Abstract
Antipsychotic drugs are extensively prescribed for the treatment of schizophrenia and other related psychiatric disorders. These drugs produced their action by blocking dopamine (DA) receptors, and these receptors are widely present throughout the brain. Therefore, extended antipsychotic use also leads to severe extrapyramidal side effects. The short-term effects include parkinsonism and the later appearing tardive dyskinesia. Currently available treatments for these disorders are mostly symptomatic and insufficient, and are often linked with a number of detrimental side effects. Antipsychotic-drug-induced tardive dyskinesia prompted researchers to explore novel drugs with fewer undesirable extrapyramidal side effects. Preclinical studies suggest a role of 5-hydroxytryptamine (serotonin)-1A and 2A/2C receptors in the modulation of dopaminergic neurotransmission and motivating a search for better therapeutic strategies for schizophrenia and related disorders. In addition, adjunctive treatment with antioxidants such as vitamin E, red rice bran oil, and curcumin in the early phases of illness may prevent additional oxidative injury, and thus improve and prevent further possible worsening of related neurological and behavioral deficits in schizophrenia. This review explains the role of serotonergic receptors and oxidative stress, with the aim of providing principles for prospect development of compounds to improve therapeutic effects of antischizophrenic drugs.
Introduction
Antipsychotic drugs
Antipsychotics were initially known as “neuroleptics” (from Latin, to take a grip of the neuron), since extrapyramidal side effects (EPSE) were considered fundamental for their curative properties.Citation1 The antipsychotic is a group of drugs used to treat mental health conditions such as schizophrenia, agitation, severe anxiety, mania, and violent impulsive behavior in both adults and adolescents. These drugs produced their effects particularly by blocking dopamine (DA) receptors in the brain. Antipsychotic drugs can not only be categorized by their chemical structure but also be classified according to their pharmacology and clinical properties.
Types of antipsychotic drugs
Typical antipsychotic drugs
These are the most primitive neuroleptic drugs, dating from the early 1950s. The typical antipsychotic properties are accomplished through its antagonism at dopaminergic receptors. Haloperidol, the most commonly used conventional antipsychotic, is primarily effective in treating the positive signs of schizophrenia. The typical antipsychotic medications (eg, haloperidol or thioridazine), with or without psychosocial and environmental interventions, are frequently used to treat symptoms that occur in a majority of older patients with dementia during their illness courses and have been the mainstay of psychopharmacologic treatment during the past several decades.Citation2
Atypical antipsychotic drugs
The “second generation” of neuroleptics was developed between the early 1960s and the 1980s. Initially, clozapine appeared as an innovatory drug with improved effectiveness over existing agents.Citation3 The atypical neuroleptics are different from the older drugs concerning their mechanism of action. They exhibit less EPSE due to their combined effects on both dopaminergic and serotonergic receptors. Formerly, they were called atypical, because their propensity to cause dyskinetic symptoms in some patients was absent. It is now known that atypical neuroleptics also cause symptoms of tardive dyskinesia (TD), but such symptoms may take longer to manifest.
Third-generation antipsychotic drugs
These medications are the most recent to be developed and still carry the risk of causing dyskinesia. Since these are the newest medications available (developed since 2001), they are also the most costly. Aripiprazole is a fairly new antipsychotic drug that is used for the treatment of bipolar disorder and clinical depression but it is still not free from side effects.Citation4
Typical versus atypical antipsychotic drugs
First-generation antipsychotics (FGAs) are classified according to their chemical structure, while second-generation antipsychotics (SGAs) are categorized according to their pharmacologic properties. The FGAs were effective, but the EPSE were often excruciating, which improved the usage of SGAs, which seem to have less risk for these effects. Due to this reason, conventional antipsychotics are usually substituted by the atypical antipsychotics.Citation5 Some clinical studies have been conducted to find out the efficacy and safety of these drugs on neuropsychiatric symptoms, but contradictory results were obtained.Citation6 Some meta-analyses and systematic reviews have provided only limited support for the superiority of SGAs.Citation7–Citation9 There was no substantial evidence that second-generation drugs were more effective and better tolerated for the treatment of negative symptoms or cognitive deficits.Citation10 In this perspective, it has been suggested that typical antipsychotics are still as useful as atypicals in the treatment of schizophrenia.Citation11 It has been found that none of the more recently developed antipsychotics except olanzapine have been shown to be superior to FGAs or even to achieve the level of clinical efficacy as attained with clozapine.Citation12,Citation13 The results are also equivocal regarding the incidence of EPSE in which, except for clozapine, no clear improvement was observed in EPSE profiles with newer antipsychotics.Citation14,Citation15 This failure is perhaps not surprising given the lack of clarity/validity of the difference between typical versus atypical antipsychotics, or the distinction between FGAs, SGAs, and third-generation antipsychotics.
