III trial, the safety profile of REV was similar to formoterol (FORM) alone when delivered sequentially or combined. There was no change in the safety profile when REV was added to FORM, whether administered sequentially or combined via a standard jet nebulizer. There were significant improvements in trough FEV1 from baseline when REV was given sequentially or combined with FORM.Citation21
To date, the RARICO Study, a phase II, pragmatic, randomized, controlled, double-blinded, multi-centre trial to evaluate the safety and feasibility of nebulized REV in comparison to nebulized IPRA in patients with COPD and acute respiratory failure requiring invasive mechanical ventilation has been registered on clinicalTrial.gov as NCT04315558 but is not recruiting yet.
CHF 5407
Chiesi Farmaceutici began developing a nebulised formulation of CHF-5407, a novel quaternary ammonium salt acting as a potent antagonist of human M3 receptors.Citation22 The only previous data are from a 2010 in-vitro study that showed that the dissociation t½ from M3 receptors was comparable to TIO, but with a more rapid dissociation from M2 receptors. Moreover, in isolated human bronchus CHF-5407 produced a similar inhibition of carbachol-induced contractions to TIO bromide and a longer lasting inhibition compared to glycopyrronium bromide (GLY). CHF-5407 has subnanomolar affinity for human M1-3 receptors and no selectivity across receptor subtypes, similar to other LAMAs in development. In vivo, the bronchodilator profile of CHF-5407 is comparable to TIO.Citation23 A phase I clinical trial on CHF-5407 was reported, although no data was published and no other studies are registered on Clintrial.gov.Citation22 Although these preliminary data were promising, research on this molecule is currently at a standstill, probably due to the developing pharmaceutical company’s use of GLY.
Bencycloquidium
Bencycloquidium (BCQB) is a quaternary ammonium salt with anticholinergic properties and a high affinity for the M3 receptor. BCQB showed a faster onset and slower offset than TIO in function studies, and treatment with intratracheally instilled or inhaled BCQB protected against methacholine or antigen-induced bronchoconstriction in a dose-dependent manner in normal and sensitized guinea pigs in vivo.Citation24 In mice sensitized and challenged with ovalbumin to develop airway inflammation, inhalation administration of BCQB significantly reduced ovalbumin-induced airway hyperresponsiveness and prevented the ovalbumin-induced increase in total cell counts and eosinophil counts.Citation25
An intranasal formulation of BCQB has been developed for the treatment of rhinorrhoea and is under clinical evaluation. BCQB proved to be safe and well tolerated in healthy Chinese subjects when administered intranasally with single and multiple doses across the doses studied. The pharmacokinetics, safety and tolerability profiles of BCQB pose it as a good candidate for further development in the treatment of rhinorrhoea in rhinitis.Citation26 In the ChiCTR2000030924 study, BCQB nasal spray four times daily per nostril proved effective and safe in the treatment of rhinorrhoea as well as sneezing, nasal congestion and itching for patients with persistent allergic rhinitis.Citation27 Although an inhaled form for patients with COPD was tested in 2015 in a cigarette-exposed murine model,Citation28 no further studies for a lung formulation have been published.
QAX028
Novartis registered two studies on QAX028, a novel once-daily LAMA which was under development.Citation29 NCT00568503, a phase I study, explored the safety, tolerability, PK and pharmacodynamics of QAX028 compared to open-label TIO as positive control and placebo in mild-to-moderate COPD patients. NCT01068613, a phase II trial, assessed the bronchodilator effects of multiple doses of QAX028 at two different dose levels (20 and 60 μg) when compared to TIO and placebo in a moderate-to-severe COPD population. No results from these studies are posted on clinicalTrial.gov and the last update was on May 2nd, 2012. It is possible that the adoption of an already-tested molecule such as GLY has made the company desist from continuing the research on this drug.
AZD9164
AZD9164 is a selective, competitive antagonist of the human M3 receptor, developed in collaboration by AstraZeneca Discovery and Pulmagen Therapeutics Limited (formerly Argenta Discovery Limited) as a potential novel inhaled, once-daily, long-acting antimuscarinic bronchodilator. AZD9164 was the first compound to be extensively evaluated in the clinical setting of a development program for a novel LAMA to treat patients with COPD. AZD9164 is a potent and competitive M3 receptor antagonist in human, guinea pig, rat, and dog in-vitro models, with a longer duration of effect than IPRA, antagonizing methacholine-induced bronchoconstriction in an in-vivo guinea pig model.Citation30 In vitro and in vivo, AZD9164 demonstrates high potency with receptor binding and functional assay which is equivalent to TIO. It is over 98% bound to human plasma protein and has high human liver microsomal intrinsic clearance, which should minimize systemic exposure and potential side effects.Citation31
The NCT00847249 study was performed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of AZD9164 following single ascending dose administrations to healthy male subjects. In this phase I study, AZD9164 showed a good safety profile via inhalation administration and the bronchodilator response was observed at doses greater than 700μg. AZD9164 has been evaluated in other phase I (NCT01016951 and NCT01096563) and phase II (NCT00939211) trials.
Two Phase I, randomized, double-blind, placebo-controlled, multiple ascending dose trials (Global multiple ascending dose, GMAD; Japanese multiple ascending dose, JMAD) were conducted in Europe and Japan, respectively. In the JMAD study, AZD9164 inhaled via Turbohaler significantly increased mean peak FEV1 compared to placebo and similar findings were seen in the GMAD trial. Transient paradoxical bronchoconstriction followed by sustained bronchodilation was observed.Citation30
The NCT00939211 phase II study concluded that AZD9164 could increase the magnitude and the duration of lung function improvements without increasing systemically mediated adverse events compared to TIO, even if an initial bronchoconstrictor effect was observed in COPD patients who received AZD9164.Citation32 The study safety review process resulted in cessation of further activities on AZD9164.
AZD8683
AZD8683 is another selective M3 receptor antagonist that was in clinical development by AstraZeneca as a solution for inhalation by nebulization and as a dry powder inhalation (DPI) via Turbohaler for patients with COPD.
PK were evaluated in guinea pig models during compound design. AZD8683 displayed high intrinsic metabolic clearance and human plasma protein binding (>95%) with a t½ of 25.6 hours and 84% inhibition of bronchoconstriction at 48 hours after intratracheal instillation in guinea pigs.Citation33 The NCT00979849 phase I study assessed the safety and PK of single ascending dose nebulized AZD8683 in healthy males. Other two Phase I randomized, double-blind, placebo-controlled trials assessed the safety and PK of single ascending dose inhaled AZD8683 via Turbohaler in both healthy participants (NCT01419600; NCT01584739) and COPD volunteers (NCT01584739).
