Abstract
Background
Oseltamivir phosphate (OP, Tamiflu®) is a widely used drug in the treatment of influenza with fever. However, case reports have associated OP intake with sudden abnormal behaviors. In rats infected by the influenza A virus (IAV), the electroencephalogram (EEG) displayed abnormal high-voltage amplitudes with spikes and theta oscillations at a core temperature of 39.9°C to 41°C. Until now, there has been no information describing the effect of OP on intact brain hippocampal activity of IAV-infected animals during hyperthermia.
Objective
The aim of the present study was to examine the effect of OP on abnormal EEG activities in the hippocampus using the rat model of influenza-associated encephalopathy.
Methods
Male Wistar rats aged 3 to 4 weeks were used for the study. Influenza A/WSN/33 strain (1 × 105 plaque forming unit in PBS, 60 µL) was applied intranasally to the rats. To characterize OP effects on the IAV-infected rats, EEG activity was studied more particularly in isoflurane-anesthetized IAV-infected rats during hyperthermia.
Results
We found that the hippocampal EEG of the OP-administered (10 mg/kg) IAV-infected rats showed significant reduction of the high-voltage amplitudes and spikes, but the theta oscillations, which had been observed only at >40°C in OP non-administered rats, appeared at 38°C core temperature. Atropine (30 mg/kg) blocked the theta oscillations.
Conclusion
Our data suggest that OP efficiently reduces the abnormal EEG activities after IAV infection during hyperthermia. However, OP administration may stimulate ACh release in rats at normal core temperature.
Introduction
Influenza A virus (IAV) is a common infectious pathogen in humans, which occasionally causes influenza-associated encephalopathy (IAE). IAE is characterized by severe neurological complications, such as convulsive seizures, loss of consciousness, and abnormal behaviors.Citation1,Citation2
Oseltamivir phosphate (OP) is a selective neuraminidase inhibitor that prevents influenza virus replication. It is prescribed for seasonal influenza and was the recommended drug for treating the anticipated pandemic of swine influenza (H1N1) in 2009.Citation3–Citation5 OP works effectively in humans when used within 48 hours following the first appearance of symptoms (fever).Citation6 However, case reports have associated OP intake with sudden abnormal behaviors.Citation7–Citation9
Recent studies have shown that OP and its metabolite, OP carboxylate (OC), cross the blood-brain barrier.Citation10,Citation11 They have been shown to induce neuronal excitability and synchrony in hippocampal slices and slice cultures.Citation7,Citation12 Moreover, OP increases dopamine levels in the medial prefrontal cortex of rats.Citation13 Most animal model studies of the action of OP on the brain have been conducted in normal rather than IAV-infected rats or mice, and the animals functioned at the normal core temperature. Until now, there has been no information describing the effect of OP on intact brain hippocampal activity of IAV-infected animals during hyperthermia.
Despite the efficacy of OP in clinics, the putative side effects associated with OP have made the use of this drug controversial. In clinics, IAE patients’ electroencephalograms (EEG) are characterized by abnormal high-voltage EEG activity with spikes and theta oscillations during high fever.Citation14–Citation16 These clinical abnormal EEG activities (high-voltage amplitudes, increased EEG spikes, and theta oscillations) were reproduced in the rat model of IAE under hyperthermia (39.9°C–41°C).Citation17
Rats and mice do not normally develop fever after IAV infection.Citation17,Citation18 In a previous study,Citation17 abnormal EEG activities were not observed in non-anesthetized IAV-infected rats at the normal core temperature (37°C–38°C). Thus, hyperthermia was a precondition to observe abnormal EEG activities and was induced using a heating system, as previously reported.Citation17 In the present study, we examined the effect of OP on abnormal EEG activities in the hippocampus of isoflurane-anesthetized rats during hyperthermia, using the rat model of IAE.Citation17
Methods
Animals, virus infection and electrophysiology
This study was performed in accordance with the guidelines for animal care and use approved by the animal care committee of The University of Tokushima. Since our goal was to model IAE in children, male Wistar rats aged 3 to 4 weeks were used because rats in this range correspond roughly to the human age range of 2.5 to 3 years.Citation19
For viral infection, the rats were anesthetized with ketamine-xylazine (62.6 mg/kg–12.4 mg/kg). Influenza A/WSN/33 strain was stocked (frozen) at −82°C and diluted to obtain the dose (1 × 105 plaque forming unit in PBS, 60 µL), which was then applied intranasally to the rats, as previously reported.Citation17 The rooms for virus stockage, viral infection, and handling of the infected animals were designed in accordance with the guidelines for animal care and use of The University of Tokushima.