Antipsychotic-drug-induced side effects
Drug-induced side effects can sometimes be troublesome. There is often a trade-off between easing symptoms and having to put up with some side effects from treatment. Different antipsychotic medicines can have different types of side effects. Sometimes one medicine causes side effects in some people but not in others. At present, none of the antipsychotic is absolutely free from side effects; thus, the treatment with these agents involves balancing risks and benefits. We need to have better understanding of pharmacological principles that could enhance the efficacy and minimize the side effects of these agents. These medications induced side effects including TD and other related movement disorders.Citation16–Citation21 Many of them have other serious side effects such as agranulocytosis, a type of autoimmune disease in which the number of infection-fighting white blood cells are decimated. Neuroleptic malignant syndrome is a rare but potentially fatal disease that is associated with all classes of antipsychotic medication.Citation22 The risk of sudden cardiac death is increased >2.4 times with both typical and atypical antipsychotic drugs.Citation23 Extrapyramidal effects include parkinsonism, dystonia, and oropharyngeal dysphagia leading to an increased risk of pneumonia.Citation24 Other side effects of atypical neuroleptics may include diabetes, inflammation of the pancreas, and weight gain. It is also known to cause insomnia as well as drowsiness, constipation, and vision problems. However, meta-analyses of clinical trials in participants with chronic schizophrenia have suggested a limited advantage of the newer agents in terms of efficacyCitation7–Citation9 and two recent large trials failed to find a difference between these two classes of antipsychotics.Citation25,Citation26 The quest for the search of more effective antipsychotic drugs with a lower occurrence of EPSE, therefore, remains highly relevant and represents an unmet research requirement for the finding of novel treatment strategies for schizophrenia and other disorders in which psychosis represents an important aspect, such as Huntington’s disease.Citation27,Citation28 In this review, we focused on the typical antipsychotic-induced movement disorders and possible therapeutic strategies for alleviating their undesirable side effects.
Antipsychotic-induced parkinsonism and its treatment
What is parkinsonism?
Major symptoms of parkinsonism include tremor, rigidity, akinesia, and postural instability. Currently, the term drug-induced parkinsonism is nearly identical with antipsychotic-induced parkinsonism. However, drug-induced parkinsonism is a recurrent undesirable effect of several drugs that impede dopaminergic function particularly in the basal ganglia,Citation29,Citation30 including calcium antagonists, orthopramides, and substituted benzamides.Citation29,Citation31,Citation32 DA receptors are extensively dispersed in the brain, and typical antipsychotics bind with dopaminergic receptors mainly in the striatum. Consequently, patients using these drugs are mostly at high risk of exhibiting parkinsonism and other related EPSE. Typical neuroleptic drugs such as haloperidol are extensively prescribed for the treatment of schizophrenia; their therapeutic effects are also associated with movement disorder,Citation33 for instance, parkinsonian-like effects and TD are frequently described as EPSE.Citation17,Citation19,Citation34 On the other hand, the risk for the development of EPSE was considered to be low for atypical antipsychotics such as clozapine, risperidone, olanzapine, and aripiprazole. It was believed that their relatively low incidence to induce EPSE was due to their potential antagonist activity at 5-hydroxytryptamine (5-HT)-2A receptors than DA receptors.Citation35 Although, treatment with neuroleptics could elicit EPSE, these drugs still prove to be efficient in treating and managing schizophrenia and related behavioral disorders.
Animal model of parkinsonism
Two standard behavioral preclinical tests are used to evaluate the following:
When a D2 receptor antagonist such as haloperidol is administered to a rat, it will remain immobile for a given period of time when placed on an inclined surface. This immobility and/or frozen body posture is called catalepsyCitation17,Citation18,Citation20,Citation36,Citation37 and caused by the blockade of DA receptors within the striatum and is considered to be a predictor of EPSE inducing potential in humans.Citation36,Citation38