As for AZD9164, the NCT01708057 phase IIa study was prematurely terminated due to the findings of an initial drop in FEV1 after administration of AZD8683 in a phase I multiple ascending dose study in healthy volunteers and COPD patients. In a double-blind, placebo-controlled, randomised, multi-centre, 3-way cross-over, single-dose phase II study (NCT01205269), two different doses of inhaled AZD8683 (50 μg and 200 μg) via Turbohaler were compared with placebo in 27 COPD patients. The FEV1 maximum increased of 0.09 L with AZD8683 200 µ compared to placebo. The results are published on clinicaltrial.gov.
NCT01666613 was registered as a phase I, single-centre, open-label, partly randomised, cross-over single-dose study in healthy volunteers to evaluate the absolute pulmonary bioavailability of AZD8683 when administered inhaled via a new dpi and via turbuhaler but never enrolled patient due to suspension of clinical investigation on AZD8683.
KRP197/Imidafenacin
Imidafenacin, 4-(2-methyl-1-H-imidazol-1-yl)-2,2-diphenyl butanamide, is an oral LAMA with high affinity for the M3 and M1 muscarinic receptor subtypes and low affinity for the M2 subtype.Citation34 Oral bronchodilators may potentially be an interesting therapeutic opportunity for less compliant patients and for those who have difficulty using inhalation devices. Inhibition of methacholine-induced bronchial constriction in a dose-dependent manner in guinea pigs was observed in preclinical studies after oral administration of imidafenacin.Citation35
A multicentre, randomized, double-blind, placebo-controlled 3×3 crossover Phase II trial was performed to evaluate the efficacy and safety of oral administration of the antimuscarinic agent imidafenacin in patients with COPD (JapicCTI-121,760). Two different dosages of oral imidafenacin were tested in twenty-seven male COPD patients with FEV1 ≥30% and <80%. Plasma imidafenacin level was positively correlated with change in FEV1. All subjects completed the trial without moderate nor severe AEs. Both doses of imidafenacin significantly improved lung function from baseline to 24 hrs after administration when compared to placebo, and area under the curve in FEV1 during 24 hrs after administration with 0.2 mg but not 0.1 mg dose was significantly improved in a dose-dependent manner. No moderate or severe AEs were reported and all subjects completed the study, suggesting the potential usefulness and tolerability of oral imidafenacin for improving the pulmonary function of patients with COPD.Citation35
ALKS27/Trospium
ALKS27/Trospium is a mAChR antagonist initially developed and commercialized as an oral agent for the treatment of overactive bladder. In 2007, Alkermes, Inc. and Indevus Pharmaceuticals, Inc. announced positive preliminary results from a randomized, double-blind, placebo-controlled phase IIa clinical study of an inhaled formulation of ALKS27 delivered by Alkermes’ proprietary AIR pulmonary delivery system in patients with COPD. In the study, single doses of ALKS27 demonstrated a rapid onset of action and produced a significant improvement in lung function over 24 h compared to placebo.Citation29 NCT00801684, a Phase II study performed with the doses of 100 and 400 μgCitation24 documented the rapid onset of action of ALKS27, observed as early as 15 min post treatment. Maximum improvement in FEV1 following ALKS27 administration occurred at 2 h, and statistically significant improvement in FEV1 was maintained at 24 h for both treatments with no significant differences between doses of 100 or 400 μg.Citation36 Presently, ALKS27 is not included in the R&D pipeline of Alkermes or Indevus Pharmaceuticals.
Dual LAMA/LABA (MABA)
There are currently six MABAs in clinical development, aiming to harness the synergy between the mechanisms and take advantage of a potentially simpler technical and clinical development pathway than LAMA/LABA combination therapiesCitation37 (, ). Bifunctional aromatic compounds are claimed to contain a β2-agonist pharmacophore linked by a flexible lipophilic spacer incorporating a trans-4-aminocyclohexanol group to a muscarinic antagonist pharmacophore. The main issue of these compounds is to find the best balance between antimuscarinic inhibition and adrenergic stimulation.
Table 2 Bi-Functional (or Dual Pharmacophore) Muscarinic Antagonist/β2-Agonist (MABA) in Clinical Development
Figure 2 Muscarinic receptor antagonists in clinical development for the treatment of COPD. Image of the CHF636ʹ molecule is not available.
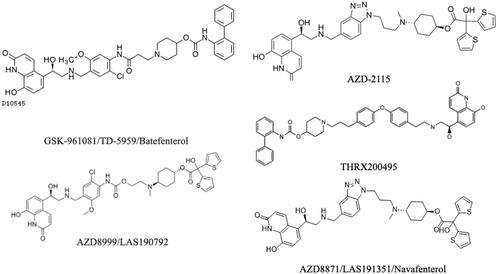
GSK961081/TD-5959/Batefenterol
GSK-961081/TD-5959/Batefenterol (BAT) is a novel first-in-class inhaled MABA for the treatment of COPD developed by GSK under licence from Theravance. To date, it is the MABA in the most advanced clinical development stage and is considered the comparator of this novel class of molecules.
BAT displayed high affinity for human M2 and M3 muscarinic receptors, but lower than TIO, and a β2-adrenoceptor affinity greater than FORM and albuterol. BAT behaved as a potent β2-adrenoceptor agonist with a high functional selectivity over β1- and β3-adrenoceptors. In isolated guinea pig tracheal tissues, BAT produced smooth muscle relaxation through a dual mechanism. In the guinea pig bronchoprotection assay, inhaled BAT produced potent and dose-dependent inhibition of bronchoconstrictor responses persistent for up to 7 days after dosing.Citation38
Eleven trials have been registered and completed on clinicaltrial.gov, six phase I and five phase II studies.
In a phase I single-dose study in 6 healthy subjects, BAT exhibited low systemic bioavailability after inhaled and oral administration, and high faecal excretion and low urinary excretion following intravenous and oral administration.Citation39
In a 4-week, multicentre, randomised, double-blind, double-dummy, three twice-daily doses and three once-daily doses of BAT were assessed and compared to placebo (NCT01319019). The study recruited 436 moderate to severe COPD patients. BAT showed statistically and clinically significant differences from placebo in all doses and regimens for trough FEV1 and the optimal total daily dose was 400 µg, either as 400 µg once daily or as 200 µg twice daily. No effects were observed on glucose, potassium, heart rate, blood pressure and no dose–response effect was seen on corrected QT elongation, resulting in a well-tolerated compound.Citation40 A sub-study was conducted to characterize the population’s PK and pharmacodynamics of three once-daily doses (100, 400, and 800 µg) and three twice-daily doses (100, 200, and 400 µg) of BAT Diskus and to investigate the relationship between the systemic exposure to BAT and cardiac safety. A two-compartment disposition PK model with first-order absorption adequately described the plasma BAT concentration–time data. No clear relationships between BAT plasma drug levels and cardiac-related safety parameters were shown.Citation41
In a 14-day, randomised, double-blind, double-dummy, crossover phase II study (NCT00478738), two different doses of BAT (400 µg and 1200 µg) once daily were compared to TIO 18 µg once daily plus salmeterol 50 µg twice daily (TIO plus SAL), versus placebo in 50 moderate COPD patients. Both dosages resulted in a significant increase in FEV1 over 24 h. Onset of bronchodilation was faster for both BAT doses compared to TIO plus SAL. No clinically relevant systemic pharmacodynamic effects were observed. AEs were similar across groups; however, tremors, dysgeusia and dry mouth were reported after BAT. BAT demonstrated sustained bronchodilation similar to TIO plus SAL, but with a more rapid onset, and was well tolerated at the tested doses.Citation42
The NCT00674817 randomised, double-blind, complete, crossover phase II study evaluated the bronchodilatory effects of adding short-acting bronchodilators (SABA) to maintenance, long-acting bronchodilator therapy with the BAT 400 µg or 1200 µg in 44 patients with moderate to severe COPD.