Anesthesia and hyperthermia
The in vivo electrophysiology experiments were carried out under isoflurane anesthesia. EEG activity in isoflurane anesthetized animals is characterized by slow bursting activity followed by a burst suppression period.Citation17,Citation20
The rats were first anesthetized by low-dose of ether, then fitted to a stereotaxic frame (model SN-6N, Narishige, Tokyo). The animals were gas-anaesthetized with 1.5–1.7 minimum alveolar concentration (MAC) of isoflurane mixed with 30% O2 and 70% N2, which allowed spontaneous respiration. During the EEG recordings, a heating pad system (model 21051-00; Fine Science Tools Inc, Foster City, CA) with a feedback control probe was inserted rectally. The body temperature of rats placed on the heating pad gradually increased from 37°C to 41°C in a period of 20 minutes, as previously reported.Citation17
EEG recordings
EEG activity was recorded using enamel-coated tungsten wire electrodes with an uncoated diameter of 120 µm (MT Giken Co, Tokyo, Japan). Craniotomy was performed without damaging the underlying dura using a standard miniature drill equipped with 0.5 mm diameter drill bit. The electrodes were inserted based on the rat brain in stereotaxic coordinates of Paxinos and Watson.Citation21 To record the hippocampal activity, an electrode was lowered into the CA1-CA3 area (left hemisphere) at 2.8 to 3.0 mm posterior to the bregma, 2.7 to 2.9 mm lateral from midline, and 2.6 to 3.0 mm below the dura. Signals were recorded using a dual microprobe system (WP Manufacturing, Inc, Longmont, CO), and a homemade amplifier (×1000). The baseline was adjusted to zero-level with a slow voltage clamp system with a time constant of 2.2 seconds. The signal was low-pass filtered at 0.5 to 3 kHz, sampled at 1 kHz, and recorded using Axopatch software (Axon Instruments, Palo Alto, CA). To verify the electrode position, the electrode tip was coated with a lipophilic tracer dissolved in dimethylsulfoxide at a concentration of 1 mg/mL to 2.5 mg/mL before insertion into the brain. After removal of the electrodes, the rats were anesthetized with ketamine-xylazine (62.6 mg/kg–12.4 mg/kg) and were transcardially perfused with saline, followed by fixation (4% paraformaldehyde). The brain was removed and immediately put in sucrose and kept in a 4°C room. Sections 800 µm thick were then prepared. Red traces of the dye left by the electrodes were observed under the microscope and photographed.
The electrophysiology EEG data analysis was performed using IGOR Pro 4 (WaveMetrics, Inc, Lake Oswego, OR) and the fast Fourier transformation (FFT) of EEG activity was computed for 30-second periods.
Slow and distinctive EEG waves were monitored continuously during the experiments to ensure that the rat was well anesthetized and without pain. At the end of experiments, the rats were given a lethal dose of pentobarbital (50 mg/kg).