The second test, the impairment of motor coordination, is also used as a predictor of antipsychotic-induced EPSE. A day before the treatment, animals are trained in a single session until they attain 150 seconds on the Rotor-Rod. Anti-psychotic agents will impair its ability to attain 150 seconds on Rota-Rod. The latency to fall in a test session of 150 seconds will be taken as a measure of motor coordination.Citation19–Citation21
Pathophysiology of parkinsonism
Antipsychotic drugs have potential DA D2 receptor blocking capability, and the beneficial therapeutic effects of these drugs on psychosis are linked with their action on the reduction in dopaminergic transmission particularly in the limbic system. Antipsychotic-drug-induced striatal DA D2 receptor blockade produces initial disinhibition of gamma amino butyric acid (GABA)- and encephalin-containing striatal neurons after the disinhibition of the subthalamic nucleus. This leads to augmented thalamocortical GABAergic inhibition by enhancing the inhibitory projection from the globus pallidus/substantia nigra pars reticulata. This pathway is similar to the model of interruption of the basal ganglia motor loop in Parkinson’s disease. Likewise, >80% of D2 receptors were reported to be occupied in patients with EPSE who were using neuroleptics.Citation39 Therefore, clinical symptoms of Parkinson disease started to appear when >80% of nigral dopaminergic neurons had degenerated. In this respect, neuroleptic-induced motor disorders are known as parkinsonian-like symptoms.Citation40
Role of serotonin in parkinsonism
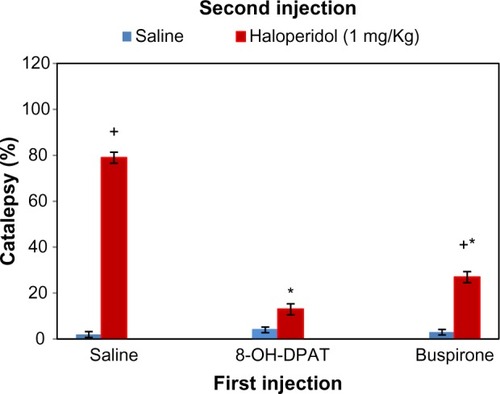
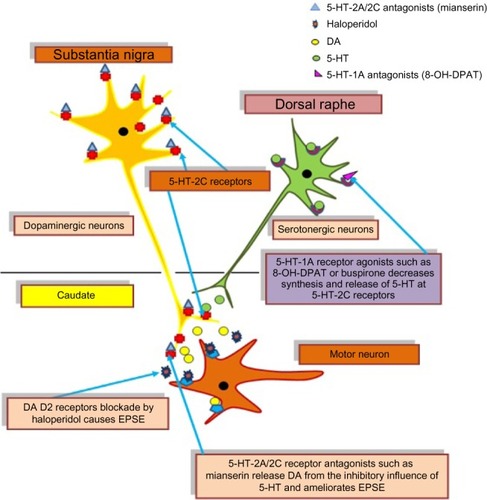
The role of serotonergic system has been identified in the toning of dopaminergic neuronal activity. 5-HT (serotonin)-1A agonists and partial agonists are identified to elicit the anticataleptogenic effects,Citation17,Citation19,Citation41–Citation43 which is positively correlated with the rank order of their intrinsic activity at 5-HT-1A receptors.Citation44 In order to find out the prospective role of somatodendritic and/or postsynaptic 5-HT-1A receptors in the modulation of haloperidol-induced catalepsy, weCitation4 found a diminished availability of 5-HT in the striatum by the administration of partial agonist buspirone and selective 5-HT-1A agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) in rats owing to the stimulation of somatodendritic 5-HT-1A receptors. Therefore, dopaminergic neurotransmission was released from the inhibitory effect of 5-HT to attenuate haloperidol-induced catalepsy (). On the other hand, postsynaptic 5-HT-1A receptors were occupied by 8-OH-DPAT and exerted an extra stimulatory influence on dopaminergic neuronal activityCitation45 leading to the additional anticataleptogenic effects of 8-OH-DPAT compared with buspirone.Citation17 By and large, these studies provide evidence that the stimulation of presynaptic in addition to postsynaptic 5-HT-1A receptors augments DA neurotransmission particularly in the striatum. 5-HT-2C receptors are known to exert serotonergic inhibitory influence on the activity of dopaminergic neurons. These receptors are located on the cell body and terminal region of dopaminergic neurons.Citation46,Citation47
Figure 1 Cataleptic effects of haloperidol (1 mg/kg) in saline, 8-OH-DPAT (0.25 mg/kg), or buspirone (1 mg/kg) pre-injected rats.
Abbreviations: ANOVA, analysis of variance; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino) tetralin; SD, standard deviation.
Therefore, the administration of mianserin and mesulergine, 5-HT-2A/2C receptor antagonists ( and ), possibly releases DA neurons from the inhibitory effects of 5-HT to ameliorate parkinsonian-like effects of the neuroleptics drug.Citation20,Citation48 This effect is illustrated in .
Figure 2 Effects of mianserin (2.5 and 5.0 mg/kg) on haloperidol-induced deficits of exploratory activity (in an open field [A]) and motor coordination (B) on Rota-Rod in rats.
Abbreviation: SD, standard deviation.
![Figure 2 Effects of mianserin (2.5 and 5.0 mg/kg) on haloperidol-induced deficits of exploratory activity (in an open field [A]) and motor coordination (B) on Rota-Rod in rats.](/cms/asset/3188b9be-b69a-4fea-add5-108a8a587417/djep_a_63553_f0002_c.jpg)
Figure 3 Effects of mesulergine (1.0 and 3.0 mg/kg) on haloperidol-induced deficits of exploratory activity (in an open field [A]) and motor coordination (B) on Rota-Rod in rats.
Abbreviation: SD, standard deviation.
![Figure 3 Effects of mesulergine (1.0 and 3.0 mg/kg) on haloperidol-induced deficits of exploratory activity (in an open field [A]) and motor coordination (B) on Rota-Rod in rats.](/cms/asset/afc9e9ef-cb70-4e29-9cef-ce68cbd2c320/djep_a_63553_f0003_c.jpg)
Figure 4 Role of 5-HT receptors in the modulation of haloperidol-induced EPSE.