The additional bronchodilation achieved following supratherapeutic doses of salbutamol and IPRA on top of single doses of BAT 400 µg or 1200 µg was comparable for the two agents and neither were associated with any clinically relevant systemic pharmacodynamic effects other than a small transient hypokalaemia, suggesting that short acting bronchodilators could potentially be used as rescue medication on top of BAT therapy.Citation43
The primary objective of the NCT02570165 randomized, double-blind, placebo-controlled, active comparator, Phase IIb study was to model the dose–response of BAT and select a dose for Phase III development. Patients aged ≥40 years with COPD and FEV1 ≥30% and ≤70% predicted normal were equally randomized to BAT 37.5, 75, 150, 300, or 600 µg, placebo, or umeclidinium bromide (UMEC)/vilanterol (VI) 62.5/25 µg once daily. All BAT doses demonstrated statistically and clinically significant improvements from baseline vs placebo in the primary and secondary endpoints, weighted-mean FEV1 over 0–6 hours post-dose and trough FEV1, respectively, with a relatively flat dose–response. Lung function improvements with BAT ≥150 µg were comparable to those with UMEC/VI. BAT was well tolerated and no new safety signals were observed, suggesting that BAT 300 µg may represent the optimal dose for Phase III studies.Citation44
Moreover, several studies have investigated the association of BAT with an ICS, either fluticasone furoate (FF) or fluticasone propionate (FP) via Ellipta® or Diskus® DPI. The NCT02666287 phase I study supported the feasibility of developing BAT/inhaled corticosteroid triple therapy in a single inhaler.Citation45 The NCT01449799 study showed that there was no PK or pharmacodynamic interaction when BAT and FP were administered concurrently.Citation46 The NCT02064504 study showed that BAT 1200 µg administrated to 48 healthy subjects via Ellipta, alone or in combination with FF, resulted in similar systemic exposure compared to BAT administered by Diskus. FF exposure was reduced when administered in combination with BAT, compared with FF alone.Citation47
The NCT02573870 phase II, randomized, placebo-controlled, double-blind study evaluated the safety and tolerability of BAT 300 μg with FF 100 μg, administered via the ELLIPTA inhaler (BAT/FF 300/100). Patients with stable COPD were randomized 2:1 to receive BAT/FF 300/100 or placebo once daily for 6 weeks. BAT/FF 300/100 was well tolerated, with similar proportions of patients reporting AEs as in the placebo group, except for dysgeusia. The safety and tolerability of BAT/FF 300/100 in COPD patients were demonstrated in this study. Six weeks of BAT/FF 300/100 treatment was non-inferior to placebo in HR change from baseline, with no new clinically relevant general or cardiovascular safety signals.Citation48
AZD8999/LAS190792
AZD8999/LAS190792 (AZD/LAS) is a novel bifunctional muscarinic antagonist and β2-adrenoceptor agonist that began clinical development for COPD by Almirall and then by AstraZeneca. The compounds display nanomolar affinity to both β2 and M3 receptors and a prolonged duration of action in animal models.Citation49 AZD/LAS is selective for the human M3 receptor and for the β2-adrenoceptor, and its functional potency is similar to BAT, with a longer duration of action in isolated tissues. Nebulized AZD/LAS inhibits ACh-induced bronchoconstriction in dogs, with minimal cardiac effects and sustained bronchodilation. The affinity of AZD/LAS for the M3 receptor was 5 times lower than that of TIO, while its affinity for the β2-adrenoceptor was higher than that of olodaterol and indacaterol. AZD/LAS has the properties of being a bifunctional molecule at the receptor level with higher potency than LABA compounds and lower potency than TIO, suggesting that the β2-adrenoceptor activity of the compound might be higher than its antimuscarinic activity, very similar to BAT.Citation50
The aim of NCT02059434, a Two-part Pharmacokinetic and Pharmacodynamic Study of AZD/LAS in Patients With Asthma and COPD, was to assess the safety and tolerability of single doses of AZD/LAS administered by inhalation to patients with mild persistent asthma and moderate to severe COPD and also to assess the ability of AZD/LAS to produce bronchodilation.
Data from a randomized placebo-controlled trial on AZD/LAS in asthmatics were reported. This was an ascending-dose first-in-man trial, designed to test safety, tolerability, PK and pharmacodynamics (bronchodilation) of AZD/LAS from 5 to 400 µg. Seventeen patients were randomized and 16 completed the trial. All doses were safe and well tolerated and no stopping criteria were met. The most frequently reported adverse event was headache and only one serious adverse event not casually related to treatment occurred but did not lead to discontinuation. An improvement in the trough FEV1 change from baseline was observed for all doses of AZD/LAS ≥20 µg and bronchodilation was sustained over the 36-hour observation period, suggesting that AZD/LAS was safe, well tolerated and able to produce clinically meaningful and sustained bronchodilation at doses of 50 µg and above.Citation51
The anti-inflammatory effects of AZD/LAS in monotherapy and combined with FP in neutrophils from healthy and COPD patients is being tested. AZD/LAS shows anti-inflammatory effects in neutrophils from COPD patients and induces synergistic anti-inflammatory effects when combined with FP, supporting the use of MABA/ICS combination therapy in COPD.Citation52
AZD8871/LAS191351/Navafenterol
AZD8871/LAS191351/Navafenterol (NAV) is a dual-acting molecule with a high muscarinic component and a long residence time at the M3 receptor; it shows kinetic selectivity for the M3 over the M2 receptor and selective affinity for the β2-adrenoceptor over the β1 and β3 subtypes, resulting in dual antimuscarinic and β2-adrenoceptor functional activity sustained over time in isolated guinea pig tissue. NAV exhibits a higher muscarinic component than BAT in human bronchi, and the profile of NAV is not very different from that of BAT, with a drop in potency in human bronchi under propranolol blockade. Nebulized NAV prevents ACh-induced bronchoconstriction in both guinea pigs and dogs, with minimal effects on salivation and heart rate at doses with bronchoprotective activity. Moreover, NAV shows long-lasting effects in dogs, with a bronchoprotective half-life longer than 24 hours. Moreover, its preclinical profile in animal models suggests a once-daily dosing in humans and a favourable safety profile.Citation53 The structural differences between NAV and AZD/LAS lays in the linker region, being a N-phenylcarbamate in the former and a benzotriazole in the latter. Important to say, the linker moiety per se is not responsible for either the β2-adrenoceptor agonism or the muscarinic antagonism, but is able to modulate both when linked to the β2 and M3 motif structures. The linker present in NAV, contrarily to the one present in AZD/LAS, unbalances the activities toward M3.Citation53
NAV is an inhaled long-acting MABA in development for the maintenance treatment of COPD and 5 clinical trials regarding it have been registered on clinicaltrial.gov, three phase I trials registered as NCT03159442 (Safety, Tolerability and Pharmacokinetics of Multiple Ascending Doses of NAV in Healthy Male Japanese Subjects), NCT02573155 (Two-part Safety, Tolerability, Pharmacodynamic and Kinetic Study of Inhaled NAV in Asthmatic and COPD Subjects), and NCT02814656 (Safety, Tolerability and Pharmacokinetics of Multiple Ascending Doses of NAV in Healthy Subjects) and two phase II trials registered as NCT03645434 (A Single Inhalation Dose Study to Assess Efficacy, PK (PK), Safety and Tolerability of NAV in Patients With Long-term Lung Diseases) and NCT02971293 (Efficacy, PK (PK), Safety and Tolerability Study of Inhaled NAV). NAV is present in the AstraZeneca group’s Respiratory & Immunology (as of 30 July 2020) phase II pipeline.
