Abstract
Background and Objectives
This study aimed to evaluate the efficacy and safety of transarterial chemoembolization (TACE) in patients with unresectable early or intermediate hepatocellular carcinoma (HCC) and Child-Pugh (CP)-B liver dysfunction.
Methods
This multicenter retrospective study enrolled patients with treatment-naïve HCC treated with TACE monotherapy between January 2012 and December 2020 at six Chinese hospitals. The primary outcome was overall survival (OS), and the secondary outcomes included the objective response rate (ORR) according to the modified RECIST and adverse events (AEs). Propensity score matching (PSM) was performed to reduce bias between the CP-B and CP-A groups.
Results
A total of 847 patients were included in the study. CP-A patients had significantly longer OS (median, 22.0 vs 19.3 months, P = 0.032) than CP-B (score of 7–9) patients, but a non-significant trend compared with CP-B (score of 7) patients (median, 22.0 vs 20.5 months, P = 0.254). After PSM, the median OS was 22.7 months for CP-A patients, while it was 19.3 months for CP-B (score of 7–9) patients (p = 0.026) and 20.5 months for CP-B (score of 7) patients (p = 0.155). CP-A patients achieved a significantly better ORR (53.0% vs 35.8%, P < 0.05) compared to CP-B (score of 7–9) patients, but a non-significant trend was observed in CP-B (score of 7) patients (53.0% vs 51.1%, P > 0.05). The post-embolization syndrome rates in the CP-A and CP-B (score of 7) cohorts were 52.1% and 53.3%, respectively. No new safety concerns were observed.
Conclusion
Patients with HCC with a CP score of 7 receiving TACE showed a similar prognosis and safety profile to CP-A patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide, of which around 80% are diagnosed at an unresectable stage.Citation1–3 For patients with intermediate-stage HCC, transarterial chemoembolization (TACE) has become the standard recommendation according to global guidelines.Citation4–6 HCC occurs primarily in patients with cirrhosis, which negatively affects the prognosis.Citation7 Assessment of liver function before various treatments plays a fundamental role in HCC, as selected treatments have the potential to damage liver function.Citation7 Methods to evaluate liver function include the Child-Pugh (CP) grade as well as the albumin-bilirubin grade. The ALBI grade has showed performance similar to the CP grade.Citation8 However, the most accepted grade for liver function assessment in both clinical trials and real-world clinical practice is the Child-Pugh (CP) grade, which has been adopted by most HCC treatment guidelines. The GIDEON study, which evaluated the safety of sorafenib in advanced HCC, demonstrated that the median OS of CP-B HCC patients was significantly lower than that of CP-A HCC patients.Citation9 Meanwhile, the high unmet need of the CP-B patient was revealed by the analysis of the Korean registry.Citation10
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, the criterion for liver function in intermediate-stage HCC is just “preserved liver function”.Citation4 No detailed score or grade of liver function has been defined. In real-world clinical practice, the ideal liver function for TACE candidates is CP-A and a CP score of 7–8.Citation11–13 However, little evidence has been reported to support this ideal liver function. Patients with CP-B were eligible for inclusion in some TACE-related randomized controlled trials (RCTs).Citation14,Citation15 Additionally, there is limited evidence regarding the efficacy of TACE monotherapy. Considering the heterogeneous nature of the CP-B subset of HCC and the unmet need for the management of HCC with a certain degree of impaired liver function in real-world clinical practice, the treatment efficacy and safety of TACE for patients with HCC and CP-B liver function should be explored.Citation16–20 This study was therefore conducted to further explore the efficacy and safety of TACE monotherapy as an initial treatment for unresectable early or intermediate HCC with CP-B liver function.
Materials and Methods
Patient Criteria
This multicenter retrospective study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the Institutional Ethics Review Boards of the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu Province, China). The requirement for written informed consent was waived by the Institutional Ethics Review Boards of the First Affiliated Hospital of Soochow University because of its retrospective nature and we stated that patient data was strictly confidential. Patients with treatment-naïve HCC who received TACE monotherapy between January 2012 and December 2020 at the six participating hospitals were screened and enrolled. The clinical or pathological diagnosis of HCC was confirmed according to current guidelines.Citation12,Citation21 A multidisciplinary discussion was performed before treatment to confirm whether TACE monotherapy should be recommended as the best treatment method for the patient. The potential advantages and disadvantages of TACE were explained to the patients. The final decision on the treatment choice was made by the patients or their relatives.