In addition to drugs, certain physiological conditions may also have beneficial implications to treat or alleviate antipsychotic-induced side effects. We have foundCitation21 that repeated exposure to immobilization stress elicited a reversal of haloperidol-induced motor deficits in rats. It was proposed that somatodendritic 5-HT-1A and 5-HT-2C receptor desensitization following the exposure to uncontrolled stress contributes to the release of DA neurotransmission from the inhibitory effect of 5-HT. Conversely, an increase in the efficacy of postsynaptic 5-HT-1A receptors may also produced an excitatory influence on the activity of the dopaminergic neuron. Therefore, it was thought to be involved in the reversal of haloperidol-induced parkinsonian, like symptoms in rats exposed to repeated stress.Citation21
Antipsychotic-induced TD and its treatment
What is TD?
The term “tardive dyskinesia” was coined in 1964 by Faurbye et al,Citation49 and it is a syndrome of potentially irreversible, involuntary hyperkinetic movements particularly in the orofacial region, which develops in patients during chronic neuroleptic treatment, and also it is a major limitation of neuroleptic therapy.Citation19,Citation34,Citation49,Citation50 Classical TD involves oral–buccal–lingual masticatory movements, which is characterized by repetitive jaw, face, and lingual movements (commonly protrusion) and may involve puffing of the cheeks, lip-smacking, puckering, and pursing, choreoathetoid movements in the limbs and trunk, guitar or piano playing movements, and other flexion and extension movements of the fingers and/or wrists.Citation51 TD develops in ∼35% of patients administered long-term with typical antipsychoticsCitation52 and may be irreversible in approximately half of the patients.Citation53
Epidemiological studies show that some factors such as age, institutionalization, a psychiatric diagnosis of mood disorders, and a history of acute extrapyramidal syndromes increase the risk of developing TD. It is more common in female patients.Citation54
Animal model of TD
Animal models for TD have been developed to explore new treatment strategies for this disabling disease.Citation19,Citation21,Citation41 Studies in animal models show that rats treated with chronic administration of antipsychotics exhibit vacuous chewing movements (VCMs) that show many classical characteristics of TD, including similarities in emergence, developmental time course, and response to dopaminergic drugs.Citation55 VCMs are generally described by purposeless mouth openings in the vertical plane, with or without tongue protrusion.Citation55,Citation56 Antipsychotic-induced VCMs seem to be in the same low frequency range (1–3 Hz) as TD symptoms in humans.Citation56 Although rats are considered one of the best animal models, there are certain drawbacks of this model. Haloperidol-induced VCMs have been reported to normalize after drug withdrawal in some studies,Citation57,Citation58 but they may be irreversible in humans.Citation59
Pathophysiology of TD
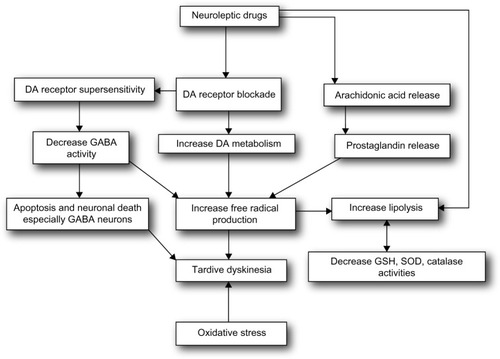
The pathophysiology of this disabling and frequently irreversible movement disorder is still vague. The long-term treatment with neuroleptics can induce some neuropathological changes in the central nervous system.Citation60 However, most studies have focused on the biochemical or behavioral changes correlated with long-term antipsychotic treatment. Several factors such as DA D2 receptor supersensitivity, GABAergic hypofunction, excitotoxicity, and oxidative stress (OS) have all been involved in the pathophysiology of TD.Citation61,Citation62 It has been hypothesized that chronic administration of antipsychotic causes postsynaptic DA receptor responsiveness in some studies, others reported an augmented behavioral response to DA agonists following long-term drug treatmentCitation63 and persistent DA D2 receptor occupancy.Citation64 In addition, it was also proposed that increase in the effectiveness of somatodendritic and postsynaptic 5-HT-1A receptors after chronic treatment with haloperidolCitation65,Citation66 might reduce the normal inhibitory serotonergic influence on locomotor activity to exhibit late-appearing dyskinesia as observed in patients on typical antipsychotic therapy.
It has been found that administration of typical antipsychotics such as haloperidol produces a shrinkage or loss of tyrosine hydroxylase immunoreactive neurons particularly in the substantia nigra. On the other hand, a decrease in the tyrosine hydroxylase immunoreactivity was also observed in rat striatum. Both of these changes may be involved in the elicitation of VCMs.Citation67,Citation68
These alterations particularly take place in the nigrostriatal pathway but not in the mesolimbic DA system.Citation69 It is therefore suggested that antipsychotic-induced brain morphological changes are the consequence of an enhanced DA metabolism that could raise intracellular DA concentration, followed by the inhibition of complex I, and increased OS.Citation70 The pathophysiology is illustrated in .