Characterization of abnormal EEG activities
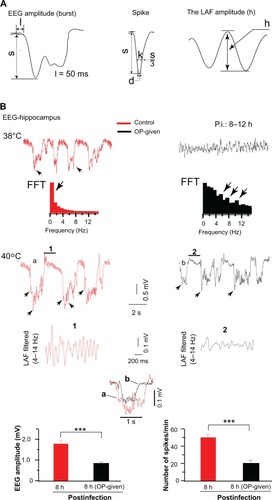
In order to examine the efficacy of OP on abnormal EEG activities during hyperthermia, we focused particularly on the following observed after IAV infection in the rats during hyperthermia:Citation17 abnormal high-voltage EEG activity, enhanced low-amplitude fluctuation (LAF) during burst suppressed periods, increased EEG spikes, and theta oscillations. The EEG activity was recorded in the hippocampus of the IAV-infected rats at 8 to 12 hours, 26 to 30 hours, and 50 to 56 hours, corresponding to the periods during which the peak abnormal EEG activities are observed in the rat model of IAE.Citation17
The EEG amplitude was measured by quantifying the number of bursts (n = 15 bursts average/point) and the EEG spike was identified as a sharp wave that usually sprouts randomly within the burst and during the burst suppressed periods, as previously described.Citation17 Theta oscillations amplitude was determined by measuring theta wave amplitude (from the positive to the peak, n = 15 waves average/points). The details of the EEG components and parameters are shown in .
Figure 1 Abnormal EEG activities were reduced in infected rats treated with OP soon after infection. (A) The characterization of abnormal EEG components (s, amplitude in mV, from the base line to depth negative, l = 50 ms); spike (1 ms < d ≤ 15 ms, 1 < k ≤ 35 ms); LAF amplitude (h, amplitude in mV). (B) The recorded abnormal EEG activities at 8 to 12 hours after infection are displayed at 38°C and 40°C body temperatures. Left: At 38°C in the control rats, EEG displayed slow activity and its corresponding dominant slow component (arrow) is depicted in the FFT plot. At 40°C, EEG displayed high-voltage slow EEG activity with enhance LAF amplitude as depicted in the filtered (4–14 Hz) and expanded segment (1, right). In the OP-administered rats, EEG shows theta oscillations (top) at 38°C and the EEG corresponding FFT plot peaked at various theta band frequencies indicated by the arrows (bottom). At 40°C, EEG displayed a low-voltage slow EEG activity. The LAF is shown in the expanded segments (2) filtered at 4–14 Hz. Note a disrupted rhythmic activity and reduce amplitude in the trace (2). Superimposed traces of bursts of the control EEG (a) and of OP-administered EEG (b) displaying a clear reduced amplitude of OP-administered trace. Bottom: Quantification of EEG amplitude (left) and EEG spikes (right) are plotted.
Abbreviations: EEG, electroencephalogram; LAF, low-amplitude fluctuation; OP, oseltamivir phosphate; FFT, fast Fourier transformation; ns, nonsignificant.
Drug and antagonist treatment
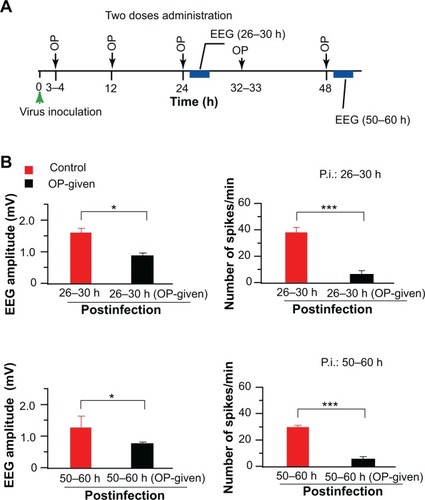
All the rats were infected with IAV. OP was not administered (control) to some of the rats, but was administered to the remaining rats. Tamiflu® capsules (75 mg) were purchased commercially from Chugai Pharmaceutical Co. (Tokyo, Japan), and the OP in the contents of the capsules was dissolved in water. OP mixture with stabilizing additives from the capsules or recrystallized OP was orally administered to the rats and they were monitored (for 1 hour postadministration) in a cage prior to the EEG recording. OP was administered to the rats in a single dose or in two doses per day ().
Figure 2 OP significantly reduced abnormal EEG activities. OP applied two doses per day. (A) OP oral administration and EEG recording time course is schematized. The green arrowhead indicates the virus inoculation time, dark arrows show the OP administration time, and the blue rectangle indicates the EEG recording time. (B) Top: EEG activity recorded at 26 to 30 hours after infection.
Abbreviations: EEG, electroencephalogram; OP, oseltamivir phosphate.