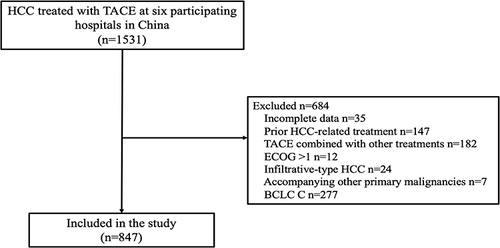
The study inclusion criteria were as follows: 1) confirmed diagnosis of unresectable early- or intermediate-stage (BCLC A or B) HCC; 2) preserved liver function, with CP grade A or B, and without hepatic encephalopathy or uncontrollable ascites; and 3) adequate renal, hematologic, and clotting functions. The exclusion criteria were as follows: 1) contraindication to TACE; 2) BCLC C; 3) undergoing ablation therapy; and 4) prior HCC-related treatment history.
TACE Procedure
All of the included patients received either conventional TACE or TACE with drug-eluting beads. To achieve better tumor control and reduce TACE-related complications, TACE was performed based on current standardization.Citation22,Citation23 Repeat TACE was performed according to the “on demand” mode, mainly according to the evidence of vital intrahepatic viable tumors by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) during follow-up. Repeat TACE was considered until: 1) liver function deteriorated to CP-C (overt hepatic encephalopathy, uncontrollable ascites, hepatorenal syndrome, or severe jaundice); 2) progression of targeted intrahepatic lesions after three consecutive TACE sessions; and 3) Eastern Cooperative Oncology Group (ECOG) performance status > 2.
Assessments
Follow-up imaging with contrast-enhanced CT and/or MRI was performed with an interval of 9–12 weeks. Laboratory tests were performed before each treatment session and during each routine follow-up. All included patients were routinely followed until death or the end of the study (June 30, 2022). Two independent experienced radiologists at each participating center used the modified Response Evaluation Criteria in Solid Tumors (mRECIST) to assess tumor response.Citation24 Safety was continuously evaluated according to laboratory test results and vital signs during follow-up. The severity of AEs was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Outcomes
The primary outcome was overall survival (OS), defined as the period from the first TACE treatment session to death from any cause. Secondary outcomes included the objective response rate (ORR) according to mRECIST and adverse events (AEs).
Statistical Analysis
The Mann–Whitney U-test (non-normally distributed data) or Student’s t-test (normally distributed data) was used to analyze continuous variables, and the chi-squared test or Fisher’s exact test was used to compare categorical variables. To reduce baseline differences and the probability of selection bias, (1:2) propensity score matching (PSM) was performed for the CP-B and CP-A groups. Gender, presence of hepatitis B virus (HBV), BCLC class, and tumor size were adjusted using a maximum propensity score distance (caliper) of 0.05. In addition, we performed (1:2) propensity score matching (PSM) for the CP-B and CP-A groups using a different method for the sensitivity analyses. Gender, presence of HBV, tumor size, and AFP > 200 ng/dl were adjusted with a maximum propensity score distance (caliper) of 0.05. OS was compared between the two groups using the Log rank test. Survival curves were generated using the Kaplan-Meier method. All the above statistical analyses were performed using SPSS (version 26.0; IBM, Somers, NY) and R language (version 4.2.0; R Project for Statistical Computing).
Results
Patient Characteristics
Eight hundred and forty-seven patients with HCC were finally included in the study, with 780 and 67 patients classified as having CP grades A and B (score of 7–9), respectively (). presents the baseline patient characteristics. In brief, the mean age was 61.37±11.13 years and 81.5% of the included patients were male. HBV infection (n=573, 67.7%) was the predominant underlying liver disease. At the start of treatment, 521 patients (61.5%) had AFP levels ≥ 200 ng/dl. Most of the patients (n=811, 95.7%) underwent conventional TACE. The baseline characteristics, including gender, ECOG status, and BCLC stage, were statistically significantly different between the CP-A and CP-B groups. After PSM, the baseline characteristics were comparable between the two groups (). An overview of patient characteristics for the CP-A and CP-B groups (score of 7) is provided in , and the baseline characteristics of the two groups were also comparable after PSM.