Role of serotonin in TD
5-HT-1A receptors are involved not only in the etiology of schizophrenia but also in the pathophysiology of TD. It has been found that postsynaptic 5-HT-1A receptors were upregulated in the postmortem brain samples of schizophrenic patients.Citation71,Citation72 Preclinical studies have also showed the role of 5-HT-1A receptors in the alleviation of TD. Thus, coadministration of buspirone, a 5-HT-1A receptor partial agonist,Citation72,Citation73 reversed reserpine-induced dyskinetic movements in a rat model of TD.Citation74 8-OH-DPAT, a selective 5-HT-1A agonist, inhibited haloperidol-induced VCMs in a dose-dependent manner.Citation75 It has been reported from our laboratory that coadministration of buspirone at low doses reversed the induction of VCMs and 5-HT-1A receptor supersensitivity followed by the chronic administration of haloperidol. Therefore, a supersensitive somatodendritic serotonergic influence on the activity of dopaminergic neurons is possibly a causative component in the onset of TD and considered a novel treatment strategy for curing schizophrenia.Citation19
OS and TD
There is a growing body of evidence that OS is involved in the pathology of major neuropsychiatric disorders. Evidence from postmortem as well as peripheral tissues indicates alterations in both free radicals and antioxidant defense mechanisms in disorders such as schizophrenia and mood disorders.Citation76 The long-term administration of typical neuroleptics, but not atypical, also decreases the levels of antioxidant enzyme, participating in the exacerbation of the oxidative events.Citation77,Citation78 In view of the OS generation, oxidative damage is detected when the production of reactive oxygen species surpasses the capability of the antioxidant system to remove them.Citation79 Additionally, the brain is more prone to the oxidative damage as compared with other organs or systems,Citation80 as it contains high concentration of membrane lipids, excitotoxic amino acids, low levels of antioxidant defenses, and autoxidizable neurotransmitters. The neuronal phospholipid membrane contains polyunsaturated fatty acids that are more vulnerable to lipid peroxidation by reactive oxygen species than other lipids such as cholesterol and saturated fatty acids.Citation81 Preclinical studies showed an increased rate of lipid peroxidation and peroxidative neuronal damage induced by the treatment with haloperidol.Citation82 It was found that OS contributed to the toxicity of haloperidol, which activated a sequence of cellular processes leading even to cell death; the production of free radicals was an integral part of this cascade.Citation76 The SGAs are reported to produce low risk of TD. But they still develop TD predominantly in older adults, perhaps due to the mechanisms that improve psychoses to overlap with those that could produce TD.Citation83,Citation84 Thus, there is a crucial requirement for better psychotic disorder management alternatives for or at least to lessen the side effects that develop with their use. Recently, the American Academy of Neurology presented the much-needed evidence-based guideline on the management of TD.Citation85
Treatment strategies for TD
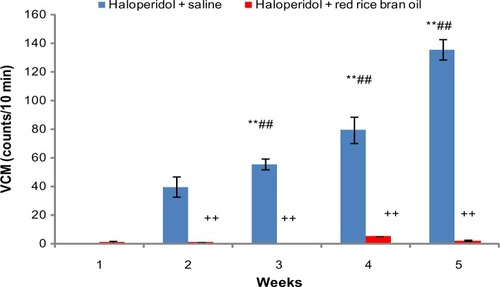
Recent studies from our laboratory have shown a reversal of haloperidol-induced parkinsonian-like effects and TD in rats cotreated with red rice bran oil (RRBO)Citation86 as shown in . It is tempting to relate protective and preventive effects of free radical scavenger RRBO with the reversal of haloperidol-induced TD. It is therefore suggested that incorporation of dietary supplementation-like RRBO having antioxidant properties can be useful to develop nutraceuticals for the treatment of schizophrenia.
Figure 6 Time course effect of RRBO on haloperidol-induced deficits of vacuous chewing movements.
Abbreviations: RRBO, red rice bran oil; SD, standard deviation; VCMs, vacuous chewing movements; W, water; S, saline; H, haloperidol.
Similarly, in other studies, antioxidant activity of hydroalcoholic extract of Brassica oleraceaCitation87 is reported to be involved in the reversal of haloperidol-induced TD suggesting it as beneficial adjuvant in the treatment of drug-induced EPSE effects and related disorders. However, other preclinical studies have reported that administration of nicotine may be useful for improving the TD associated with antipsychotic uses.Citation70 On the other hand, administration of curcumin reversed oxidative stress-induced TD in rats suggesting it as a promising therapeutic choice to treat this hyperkinetic movement disorder.Citation88 Related study presented a detailed review on positive results of nonneuroleptic agents in different trials, including tetrabenazine, amantadine, levetiracetam, piracetam, clonazepam, propranolol, vitamin B6, and Ginkgo biloba.Citation89 Clinical studies have shown that ginkgo biloba (240 mg/d for 12 weeks) was more helpful in reducing TD symptoms in people with schizophrenia.Citation90 On the other hand, the administration of vitamin B6Citation91 and vitamin ECitation92 (1,200 IU/d for 12 weeks) also proved to be effective in treating TD in clinical trials.
Conclusion
In conclusion, antioxidants are very low-risk drugs, and their use could be more beneficial as compared with the invented drugs, which, in most cases, produce undesirable side effects during their chronic treatment. Therefore, we suggest the use of antioxidants and serotonergic drugs as stand-alone intervention or as adjunct to conventional medications. They may provide attractive treatment strategic targets to manage antipsychotic-induced EPSE arising from dysfunctional serotonergic neurotransmission and OS. These studies suggest that antioxidants should be tried.