In a Phase I RCT (NCT02573155) NAV administered at 400 μg and 1800 μg via the DPI Genuair significantly improved trough FEV1 compared to placebo and both dosages were as effective as indacaterol 150 μg administered via DPI Breezhaler and TIO 18 μg administered via the DPI HandiHaler in improving trough FEV1. AEs reported in the NAV 400 μg group were common and similar to those in the TIO 18 μg group (≃3%) and no patients treated with NAV 1800 μg had AEs. In the NCT02814656 Phase I RCT, patients treated with ascending doses of NAV also did not experience any AEs.Citation54
NCT02971293, a randomized, double-blind, placebo-controlled, three-period crossover, phase IIa study investigated the efficacy, safety, and tolerability of inhaled NAV 100 µg or 600 µg once daily for 14 days in patients with moderate to severe COPD. Once-daily doses of NAV in patients with COPD delivered significant bronchodilation and clinically meaningful improvement of symptoms. No safety concerns emerged during the study. Once-daily doses of NAV 100 µg and 600 µg over 14 days produced statistically significant and dose-ordered improvements versus placebo in trough and peak FEV1, a significant decrease from baseline in mean daily Breathlessness, Cough and Sputum Scale score and a good overall safety and tolerability profile was observed.Citation37
Data from a randomized placebo-controlled trial on NAV in asthmatics were reported. This was an ascending-dose first-in-man trial, designed to test safety, tolerability, PK and pharmacodynamics (bronchodilation) of NAV. All doses from 50 to 2100 µg were safe and well tolerated with no serious AEs and no stopping criteria. The most frequent AEs were headache (10, 62.5%) and nasopharyngitis (7, 43.8%). NAV had a quick onset of action and a clear, dose ordered bronchodilator response. A sustained effect was observed over 24–36 hours following NAV doses ≥200 µg. An improvement in trough FEV1 change from baseline was observed for all doses, with a clinically meaningful response occurring at all doses ≥200 µg suggesting that NAV was safe, well tolerated and able to produce a sustained bronchodilation at doses >200 µg.Citation55
AZD2115
AstraZeneca is active in the development of MABAs with its lead compound AZD-2115 which has been investigated in phase II studies in COPD patients. AZD-2115 is one of the outcomes of AstraZeneca’s collaboration with Argenta Discovery (now Pulmagen Therapeutics) with seven patent applications claiming multiple MABAs.Citation56
Five RCTs are registered as completed on clinicaltrial.gov, three phase I studies (NCT03097380, NCT01283984, NCT01445782) and two phase II studies (NCT01498081, NCT02109406).
NCT03097380 is a phase I open-label study using positron emission tomography (PET) to explore the binding of AZD2115 and TIO to muscarinic receptors in the lungs in healthy volunteers after inhalation. The study results suggest that PET imaging of muscarinic receptor binding in lungs using the radioligand [11C]VC-002 is feasible and that a 60-min acquisition time allows for calculation of binding parameters which appear to be robust.Citation57
NCT01498081, a single-dose phase IIa study investigated the effects of three different doses of inhaled AZD2115 in COPD patients compared to indacaterol 150 µg and indacaterol 150 µg plus TIO 18 µg. Inhalation of single doses of AZD2115 (25 µg, 80 µg, and 240 µg) resulted in a rapid dose-related increase in FEV1 with distinct peaks within 2 hours post-dose, followed by a progressive decrease. For the two lowest doses, the bronchodilatory effect was not sustained for 24 hours post-dose. Overall, there were few AEs observed in the study. The proportion of patients with AEs was higher during AZD2115 240 µg and AZD2115 80 µg treatment periods as compared with AZD2115 25 µg, indacaterol 150 µg plus TIO 18 µg, indacaterol 150 µg, and placebo treatment periods. There were no AEs with a fatal outcome in this study. There were no clinically relevant safety changes with respect to FEV1 and FVC in the study. No case of post-dose bronchoconstriction was observed in this study. AZD2115 was generally well tolerated at single inhaled predicted lung-deposited doses up to 240 µg in COPD patients. (Clinical Study Report Synopsis Drug Substance AZD2115 Study Code D3060C00003 Edition Number 1)
CHF6366
CHF6366 is a novel bifunctional compound for the treatment of COPD displaying both muscarinic receptor antagonist and β2-adrenergic receptor agonist properties in early clinical development by Chiesi Farmaceutici.
The bronchoprotective activity of CHF6366 and its safety profile have been assessed in a model of ACh-induced bronchoconstriction in dogs in comparison with BAT, TIO and FORM. CHF6366 (0.1–3µg/kg), administered intratracheally via nebulizer in anaesthetised dogs. It exerted a dose-dependent prevention of ACh-induced bronchospasm during the first two hours post-dose and this activity was significantly maintained up to 24 hours, comparable to that of TIO. In this dog model, CHF6366, but not BAT nor FORM, provided full, 24-hour bronchoprotection without systemic side effects.Citation58
The bronchoprotective effect of CHF6366 was also tested in a model of bronchospasm in anaesthetised guinea pigs that allowed discrimination of the two pharmacological activities by using the spasmogens ACh, -/+ β2 blocker for the assessment of the MABA and antimuscarinic effect, respectively, and histamine (β2 effect). BAT was used as a MABA comparator in this study. CHF6366 (0.3–1nmol/kg), administered intratracheally, showed good balance of the two activities, the peak effect was observed at 5 minutes post-dose with both activities fully effective within the first 15 minutes, differently than BAT which resulted less balanced compared to CHF6366, with a predominance of β2 activity.Citation59
NCT03378648, a first-in-human randomised, double-blind, placebo-controlled phase I/phase IIa study of single ascending doses in healthy male volunteers and repeated ascending doses in asthmatic patients followed by a 3-way cross-over, placebo-controlled, single-dose in COPD patients investigated the safety, tolerability, pharmacokinetics, and pharmacodynamics of CHF6366 and has been completed. To date, no data are posted on clincaltrial.gov.