Atropine (30 mg/kg; Nacarai, Kyoto, Japan), an mAChR agonist, was dissolved in saline (0.9% NaCl) and was given via intraperitoneal (ip) injection.
Statistical analyses
Numerical values were expressed as mean ± SD. P value was obtained by Student’s paired t-test (using SigmaPlot 10; Systat Software Inc, San Jose, CA), and P < 0.05 was considered statistically significant.
Results
In single dose cases, OP was administered 3 hours after infection (n = 5) and EEG was recorded at 8 hours to 12 hours after infection. In the control rats (IAV-infected rats not given OP), the EEG showed slow oscillations at 38°C core temperature; the corresponding slow component (<1 Hz) is depicted in the FFT plot (, top left). At 40°C core temperature, the EEG showed high-voltage slow EEG activity (or theta-like oscillations in two of the five rats) with increased spikes (arrowheads in the EEG traces) and enhanced amplitudes of the LAF, as shown in the expanded trace (segment 1) (, left bottom). In this study and a previous study,Citation17 theta-like oscillations in the control rats were observed only during hyperthermia (39.9°C–41°C). In contrast, in the OP-administered rats, the EEG displayed theta-like oscillations at the normal core temperature (38°C) in two out of the five rats (, top right). The FFT plot shows the corresponding theta frequency distributions (, bottom right). At 40°C, the EEG amplitude was reduced and the rhythmic activity of the LAF was disrupted while the amplitude of the LAF was reduced, as shown in the expanded trace (segment 2) shown in . There was a significant difference (n = 3, P < 0.001) between the amplitude of LAF values of the OP-administered rats (0.14 ± 0.05 mV) and that of the control rats (0.39 ± 0.06 mV). The quantified abnormal EEG amplitudes (left) and EEG spikes (right) were reduced in the OP-administered rats compared with the control rats (, bottom).
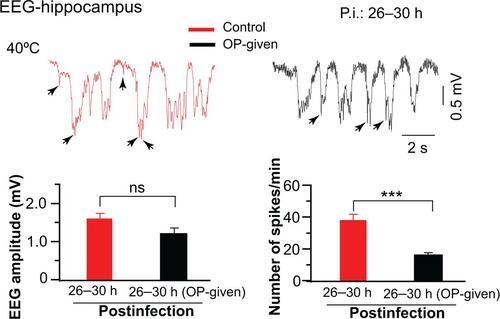
The EEG was then recorded at 26 to 30 hours after infection. Abnormal EEG amplitudes in OP-administered rats were reduced (n = 5, supplementary ), but the reduction was not significant. However, the amplitude of LAF values (0.26 ± 0.04) was significantly (n = 3, P < 0.05) reduced in the OP-administered rats compared to the control rats (0.14 ± 0.04), and the EEG spikes were significantly (P < 0.001) reduced in OP-administered rats. This suggests that the efficiency of the single-dose OP administration may be weakened after 26 hours of infection.
When two doses of OP were administered per day, abnormal EEG amplitudes (P < 0.05) and EEG spikes (P < 0.001) recorded at 26 to 30 hours after infection were more significantly reduced compared with the control rats (n = 3; , top). Those at 50 to 60 hours after infection were also reduced in comparison with the control rats (n = 3, , bottom). This finding suggests that two doses of OP administered per day were more efficient than a single dose in reducing abnormal EEG activities in IAV-infected rats.