Table 1 Patient Characteristics of CP-A/B (Score of 7–9) Patients with TACE Therapy Before Propensity Score Matching
Table 2 Patient Characteristics of CP-A/B (Score of 7–9) Patients with TACE Therapy After Propensity Score Matching
Table 3 Baseline Characteristics of CP-A/B (Score of 7) Patients with TACE Therapy Before and After Propensity Score Matching (PSM)
Efficacy
Median OS was 21.6 months (95% CI, 20.5–22.7) in the overall population after a median follow-up period of 27.3 months. Patients with CP-A liver function had a significantly longer OS than those with CP-B (score of 7–9) patients [median, 22.0 (95% CI 20.6–23.4) vs 19.3 (95% CI 16.5–22.2) months, P = 0.032, Figure S1] and those with CP-B (score of 8–9) patients [median, 22.0 (95% CI 20.6–23.4) vs 18.8 (95% CI 12.9–24.6) months, P = 0.016, Figure S2], whereas a non-significant trend was observed compared with CP-B (score of 7) patients [median, 22.0 (95% CI 20.6–23.4) vs 20.5 (95% CI 17.4–23.6) months, P = 0.254, Figure S3]. When comparing across CP classes after PSM between the two groups, CP-A patients achieved a median OS of 22.7 months (95% CI, 17.4–28.0) versus 19.3 months (95% CI, 16.5–22.2) in CP-B (score of 7–9) patients (p = 0.026) (), 18.8 months (95% CI, 12.9–24.6) in CP-B (score of 8–9) patients (p = 0.014) (), and 20.5 months (95% CI, 17.4–23.6) in CP-B (score of 7) patients (p = 0.155) (). The median OS of the CP-A group was remarkably better than that of the CP-B (score of 7–9) group before and after PSM. Notably, the CP-A group did not have a significantly prolonged median OS compared to the CP-B (score of 7) group before or after PSM. The independent risk factor for OS was CP grade and tumor size based on multivariable Cox regression (Table S1). The complete response was 16.2% and objective response rate was 56.3% (The complete response rates for BCLC A and BCLC B were 27.8% and 9.0%, respectively; The objective response rates for BCLC A and BCLC B were 63.4% and 51.5%, respectively). After PSM, CP-A patients had a better ORR (53.0% vs 35.8%, P < 0.05) than CP-B (score of 7–9) patients, whereas a non-significant trend was observed in CP-B (score of 7) patients (53.0% vs 51.1%, P > 0.05) (Table S2).
Figure 2 Kaplan–Meier survival curves stratified by the Child-Pugh class [Child-Pugh (A) vs Child-Pugh (B) (score of 7–9)] after propensity score matching.
![Figure 2 Kaplan–Meier survival curves stratified by the Child-Pugh class [Child-Pugh (A) vs Child-Pugh (B) (score of 7–9)] after propensity score matching.](/cms/asset/8125fcc3-b36f-4882-a53b-7e67a995ee92/djhc_a_12295834_f0002_c.jpg)
Safety
After PSM, 105 patients (52.2%) developed any-grade AEs. No new safety concerns were observed. Post-embolization syndrome, including fever, pain, nausea, vomiting, and liver enzyme abnormalities, was the most common AE related to TACE in both groups (52.1% and 55.2% in the CP-A and CP-B [score of 7–9] groups, P > 0.05). In addition, patients with CP-B (score of 7) showed similar AEs related to TACE to patients with CP-A (53.3% vs 52.1%, P > 0.05) (Table S3). With supportive care, the syndrome became self-limiting after TACE. In addition, the hepatic dysfunction induced by TACE also improved and returned to baseline within a week.
Sensitivity Analyses
After we matched according to the methodology required for the sensitivity analysis, 201 patients remained in the study cohort (134 and 67 patients were classified as CP grade A and B (score of 7–9), respectively). Median OS was 25.1 months (95% CI, 18.8–31.4) in patients with CP-A liver function, which was significantly longer than that in the CP-B (score of 7–9) cohort (19.3 months [95% CI, 16.5–22.2], P = 0.010) (Figure S4A), whereas a non-significant trend was observed in the CP-B (score of 7) cohort (20.5 months [95% CI, 17.4–23.6], P = 0.092) (Figure S4B).