THRX200495
Theravance Incorporated has developed THRX200495 in preclinical studies as a single agent acting as a muscarinic receptor antagonist and a β2-adrenoceptor agonist. Using an in vivo model of bronchoprotection in guinea pigs, THRX-200495 was compared to a fixed-dose combination of a short-acting muscarinic receptor antagonist (IPRA) and a β2-adrenoceptor agonist (albuterol). Conscious guinea pigs received aqueous nebulized solutions of vehicle or test compound by aerosol exposure and bronchoprotective potency was estimated in anesthetized, tracheotomized and ventilated guinea pigs at predetermined time points after aerosol exposure by measuring changes in ventilation pressure. THRX-200495 exhibited a potent dual effect as an anti-muscarinic and adrenoceptor agonist that persisted for over 24 h, demonstrating potent, balanced and long-lasting bronchodilation in a guinea pig model of bronchoprotection that was greater than either the IPRA or albuterol mechanisms alone.Citation60
Conclusions
LAMAs are now one of the leading therapies in COPD patients of all stages. In consideration of the variety of LAMAs currently on the market and the high failure rate of new molecules in more or less advanced research phases, new experimental models are necessary to optimize research investments. MABAs are currently the most promising new class of bronchodilators.
Expert Opinion
COPD is now the third cause of death worldwide and is still growing. The world market for COPD drugs is estimated to reach $14.1 billion by 2025. Nevertheless, on such a prevalent condition and in such a leading market, the search for new molecules has seen a setback in the last years. Of the 10 LAMAs that have undergone a phase I or II trial in the past decade, only REV has been approved by the FDA, but not by the EMA yet. Generally, drug discovery is a very long and difficult process, and bringing a new drug on the market is very expensive. It is calculated that for one approved drug, more than 10,000 chemicals are lost.Citation61 AZD9164 developed by AstraZeneca was stopped because of safety concerns. In the GMAD and the JMAD studies, healthy subjects reported similar AEs with AZD9164 and placebo and no severe cardiac AEs were reported, but in the GMAD study, a small, transient decrease of FEV1 was observed 15 minutes after the administration of AZD9164 (on average, 2.3% decrease after a single dose of 400 μg, 5.5% decrease after 2800 μg) and one subject quit the study due to a decrease greater than 30%. Similar outcomes were found in the JMAD study. Moreover, in the GMAD COPD study, all three COPD patients who received AZD9164 had a transient (5 to 15 minutes) bronchoconstriction. Because of these findings, the development of AZD9164 was discontinued. The reasons for this transitory AE were not clear and were not foreseen in the pre-clinical studies. A partial agonist activity of the compound on the M1 receptor or an early M2 antagonist effects was hypothesized to explain the bronchoconstriction before the onset of the therapeutic effects on M3 receptors. Otherwise, a non-pharmacological hypothesis was based on a possible local irritant effect of AZD9164 or its other components. However, the overall result was that a very promising drug was discontinued and a similar fate also befell AZD8683. The experience of these two molecules may have highlighted the importance of including early timepoint measurements of lung function when investigating novel inhaled treatments, as some authors suggested,Citation30 but it is also possible that new study models that can provide a better representation of the response in humans need to be introduced. For example, ex vivo bronchial studies are now widely used to evaluate the synergistic effect of various bronchodilators and have proven to be reliable in predicting the possible response of bronchi in humansCitation62 and it is likely that future pharmacological research in the respiratory field will not be able to refrain from early testing of drugs on ex vivo bronchi, not only to evaluate the bronchodilator effect but also to evaluate the right balance between LAMA and LABA, such as to find the best dosage of LAMA/LABA combinations or for MABAs.
Another reason why research on LAMAs has slowed in recent years may also be due to the expiration of two widely tested LAMA patents (TIO and GLY) with no significant AEs. There are 4 LAMAs in the market so far (TIO, GLY, UMEC, and ACLI), which have different formulations and different durations of action. Even if with some differences in onset time, all of them are effective, mainly selective for M3 receptors and with neglectable AEs.Citation63
AstraZeneca, Novartis and Chiesi Farmaceutici quitted the experimentation on AZD9164/AZD8683, QAX028 and CHF-5407, respectively, and adopted GLY in their combinations. Studies on BCQB and Mequitazine in COPD were stopped in 2015 during preclinical or phase II trials, but BCQB continued to be developed for rhinitis. The last available data on QAX028 dates back to 2012, while Alkermes and Indevus Pharmaceuticals stopped testing Trospium. The availability of worthwhile alternatives has probably outweighed the need to search for new molecules of the same class.
REV is the last LAMA approved by the FDA, but not by EMA. The mean peak FEV1 improvement after the first administration was >120 mL compared to placebo.Citation20 It offers the advantage of being the first once-daily LAMA via a standard jet nebulizer in patients with COPD and it can be used in a sequential or combined administration with LABA in hospitalised, ventilated or non-compliant COPD patients who need dual therapy.Citation21
Conversely, Imidafenacin is an oral antimuscarinic developed for the treatment of overactive bladder syndrome, only marketed in Japan. Because of its high affinities for the M3 and M1 muscarinic receptor subtypes and a low affinity for M2 receptors, it has been tested in COPD.Citation35 Although it is less effective in improving lung function compared to approved inhaled LAMAs, it would be the first available oral LAMA on the market and could be indicated in non-adherent patients or in patients with poor coordination.
MABA is a new class of drugs for COPD, which have a β2-agonists component along with the LAMA component. The are many reasons why companies should develop MABAs: a single molecule that confers both therapeutic effects can speed up the approval of a double bronchodilation and this would support the approval of each individual component, separately or subsequently, on the one hand, and it would favor adherence to treatment in patients who need both compounds, on the other hand. Moreover, due to MABAs’ intrinsic characteristic of acting simultaneously on two different pathways in the same cell, their investigation could open yet unknown and unexplored fields of research.