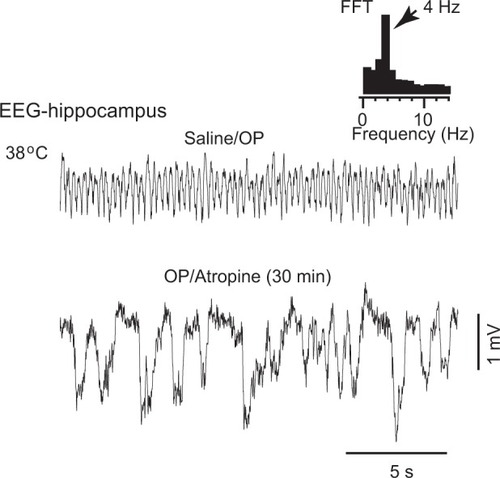
Two types of theta are known: atropine sensitive (a muscarinic receptor blocker) and atropine resistant.Citation22,Citation23 We studied the effect of atropine on the OP-induced theta oscillations in IAV-infected rats. We first confirmed that EEG displayed theta oscillations at 38°C. Then, maintaining this temperature, a saline ip solution was injected, and 30 minutes after the saline injection, atropine (30 mg/kg, ip) was administered. As can be seen in , atropine blocked the theta oscillation in all the rats examined (n = 3), revealing that the OP-induced theta oscillation is atropine sensitive.
Figure 3 Atropine blocked theta oscillations. The EEG activity was recorded in the hippocampus at 38°C rat body temperature. OP-induced theta oscillations were suppressed by intraperitoneal injection of atropine.
Abbreviations: EEG, electroencephalogram; OP, oseltamivir phosphate; FFT, fast Fourier transformation.
Discussion
The present work is the first study to report the effect of OP on rats infected with IAV under hyperthermia in vivo.
We found that one or two orally administered doses of OP were efficient when given soon (~3 hours) after IAV infection. OP efficiently reduced the abnormal EEG activities (ie, enhanced amplitude of the LAF, high-voltage EEG amplitudes, increased EEG spike) in the IAV-infected rats. However, we also observed theta oscillations at 38°C core temperature in the OP-administered rats.
Although the mechanisms by which OP reduces abnormal EEG activity are not clear, we presume at least that the N-methyl-D-aspartate (NMDA) receptor and the blockage of the virus spread from the host cells could play important roles in reducing the high-voltage amplitudes and EEG spikes, as explained below.
IzumiCitation7 reported that OP induces neuronal excitability via NMDA receptor activation. Our recent study also found that in uninfected rats the activation of NMDA receptors peaked at 2 hours after OP administration and declined at 4 hours after OP administration (data not shown). This suggests the inactivation of the NMDA receptors at 4 hours after OP administration, and this inactivation may prevent calcium influx into the neurons. It is also known that OP is a sialic acid analogue, which inhibits the influenza neuraminidase enzyme and prevents the release and spread of the virus from infected host cells during budding.Citation4,Citation6 OP treatment (1 hour after infection at daily base) significantly reduces the infection rate in multiple cell types and reduces the progression of the virus in mice.Citation24 These previous findings suggest that the early administration of OP after IAV infection within a period before the virus proliferates, in addition to the inactivation of the NMDA receptors at a later time (>4 hours) after OP administration, could be possible factors in explaining how OP works to reduce the abnormal EEG activities.
The observation of theta oscillations at 38°C in OP-administered rats is an important finding for confirming the possible action of OP on muscarinic acetylcholine (mACh) receptors. We have observed a similar finding in normal uninfected rats; the OP administered to these rats induced theta oscillations (2 Hz to 6 Hz) at 38°C core temperature (data not shown). Theta oscillations were atropine sensitive and prevented fast hippocampal activity, which resulted in a slow EEG activity (). This result is in full agreement with a previously reported in vitro study in which OP was found to activate mACh receptors.Citation12 The activation of mACh receptors may suggest the stimulation of acetylcholine containing cholinergic neurons, which have been known to play important roles in cortical activation and in regulating states of consciousness.Citation25,Citation26 ACh is a major excitatory neurotransmitter in the central nervous system. It plays key roles during synaptic transmission, and constitutes a system with other neurotransmitter/modulators to regulate brain states of vigilance. Citation25
Because the EEG recording from the hippocampus in nonanesthetized rats was not possible, the effect of anesthesia could be an issue to consider during the interpretation of the data. Isoflurane has a neuroprotective effect on the brain. It reduces excitatory synaptic transmission in the hippocampus,Citation27–Citation29 and it enhances inhibitory synaptic potentials at concentrations above 0.5 MAC (1%).Citation29 Isoflurane alters the ACh release in a dose-related manner, and that at 1.5 MAC, it significantly decreases ACh release in the cortex and striatum of rats.Citation30,Citation31 With such concentrations, EEG activity is generally characterized by slow (bursting) activity followed by burst-suppressed periods.Citation17,Citation20 In the present and previous studies,Citation17 1.5–1.7 MAC isoflurane was used. Thus, it is unlikely that the theta oscillations were isoflurane dependent.