Disclosure
The author reports no conflicts of interest in this work.
References
- BrilesJJRosenbergDRBrooksBARobertsMWDiwadkarVAReview of the safety of second-generation antipsychotics: are they really “atypically” safe for youth and adults?Prim Care Companion CNS Disord201214311r01298
- ButlerRRadhakrishnanRDementiaClin Evid201291001
- KaneJHonigfeldGSingerJMeltzerHClozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazineArch Gen Psychiatry1988457897963046553
- FountoulakisKNVietaEEfficacy and safety of aripiprazole in the treatment of bipolar disorder: a systematic reviewAnn Gen Psychiatry20098161619635147
- DeclercqTPetrovicMAzermaiMWithdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementiaCochrane Database Syst Rev20133CD00772623543555
- BallardCWaiteJThe effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s diseaseCochrane Database Syst Rev20061CD00347616437455
- GeddesJFreemantleNHarrisonPBebbingtonPAtypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysisBMJ20003211371137611099280
- LeuchtSWahlbeckKHamannJKisslingWNew generation antipsychotics vs low-potency conventional antipsychotics: a systematic review and metaanalysisLancet2003361581158912747876
- DavisJMChenNGlickIDA meta-analysis of the efficacy of second-generation antipsychoticsArch Gen Psychiatry20036055356412796218
- KeefeRSBilderRMDavisSMCATIE InvestigatorsNeurocognitive Working GroupNeurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trialArch Gen Psychiatry20076463364717548746
- LewisSLiebermanJCATIE and CUTLASS: can we handle the truth?Br J Psychiatry2008192316116318310570
- AttardATaylorDMComparative effectiveness of atypical antipsychotics in schizophrenia: what have real-world trials taught us?CNS Drugs20122649150822668246
- HartlingLAbou-SettaAMDursunSAntipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysisAnn Intern Med201215749851122893011
- PringsheimTDojaABelangerSPattenSTreatment recommendations for extrapyramidal side effects associated with second-generation antipsychotic use in children and youthPaediatr Child Health20111659059823115503
- HaddadPMDasAKeyhaniSChaudhryIBAntipsychotic drugs and extrapyramidal side effects in first episode psychosis: a systematic review of head-head comparisonsJ Psychopharmacol2012265Suppl152622057019
- LiCRChungYCParkTWClozapine-induced tardive dyskinesia in schizophrenic patients taking clozapine as a first-line antipsychotic drugWorld J Biol Psychiatry20091091992419995222
- HaleemDJShireenEHaleemMASomatodendritic and post-synaptic serotonin-1A receptors in the attenuation of haloperidol-induced catalepsyProg Neuropsychopharmacol Biol Psychiatry20042881323132915588759
- HaleemDJSerotonergic modulation of DA neurotransmission: a mechanism for enhancing therapeutics in schizophreniaJCPSP200616855656216899192
- HaleemDJSamadNHaleemMAReversal of haloperidol induced extrapyramidal symptoms by buspirone; a time related studyBehav Pharmacol20071814715317351421
- ShireenENaeemSInamQUHaleemDJOral administration of haloperidol at clinically recommended doses elicits smaller parkinsonian effects but more tardive dyskinesia in ratsPak J Pharm Sci201326227127623455196
- ShireenEPervezSMasroorMReversal of haloperidol induced motor deficits in rats exposed to repeated immobilization stressPak J Pharm Sci20142751459146625176240
- StrawnJRKeckPECaroffSNNeuroleptic malignant syndromeAm J Psychiatry200716487087617541044
- RayWAChungCPMurrayKTHallKSteinCMAtypical antipsychotic drugs and the risk of sudden cardiac deathN Engl J Med200936022523519144938
- KnolWvan MarumRJJansenPASouvereinPCSchobbenAFEgbertsACAntipsychotic drug use and risk of pneumonia in elderly peopleJ Am Geriatr Soc20085666166618266664
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med20053531209122316172203
- JonesPBBarnesTRDaviesLRandomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1)Arch Gen Psychiatry2006631079108717015810
- AlpayMKoroshetzWJQuetiapine in the treatment of behavioral disturbances in patients with Huntington’s diseasePsychosomatics200647707216384811
- JohnstonTGRisperidone long-acting injection and Huntington’s disease: case series with significant psychiatric and behavioural symptomsInt Clin Psychopharmacol20112611411921119522
- Marti-MassoJFCarreraNUrtasunMDrug-induced parkinsonism: a growing list [letter]Mov Disord199381258419801
- GershanikOSDrug-induced parkinsonism in the aged: recognition and preventionDrugs Aging199451271327981484
- Perez-GilabertYMateoDGimenez-RoldanSActividad asistencial en una consulta hospital aria especializada en enfermedad de Parkinson y transtornos del movimiento: un estudio prospectivo durante anos [Care activities in a hospital clinic specializing in Parkinson’s disease and movement disorders: a prospective study for years]Neurologia19949317323 Spanish7803048