However, the development of MABAs has some pharmacological issues that must be overcome. The construction of a molecule with two functions needs to provide a good balance of both the antimuscarinic and the adrenergic properties, otherwise the risk is that only one of the two is truly predominant and clinically active, and the molecule will simply become a LAMA or a LABA. BAT, which is the most advanced MABA in course of development, behaves as a potent LABA but is less potent than TIO.Citation38 Similarly, AZD/LAS was unbalanced towards the adrenergic component, differently NAV showed a more marked antimuscarinic activity.
Currently, we are still a long way from the cure for COPD and the development of new classes of drugs is necessary in order to better control the symptoms and to improve patient conditions. Despite this, the search for innovative molecules presents a high economic investment and is not without huge losses. Only the application of new in vitro and ex vivo models can help improve the development system and make it more efficient.
In conclusion, REV is the only authorized new LAMA, administered through nebulization; Imidafenacin, an M3-selective LAMA still awaiting approval in Europe and the USA, would be the first available oral LAMA on the market; and MABA is a new class of bronchodilators which combine LAMA and LABA, the main issue is formulating a balanced compound with similar LAMA and LABA properties.
Abbreviations
Ach, acetylcholine; AE, adverse effect; ASM, airway smooth muscles; AZD/LAS, AZD8999/LAS190,792; BAT, GSK-961,081/TD-5959/Batefenterol; BCQB, bencycloquidium bromide; Cmax, peak plasma concentrations; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FDA, Food and Drug Administration; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FORM, formoterol; FP, fluticasone propionate; GLY, glycopyrronium bromide; ICS, inhaled corticosteroid; IPRA, ipratropium bromide; LABA, long-acting β2 adrenoceptor agonist; LAMA, long-acting muscarinic antagonists; MABA, muscarinic antagonist/β2-agonist; mAChR, postjunctional muscarinic receptors; NAV, AZD8871/LAS191351/Navafenterol; PK, pharmacokinetics; REV, Revefenacin/TD-4208; TIO, tiotropium bromide; UMEC, umeclidinium bromide; VI, vilanterol.
Article Highlights
Currently, just a few LAMA molecules are under study while many of them were stopped about five years ago.
Revefenacin is the last LAMA approved by the FDA and it offers the advantage of being the first once-daily LAMA for nebulization in patients with COPD, plus it can be used in hospitalised or ventilated COPD patients.
Imidafenacin is an M3-selective LAMA, already used for overactive bladder, currently under investigation for COPD. It would be the first available oral LAMA on the market, although its effect on improving FEV1 is modest.
MABA is a new class of bronchodilators, which combine long-acting muscarinic antagonism with β2-agonist activity.
The main issue on MABAs is formulating a balanced compound with similar antimuscarinic and β-adrenergic activities.
New in vitro ed ex vivo models are necessary to improve and make the development of new drugs more efficient.
Authorship
All authors (JO, AC, MC, LC, PR):
Made a significant contribution to the work reported.
Have drafted, written, and revised the article.
Have agreed on the journal to which the article was submitted.
Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
Agree to take responsibility and be accountable for the contents of the article.
Disclosure
JO participated as a speaker in scientific meetings and courses under the sponsorship of AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Novartis, Zambon. AC participated as a speaker in scientific meetings and courses under the sponsorship of AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, and Zambon. MC reports personal fees from AstraZeneca, GlaxoSmithKline, and Cipla, and grants and personal fees from Novartis, during the conduct of the study; and grants and personal fees from Almirall, Boehringer Ingelheim, Novartis, and Zambon, and personal fees from ABC Farmaceutici, AstraZeneca, Biofutura, Chiesi Farmaceutici, Cipla, Edmond Pharma, GlaxoSmithKline, Lallemand, Menarini, Mundipharma, Ockham Biotech, Pfizer, Sanofi, and Verona Pharma, outside the submitted work. LC has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis, received non-financial support by AstraZeneca, received a research grant partially funded by Boehringer Ingelheim, Novartis and Almirall, and is or has been a consultant to Edmond Pharma, Zambon and Verona Pharma. His department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon. PR participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon. The authors report no other potential conflicts of interest for this work.
Additional information
Funding
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf. Accessed November 25, 2020.
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101(3):971–985. doi:10.1152/japplphysiol.00313.2006
- Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504.
- Barnes PJ. Muscarinic receptor subtypes in airways. Life Sci. 1993;52(5–6):521–527. doi:10.1016/0024-3205(93)90310-Y
- Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690.
- Calzetta L, Rogliani P, Ora J, et al. LABA/LAMA combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev. 2017;26:160043.
- Calzetta L, Matera MG, Cazzola M. Pharmacological mechanisms leading to synergy in fixed-dose dual bronchodilator therapy. Curr Opin Pharmacol. 2018;40:95–103. doi:10.1016/j.coph.2018.03.011
- Heusler P, Cussac D, Naline E, et al. Characterization of V0162, a new long-acting antagonist at human M3 muscarinic acetylcholine receptors. Pharmacol Res. 2015;100:117–126. doi:10.1016/j.phrs.2015.07.033
- Devillier P, Garrigue E, D’Auzers G, et al. V0162 a new long-acting bronchodilator for treatment of chronic obstructive lung diseases: preclinical and clinical results. Respir Res. 2015;16(1):68. doi:10.1186/s12931-015-0227-1
- Theravance Biopharma. Theravance biopharma and mylan receive FDA approval for YUPELRITM (revefenacin) in adults with chronic obstructive pulmonary disease [media release]. November 9, 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210598Orig1s000Approv.pdf. Accessed November 25, 2020.
- Hegde SS, Pulido-Rios MT, Luttmann MA, et al. Pharmacological properties of revefenacin (TD-4208), a novel, nebulized long-acting, and lung selective muscarinic antagonist, at human recombinant muscarinic receptors and in rat, guinea pig, and human isolated airway tissues. Pharmacol Res Perspect. 2018;6(3):e00400. doi:10.1002/prp2.400
- Pulido-Rios MT, McNamara A, Obedencio GP, et al. In vivo pharmacological characterization of TD-4208, a novel lung-selective inhaled muscarinic antagonist with sustained bronchoprotective effect in experimental animal models. J Pharmacol Exp Ther. 2013;346(2):241–250. doi:10.1124/jpet.113.203554
- Heo Y-A. Revefenacin: first global approval. Drugs. 2019;79(1):85–91. doi:10.1007/s40265-018-1036-x
- Borin MT, Lo A, Barnes CN, et al. Pharmacokinetics and safety of revefenacin in subjects with impaired renal or hepatic function. Int J Chron Obstruct Pulmon Dis. 2019;14:2305–2318. doi:10.2147/COPD.S203709
- Donohue J, Feldman G, Sethi S, et al. Cardiovascular safety of revefenacin for nebulization: a review of randomized controlled trial data. Chest. 2018;154(4):734A–735A. doi:10.1016/j.chest.2018.08.664
- Borin MT, Barnes CN, Darpo B, et al. Revefenacin, a long-acting muscarinic antagonist, does not prolong QT interval in healthy subjects: results of a placebo- and positive-controlled thorough QT Study. Clin Pharmacol Drug Dev. 2020;9:130–139.