The physiological significance of high-voltage slow and theta oscillations in influenza patients in the clinic is not well documented. However, in the rat model of IAE, we speculated that the alternation between these two oscillations may lead to brain instability, and that this may explain the abnormal behaviors observed in some patients.Citation17 In the present study, the OP-induced theta oscillations were similar to those observed during hyperthermia in the IAV-infected rats not given OP. Thus, both theta oscillations suggest the stimulation of ACh release. Excessive release of ACh may affect synaptic transmission and oscillation patterns in the brain, which may lead to abnormal behavior in influenza patients. Under such conditions, atropine may play a therapeutic role in stabilizing the brain states from fast (2–6 Hz) to slow (<1 Hz) oscillations.
In the present study, because hyperthermia was a precondition for observing EEG abnormalities in the control rats, the effect of OP on core temperature before and during EEG recording was not investigated. However, recent studies showed that OP induces hypothermia in miceCitation32 and OP administered in ethanol-injected rats significantly augmented the hypothermia effect.Citation7 Hypothermia has also been reported after OP ingestion in clinics.Citation33
Conclusion
Our data show that OP, when administered in the early phase of an infection, can efficiently reduce abnormal EEG activities in IAV-infected rats. However, OP administration may stimulate ACh release in rats at the normal core temperature.
Future studies should investigate the biomolecular basis of NMDA and mAChRs expression levels and measure ACh release in OP-administered rats, especially in the cortex and the hippocampus. Such an investigation could improve our understanding of protecting the brain during the early phase of IAV infection against neuronal excitability and neurological complications, and thus prevent abnormal behaviors (which can lead to fatal accidents) in influenza patients with high fever.
Acknowledgments
This research was supported by Grants-in-Aid (20611013) and the Special Coordination Funds for Promoting Science and Technology of the Ministry of Education, Culture, Science and Technology of Japan.
Supplementary figure
Figure S1 OP significantly reduced EEG spikes but not EEG amplitudes.
Notes: The effect of OP was weakened after 26 to 30 hours postinfection. Note a decrease of EEG amplitude in the OP-administered rats compared with the control rats. However, the difference was not significant. In contrast, a significant decrease in the number of spikes was observed in the OP-administered rats.
Abbreviations: OP, oseltamivir phosphate; EEG, electroencephalogram.
Disclosure
The authors report no conflicts of interest, direct or indirect, in this work.
References
- KasaiTTogashiTMorishimaTEncephalopathy associated with influenza epidemicsLancet20003551558155910801205
- MorishitaTTogashiTYokotaYEncephalitis and encephalopathy associated with an influenza epidemic in JapanClin Infect Des200235512517
- GubarevaLOkomo-AdhiamboMUpdate: Drug susceptibility of swine-origin influenza A (H1N1) viruses. Center for Disease Control and Prevention (CDC)MMWR Morb Mortal Wkly Rep20095843343519407738
- MosconaANeuraminidase inhibitors for influenzaN Engl J Med20053531363137316192481
- SawabuchiTSuzukiSIwaseCBoost of mucosal secretory immunologlobulin: a response by clarithromycin in paediatric influenzaRespirology2009141173117919909463
- McNichollIRMcNichollJJNeuraminidase inhibitors: zanamivir and oseltamivirAnn Pharmacother200135577011197587
- IzumiYTokudaKO’DellKAZorumskiCFNarahashiTNeuroexcitatory action of tamiflu and its carboxylate metaboliteNeurosci Lett2007426545817884292