- Jimenez-JimenezFJOrti-ParejaMAyuso-PeraltaLDrug-induced parkinsonism in a movement disorders unit. A four-year surveyParkinsonism Relat Disord1996214514918591033
- GrohmannRKochRSchmidtLGExtrapyramidal symptoms in neuroleptic recipientsAgents Actions Suppl19902971821969222
- CaseyDETardive dyskinesia: pathophysiology and animal modelsJ Clin Psychiatry2000615910739324
- KurokiTNagaoNNakaharaTNeuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesisProg Brain Res200817219921218772034
- HaleemDJBatoolFKhanNHDifferences in the effects of haloperidol and clozapine on rat brain serotonin and dopamine metabolism and on tests related to extrapyramidal functions in ratsMed Sci Monit2002833543361
- ShireenEHaleemDJReversal of haloperidol-induced motor deficits by mianserin and mesulergine in ratsPak J Pharm Sci201124171221190911
- PorsoltRDMoserPCCastagnéVBehavioral indices in antipsychotic drug discoveryJ Pharmacol Exp Ther201033363263820200119
- FardeLNordströmALWieselFAPauliSHalldinCSedvallGPositron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effectsArch Gen Psychiatry1992495385441352677
- ShinH-WChungSJDrug-induced parkinsonismJ Clin Neurol20128152122523509
- HaleemDJSamadNHaleemMAReversal of haloperidol-induced tardive vacuous chewing movements and supersensitive somatodendritic serotonergic response by buspirone in ratsPharmacol Biochem Behav20078711512117498786
- PrinssenEPKlevenMSKoekWInteractions between neuroleptics and 5-HT(1A) ligands in preclinical behavioral models for antipsychotic and extrapyramidal effectsPsychopharmacology (Berl)1999144202910379620
- HaleemDJKhanNHEnhancement of serotonin-1A receptor dependent responses following withdrawal of haloperidol in ratsProg Neuropsychopharmacol Biol Psychiatry200327464565112787852
- PrinssenEPColpaertFCKoekW5-HT1A receptor activation and anticataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsyEur J Pharmacol200245321722112398907
- HaleemDJExtending therapeutic use of psychostimulants: focus on serotonin-1A receptorProg Neuropsychopharmacol Biol Psychiatry20134617018023906987
- EspositoESerotonin-dopamine interaction as a focus of novel antidepressant drugsCurr Drug Targets2006717718516475959
- ClemettDAPunhaniTDuxonMSBlackburnsTPFoneKCImmunohistochemical localization of the 5-HT-2C receptor protein in the rat CNSNeuropharmacology200039261263
- KapurSRamingtonGJonesCHigh levels of dopamine D2 receptor occupancy with low dose haloperidol treatment: a PET studyAm J Psychiatry1996153946950
- FaurbyeARaschPJPetersenPBBrandborgGPakkenbergHNeurological symptoms in pharmacotherapy of psychosesActa Psychiatr Scand196440102714217630
- EganMFApudJWyattRJTreatment of tardive dyskinesiaSchizophr Bull1997235836099365997
- RevueltaGJCloudLAiaPGFactorSATardive dyskinesiasAlbaneseAJHyperkinetic Movement Disorders: Differential Diagnosis and TreatmentChichesterWiley-Blackwell2012331352
- DayaluPChouKLAntipsychotic-induced extrapyramidal symptoms and their managementExpert Opin Pharmacother2008991451146218518777
- Soares-WeiserKFernandezHHTardive dyskinesiaSemin Neurol200727215916917390261
- JesteDVCaliguirMPTardive dyskinesiaSchizophr Bull1993193033158100643
- WaddingtonJLSpontaneous orofacial movements-induced in rodents by very long term-neuroleptic administration: phenomenology, pathophysiology and putative relationship with tardive dyskinesiaPsychopharmacology (Berl)19901014314471975104
- KulkarniSKNaiduPSAnimal models of tardive dyskinesia-a reviewIndian J Physiol Pharmacol20014514816011480221
- MarcheseGCasuMNBartholinicFSubchronic treatment with classical but not atypical antipsychotic produced morphological changes in rat nigrostriatal dopaminergic neurons directly related to early onset vacuous chewingEur J Neurosci2002151187119611982629
- ZhangWPerryKWWongDTSynergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine or norepinephrine and DA release in rat prefrontal cortexNeuropsychopharmacology20002325026210942849
- GershanikOSGómez ArévaloGJTypical and atypical neurolepticsHandb Clin Neurol201110057959921496609
- CadetJLKahlerLAFree radical mechanisms in schizophrenia and tardive dyskinesiaNeurosci Biobehav Rev1994184574677708360
- WrightAMBempongJKirbyMLBarlowRLBloomquistJREffects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesiaBrain Res19987882152229555021
- NaiduPSSinghAKulkarniSKEffects of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunctionsPhytother Res20062014014616444668
- JennerPMarsdenCDIs the dopamine hypothesis of tardive dyskinesia completely wrong?Trends Neurosci19869259260