- Quinn D, Barnes CN, Yates W, et al. Pharmacodynamics, pharmacokinetics and safety of revefenacin (TD-4208), a long-acting muscarinic antagonist, in patients with chronic obstructive pulmonary disease (COPD): results of two randomized, double-blind, Phase 2 studies. Pulm Pharmacol Ther. 2018;48:71–79. doi:10.1016/j.pupt.2017.10.003
- Pudi KK, Barnes CN, Moran EJ, et al. A 28-day, randomized, double-blind, placebo-controlled, parallel group study of nebulized revefenacin in patients with chronic obstructive pulmonary disease. Respir Res. 2017;18(1):182. doi:10.1186/s12931-017-0647-1
- Donohue JF, Kerwin E, Barnes CN, et al. Efficacy of revefenacin, a long-acting muscarinic antagonist for nebulized therapy, in patients with markers of more severe COPD: a post hoc subgroup analysis. BMC Pulm Med. 2020;20(1):134. doi:10.1186/s12890-020-1156-4
- Siler TM, Moran EJ, Barnes CN, et al. Safety and efficacy of revefenacin and formoterol in sequence and combination via a standard jet nebulizer in patients with chronic obstructive pulmonary disease: a phase 3b, randomized, 42-Day Study. Chronic Obstr Pulm Dis. 2020;7:99–106.
- Cazzola M, Page C, Matera MG. Long-acting muscarinic receptor antagonists for the treatment of respiratory disease. Pulm Pharmacol Ther. 2013;26(3):307–317. doi:10.1016/j.pupt.2012.12.006
- Villetti G, Pastore F, Bergamaschi M, et al. Bronchodilator activity of (3R)-3-[[[(3-fluorophenyl)[(3,4,5-trifluorophenyl)methyl]amino] carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo[2.2.2]octane bromide (CHF5407), a potent, long-acting, and selective muscarinic M3 Receptor antagonist. J Pharmacol Exp Ther. 2010;335(3):622–635. doi:10.1124/jpet.110.170035
- Jiang J-X, Cao R, Deng W-D, et al. Characterization of bencycloquidium bromide, a novel muscarinic M3 receptor antagonist in guinea pig airways. Eur J Pharmacol. 2011;655(1–3):74–82. doi:10.1016/j.ejphar.2011.01.017
- Cao R, Dong X-W, Jiang J-X, et al. M3 muscarinic receptor antagonist bencycloquidium bromide attenuates allergic airway inflammation, hyperresponsiveness and remodeling in mice. Eur J Pharmacol. 2011;655(1–3):83–90. doi:10.1016/j.ejphar.2011.01.024
- Sun L, Ding L, Wang Y, et al. Pharmacokinetics, safety and tolerability of bencycloquidium bromide, a novel selective muscarinic M1/M3 receptor antagonist, after single and multiple intranasal doses in healthy Chinese subjects: an open-label, single-center, first-in-Human Study. Drugs R D. 2012;12(1):17–28. doi:10.2165/11599330-000000000-00000
- Jiang Z, Xiao H, Liu S, et al. Bencycloquidium bromide nasal spray is effective and safe for persistent allergic rhinitis: a phase III, multicenter, randomized, double-blinded, placebo-controlled clinical trial. Eur Arch Otorhinolaryngol. 2020;277(11):3067–3077. doi:10.1007/s00405-020-06183-5
- Zhang S-J, Jiang J-X, Ren -Q-Q, et al. Effects of the inhalation of the M3 receptor antagonist bencycloquidium bromide in a mouse cigarette smoke-induced airway inflammation model. Drug Dev Res. 2015;76(3):123–131. doi:10.1002/ddr.21248
- Cazzola M, Rogliani P, Segreti A, et al. An update on bronchodilators in phase I and II clinical trials. Expert Opin Investig Drugs. 2012;21(10):1489–1501. doi:10.1517/13543784.2012.710602
- Jorup C, Bengtsson T, Strandgården K, et al. Transient paradoxical bronchospasm associated with inhalation of the LAMA AZD9164: analysis of two phase I, randomised, double-blind, placebo-controlled studies. BMC Pulm Med. 2014;14(1):52. doi:10.1186/1471-2466-14-52
- Mete A, Bowers K, Chevalier E, et al. The discovery of AZD9164, a novel muscarinic M3 antagonist. Bioorg Med Chem Lett. 2011;21(24):7440–7446. doi:10.1016/j.bmcl.2011.10.002
- Bjermer L, Bengtsson T, Jorup C, et al. Local and systemic effects of inhaled AZD9164 compared with tiotropium in patients with COPD. Respir Med. 2013;107(1):84–90. doi:10.1016/j.rmed.2012.09.014
- Mete A, Bowers K, Bull RJ, et al. The design of a novel series of muscarinic receptor antagonists leading to AZD8683, a potential inhaled treatment for COPD. Bioorg Med Chem Lett. 2013;23(23):6248–6253. doi:10.1016/j.bmcl.2013.09.092
- Kobayashi F, Yageta Y, Segawa M, et al. Effects of imidafenacin (KRP-197/ONO-8025), a new anti-cholinergic agent, on muscarinic acetylcholine receptors. Arzneimittelforschung. 2011;57(02):92–100. doi:10.1055/s-0031-1296589
- Machida K, Kawayama T, Kinoshita M, et al. Imidafenacin, an orally active muscarinic receptor antagonist, improves pulmonary function in patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled 3×3 crossover phase II trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2175–2184. doi:10.2147/COPD.S223002
- Oleson L, Turncliff R, Silverman B. ALKS 27 (trospium inhalation powder) improves lung function following single administration in subjects with COPD (abstract). Am J Respir Crit Care Med. 2010;181:A4457.