- MaxwellSRTamiflu and neuropsychiatric disturbance in adolescentsBMJ20073341232123317569896
- TanabeTHaraKNakajimaMOseltamivir treatment for children showing abnormal behavior during influenza virus infectionBrain Dev20103244044419200672
- MorimotoKNakakariyaMShirasakaYOseltamivir (Tamiflu™) efflux transport at the blood-brain barrier via P-glycoproteinDrug Metab Dispos2008366917940134
- OseAKusuharaHYamatsuguKP-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrierDrug Metab Dispos20083642743418039806
- UsamiASasakiTSatohNOseltamivir enhances hippocampal network synchronizationJ Pharmacol Sci200810665966218403897
- YoshinoTNisijimaKShiodaKYuiKKatoSOseltamivir (Tamiflu) increases dopamine levels in the rat medial prefrontal cortexNeurosci Lett2008438676918457919
- SatoSKumadaSKojiTOkaniwaMReversible frontal lobe syndrome with influenza virus infection in childrenPediatr Neurol200022431832110788752
- OkumuraANakanoTDelirious behavior in children with influenza: its clinical features and EEG findingsBrain Dev20052727127415862189
- FukumotoYOkumuraAFukumotoKSerum levels of cytokines and EEG finding in children with influenza associated with mild neurological complicationsBrain Dev20072942543017287101
- CisséYWangSInoueIKidoHRat model of influenza-associated encephalopathy (IAE): study of electroencephalogram (EEG) in vivoNeurosci201016511271137
- TothLARehgJEWebsterRGStrain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized miceJ Neuroimmunol19955889997730450
- RuthEBMetamorphosis of the public symphysis. I. The white rat (Mus norgicus albinos)Anat Rec19356417
- KroegerDAmzicaFHypersensitivity of anesthesia-induced comatose brainJ Neurosci20072739105971060717898231
- PaxinosPWatsonCThe Rat Brain in Stereotaxic Coordinates5th edAmsterdam; BostonElsevier Academic Press2005
- GoutagnyRJacksonJWilliamsSSelf-generated theta oscillations in the hippocampusNat Neurosci200912121491149319881503
- KramisRVanderwolfCHBlandBHTwo types of hippocampal rhythmical slow activity in both the rabbit and the rat: Relations to the behaviour and effects of atropine, diethyl ether, urethane, and pentobarbitalExp Neurol1975491 Part 158851183532
- ManicassamyBManicassamySBelicha-VillanuevaAAnalysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virusPNAS201010725115191152420534531
- JonesBENeurotransmitter systems regulating sleep-wake statesBiological PsychiatryD’HaeninDden BoerJAWillnerPNew YorkJohn Wiley and Sons2002212151228
- CelesiaGGJasperHHAcetylcholine released from cerebral cortex in relation to state of activationNeurology199616105310635950916
- Lloyd-ThomasARColePVPriorPFQuantitative EEG and brain-stem auditory evoked potentials: Comparison of isoflurane with halot-hane using the cerebral function analsysing monitorBr J Anaesth1990653063122223358
- MacIverMBRothSHInhalation anaesthetics exhibit pathway-specific and differential actions on hippocampal synaptic responses in vitroBr J Anaesth1988606806912837263
- MiuPPuilEIsoflurane-induced impairment of synaptic transmission in hippocampal neuronsExp Brain Res1989753543602542074
- ShichinoTMurakawaMAdachiTMiyazakiYMoriKEffects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivoBr J Anaesth1998803653709623440
- ShichinoTMurakawaMAdachiTEffects of isoflurane on in vivo release of acetylcholine in the rat cerebral cortex and striatumActa Anesthesiol Scand19974113351340
- OnoHNaganoYMatsunamiNOseltamivir, an anti-influenza virus drug, produces hypothermia in mice: comparison among oseltamivir, zanamivir and diclofenacBiol Pharm Bull20083163864218379055
- Ministry of Health, Labor and Welfare, Japan (MHLW)Public opening data (documents 5-1-1 and 5-2) at the Meeting for Safety Measure Investigation CouncilApril 4, 2007 Available from: http://www.mhlw.go.jp/shingi/2007/04/s0404-2.htmlAccessed April 4, 2007 Japanese