- TurronePRemingtonGNobergaJNThe VCM model of TD revisited: is there a relationship to DA D-2 receptor occupancy?Neurosci Biobehav Rev20022636138012034136
- ShireenEQurrat-ul-AinBatoolFHaleemDJNeurochemical effects of 8-OH-DPAT in rats treated with haloperidolPak J Pharm Sci2002151718216414870
- ShireenEKhanABatoolFHaleemDJIncrease in serotonin-1A receptor responses following haloperidol withdrawalJ Basic Appl Sci2006214554
- LevinsonAJGarsideSRosebushPIMazurekMFHaloperidol induces persistent down-regulation of tyrosine hydroxylase immunoreactivity in substantia nigra but not ventral tegmental area in the ratNeuroscience1998842012119522374
- ZhangYXuHHeJQuetiapine reverses altered locomotor activity and tyrosine hydroxylase immunoreactivity in rat caudate putamen following long-term haloperidol treatmentNeurosci Lett2007420667117466452
- ReynoldsKBMac GillivrayLZettlerMRosebushPIMazurekMFRole of the dopamine transporter in mediating the neuroleptic-induced reduction of tyrosine hydroxylase-immunoreactive midbrain neuronsBrain Res20111394243221396352
- BordiaTMcIntoshJMQuikMNicotine reduces antipsychotic-induced orofacial dyskinesia in ratsJ Pharmacol Exp Ther201234061261922144565
- TauscherJKapurSVerhoeffNPBrain serotonin 5-HT-1A receptor binding in schizophrenia measured by positron emission tomography and [11C]WAY-100635Arch Gen Psychiatry20025951452012044193
- GobertARivetJMCisterilliLMelonCMillanMJBuspirone modulates basal and fluoxetine-stimulated dialysate levels of dopamine, noradrenaline and serotonin in the frontal cortex of freely moving rats: activation of serotonin-1A receptors and blockade of alpha-2-adrenergic receptors underlie its activationNeuroscience1999931251126210501449
- PeroutkaSJSelective interaction of novel anxiolytics with 5-hydroxytryptamine 1A receptorsBiol Psychiatry1985209719792862927
- QueirozCMFrussa-FilhoREffects of buspirone on an animal model of tardive dyskinesiaProg Neuropsychopharmacol Biol Psychiatry1999231405141810631766
- NaiduPSKulkarniSKEffect of 5-HT-1A and 5-HT-2A/2C receptor modulation on neuroleptic-induced vacuous chewing movementsEur J Pharmacol2001428818611779040
- PandyaCDHowellKRPillaiAAntioxidants as potential therapeutics for neuropsychiatric disordersProg Neuropsychopharmacol Biol Psychiatry20154621422323123357
- ParikhVMohammadMKSahebaraoPMDifferential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brainJ Psychiatr Res2003371435112482469
- PillaiAParikhVTerryAVMahadikSPLong-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brainJ Psychiatr Res200741537238616564057
- JenkinsRRGoldfarbAIntroduction-oxidant stress, aging, and exerciseMed Sci Sports Exerc1993252102128450723
- HalliwelBGutteridgeJMCFree Radicals in Biology and Medicine3rd edOxfordOxford University Press1999
- EvansDRParikhVVKhanMMCoussonsCBuckleyPFMahadikSPRed blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatmentProstaglandins Leukot Essent Fatty Acids20036939339914623492
- SagaraYInduction of reactive oxygen species in neurons by haloperidolJ Neurochem199871100210129721725
- CorrellCULeuchtSKaneJMLower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studiesAm J Psychiatry200416141442514992963
- TarsyDLunguCBaldessariniRJEpidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugsHandb Clin Neurol201110060161621496610
- BhidayasiriRFahnSWeinerWJEvidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of NeurologyNeurology2013846346923897874
- NazFShireenESuppression and treatment of haloperidol induced extra-pyramidal side effects and anxiety syndrome by the coadministeration of red rice bran oil in ratsInt J Endors Health Sci Res2014228292
- NagarjunaSArifullahMKumarASEvaluation of antioxidant and antiparkinsonian activities of Brassica oleracea in haloperidol-induced tardive dyskinesiaInt J Green Pharm201593143149
- BishnoiMChopraKKulkarniSKProtective effect of Curcumin, the active principle of turmeric (Curcuma longa) in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes in rat brainPharmacol Biochem Behav20088851152218022680
- CloudLJZutshiDFactorSATardive dyskinesia: therapeutic options for an increasingly common disorderNeurotherapeutics201411116617624310603
- ZhangWFTanYLZhangXYChanRCWuHRZhouDFExtract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double-blind, placebo-controlled trialJ Clin Psychiatry201172561562120868638
- LernerVMiodownikCKaptsanAVitamin B6 in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover studyAm J Psychiatry20011581511151411532741
- ZhangXYZhouDFCaoLYXuCQChenDCWuGYThe effect of vitamin E treatment on tardive dyskinesia and blood superoxide dismutase: a double-blind placebo-controlled trialJ Clin Psychopharmacol2004241838614709952