- Singh D, Fuhr R, Jimenez L, et al. A randomized trial of dual-acting bronchodilator AZD8871 for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;199(10):1282–1284. doi:10.1164/rccm.201812-2345LE
- Hegde SS, Hughes AD, Chen Y, et al. Pharmacologic characterization of GSK-961081 (TD-5959), a first-in-class inhaled bifunctional bronchodilator possessing muscarinic receptor antagonist and β2-adrenoceptor agonist properties. J Pharmacol Exp Ther. 2014;351(1):190–199. doi:10.1124/jpet.114.216861
- Ambery C, Young G, Fuller T, et al. Pharmacokinetics, excretion, and mass balance of [14C]-batefenterol following a single microtracer intravenous dose (concomitant to an inhaled dose) or oral dose of batefenterol in healthy men. Clin Pharmacol Drug Dev. 2018;7(8):901–910. doi:10.1002/cpdd.616
- Wielders PLML, Ludwig-Sengpiel A, Locantore N, et al. A new class of bronchodilator improves lung function in COPD: a trial with GSK961081. Eur Respir J. 2013;42(4):972–981. doi:10.1183/09031936.00165712
- Ambery CL, Wielders P, Ludwig-Sengpiel A, et al. Population pharmacokinetics and pharmacodynamics of GSK961081 (batefenterol), a muscarinic antagonist and β2-agonist, in moderate-to-severe COPD patients: substudy of a randomized trial. Drugs R D. 2015;15(3):281–291. doi:10.1007/s40268-015-0104-x
- Bateman ED, Kornmann O, Ambery C, et al. Pharmacodynamics of GSK961081, a bi-functional molecule, in patients with COPD. Pulm Pharmacol Ther. 2013;26(5):581–587. doi:10.1016/j.pupt.2013.03.015
- Norris V, Ambery C. Bronchodilation and safety of supratherapeutic doses of salbutamol or ipratropium bromide added to single dose GSK961081 in patients with moderate to severe COPD. Pulm Pharmacol Ther. 2013;26(5):574–580. doi:10.1016/j.pupt.2013.03.009
- Crim C, Watkins ML, Bateman ED, et al. Randomized dose-finding study of batefenterol via dry powder inhaler in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;15:615–629. doi:10.2147/COPD.S190603
- Ambery C, Young G, Fuller T, et al. Open-label, crossover study to determine the pharmacokinetics of fluticasone furoate and batefenterol when administered alone, in combination, or concurrently. Clin Pharmacol Drug Dev. 2019;8(2):188–197. doi:10.1002/cpdd.603
- Norris V, Ambery C, Riley T. Pharmacokinetics and pharmacodynamics of GSK961081, a novel inhaled muscarinic antagonist β 2 -agonist, and fluticasone propionate administered alone, concurrently and as a combination blend formulation in healthy volunteers: clinical pharmacology in drug development. Clin Pharmacol Drug Dev. 2014;3:305–313.
- Ambery C, Riddell K, Daley-Yates P. Open-label, randomized, 6-way crossover, single-dose study to determine the pharmacokinetics of batefenterol (GSK961081) and fluticasone furoate when administered alone or in combination. Clin Pharmacol Drug Dev. 2016;5(5):399–407. doi:10.1002/cpdd.274
- Crim C, Gotfried M, Spangenthal S, et al. A randomized, controlled, repeat-dose study of batefenterol/fluticasone furoate compared with placebo in the treatment of COPD. BMC Pulm Med. 2020;20(1):119. doi:10.1186/s12890-020-1153-7
- Norman P. Novel dihydroquinoline-based MABAs; clues to the identity of LAS-190792: evaluation of WO20111411802. Expert Opin Ther Pat. 2012;22(2):185–192. doi:10.1517/13543776.2012.655270
- Aparici M, Carcasona C, Ramos I, et al. Pharmacological preclinical characterization of LAS190792, a novel inhaled bifunctional muscarinic receptor antagonist /β 2 -adrenoceptor agonist (MABA) molecule. Pulm Pharmacol Ther. 2017;46:1–10. doi:10.1016/j.pupt.2017.07.003
- Astbury C, Pujol H, Massana È, et al. A Randomized Placebo Controlled Trial of AZD8999 (LAS190792) a Novel Dual Acting Bronchodilator in Asthmatics. Airway Pharmacology and Treatment. European Respiratory Society; 2017:OA4406. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/1393003.congress-2017.OA4406. Accessed November 25, 2020.
- Milara J, Contreras S, de Diego A, et al. In vitro anti-inflammatory effects of AZD8999, a novel bifunctional muscarinic acetylcholine receptor antagonist /β2-adrenoceptor agonist (MABA) compound in neutrophils from COPD patients. PLoS One. 2019;14(1):e0210188. doi:10.1371/journal.pone.0210188
- Aparici M, Carcasona C, Ramos I, et al. Pharmacological profile of AZD8871 (LAS191351), a novel inhaled dual M3 receptor antagonist/β2-adrenoceptor agonist molecule with long-lasting effects and favorable safety profile. J Pharmacol Exp Ther. 2019;370:127–136. doi:10.1124/jpet.118.255620
- Calzetta L, Ritondo BL, Matera MG, et al. Investigational treatments in phase I and II clinical trials: a systematic review in chronic obstructive pulmonary disease (COPD). Expert Opin Investig Drugs. 2020;1–16.
- Jimenez L, Astbury C, Seoane B, et al. A Randomized Placebo Controlled Trial of AZD8871 a Novel Dual Acting Bronchodilator in Asthmatics. Airway Pharmacology and Treatment. European Respiratory Society; 2017:PA1811. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/1393003.congress-2017.PA1811. Accessed November 25, 2020.
- Norman P. Evaluation of WO-2012085582 and WO-2012085583 two identified MABAs: backups to AZD-2115? Expert Opin Ther Pat. 2012;22(11):1377–1383. doi:10.1517/13543776.2012.718761
- Cselényi Z, Jucaite A, Kristensson C, et al. Quantification and reliability of [11C]VC - 002 binding to muscarinic acetylcholine receptors in the human lung — a test-retest PET study in control subjects. EJNMMI Res. 2020;10(1):59. doi:10.1186/s13550-020-00634-0
- Carnini C, Cesari N, Rudolph K, et al. Bronchoprotective Activity and Safety Evaluation of the Novel Antimuscarinic/Β2 Agonist (MABA) CHF6366 in Dogs. Airway Pharmacology and Treatment. European Respiratory Society; 2017:PA1804. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/1393003.congress-2017.PA1804. Accessed November 25, 2020.
- Miglietta D, Carnini C, Bassani F, et al. CHF6366: Characterisation of the Bronchoprotective Effect of a Novel MABA Compound in the Experimental Bronchospasm Model in Anaesthetised Guinea Pigs. Airway Pharmacology and Treatment. European Respiratory Society; 2017:OA4405. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/1393003.congress-2017.OA4405. Accessed November 25, 2020.
- McNamara A, Steinfeld T, Pulido-Rios MT, et al. Preclinical efficacy of THRX-200495, a dual pharmacology muscarinic receptor antagonist and β(2)-adrenoceptor agonist (MABA). Pulm Pharmacol Ther. 2012;25:357–363. doi:10.1016/j.pupt.2012.06.007
- Barnes PJ, Bonini S, Seeger W, et al. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45(5):1197–1207. doi:10.1183/09031936.00007915
- Cazzola M, Calzetta L, Ora J, et al. Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside. Respir Med. 2015;109(10):1305–1311. doi:10.1016/j.rmed.2015.08.005
- Ismaila AS, Huisman EL, Punekar YS, et al. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:2495–2517. doi:10.2147/COPD.S92412
- Cazzola M, Lopez-Campos J-L, Puente-Maestu L. The MABA approach: a new option to improve bronchodilator therapy. Eur Respir J. 2013;42(4):885–888. doi:10.1183/09031936.00067013