Discussion
According to several guidelines and previous studies, ideal candidates with HCC for TACE treatment should be those with CP grade A and score of 7–8 when concerned about liver function.Citation11–13 Nevertheless, little evidence has been reported on this topic, especially from routine clinical practice. This multicenter study showed that the median OS of CP-A patients was significantly longer than that of CP-B (score of 7–9) patients before or after PSM. Notably, there was no difference in the median OS for CP-A and CP scores of 7, both before and after PSM. In addition, tolerability was comparable between CP-A patients and CP-B (score of 7–9)/CP-B (score of 7) patients after PSM.
A meta-analysis showed that up to 28% of patients with CP-B could benefit from TACE therapy for unresectable HCC.Citation25,Citation26 Similarly, our study demonstrated that patients with a CP score of 7 could benefit from TACE treatment and were comparable to those with CP-A. The result was in line with the EASL guideline’s recommendation that the best candidates for TACE are patients with a single- or pauci-nodular tumor without vascular invasion or metastases, who are asymptomatic and have CP-A or CP-B (score of 7).Citation12 However, this study showed that CP-A patients achieved a remarkable survival advantage compared to CP-B (score of 7–9) patients, suggesting that CP-A patients had a better clinical benefit than patients with CP (score of 8–9) but not CP (score of 7), even though they belong to the same CP grade and would therefore have an identical prognostic weight according to the staging system.Citation4 One study showed that about half of the intermediate-stage HCC patients could receive locoregional treatment, with good clinical benefit for CP-B (score of 7).Citation27 Similarly, the important role of liver impairment in the prognosis of patients with HCC, rather than just the tumor burden, was confirmed in our study. In fact, there is a difference in prognosis between patients with CP-A and CP-B liver function as well as between CP-B (score of 7) and CP-B (score of 8–9). In addition, the ORR of CP-A patients was remarkably better than that of CP-B (score of 7–9) patients but not CP-B (score of 7) patients. These findings highlight the wide variability among patients. They cannot be considered a homogeneous group with similar prognosis, even within specific CP-B liver function.
The observational nature of this study made it impossible to determine whether TACE was detrimental to CP-B patients because it was not a randomized trial. However, the survival analysis, based on the CP score of the patients receiving TACE monotherapy, showed that the prognosis of patients with CP-B (score of 7) was so good that the potential for benefit from TACE as an antitumor treatment was not compromised by its impact on liver function. The difficulty in making recommendations for CP-B (score of 7) patients is understandable, as few trials of HCC treatment have focused specifically on this group; they are most often combined with patients with CP-A. In RCTs, prognosis and treatment allocation are usually dictated by the latter, because they usually comprise the majority of the study population.Citation26,Citation28 Future research is needed to identify the most appropriate patients for TACE within the CP-B (score of 7) grade.
Tolerability was an important concern in our study. Overall, TACE in CP-B (score of 7–9) patients led to 55.2% TACE-related post-embolization syndrome, which was consistent with that in CP-A patients (52.1%), and no new safety concerns were observed. There were also comparable AEs associated with TACE in CP-A and CP-B (score of 7) patients. In line with our study, a systematic review including 101 articles and 10,108 patients showed that the TACE-related post-embolization syndrome rate was 47.7% (95% CI 35.4–60.0).Citation29
This study has several limitations. First, the retrospective nature of the study could have led to selection bias. Second, no data were available for secondary or combined therapies for the included patients. Third, Child Pugh score contains a number of rather subjective clinical variables. Finally, the majority of patients included in the study received conventional TACE, with only a small proportion of patients receiving drug-eluting beads. Such an unbalanced TACE approach has the potential to influence study outcomes.
In conclusion, patients with a CP score of 7 receiving TACE monotherapy showed a similar prognosis and safety profile to patients with CP-A.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Jian-Qiang Jiang, Jin-Tao Huang and Wei-Dong Wang contributed equally as co-first authors for this study. Xiao-Li Zhu and Bin-Yan Zhong contributed equally as joint corresponding authors for this study.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
- Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi:10.14218/JCTH.2022.00293
- Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–1147. doi:10.1016/j.jhep.2016.07.020
- Jeon D, Song GW, Lee HC, Shim JH. Treatment patterns for hepatocellular carcinoma in patients with Child-Pugh class B and their impact on survival: a Korean nationwide registry study. Liver Int. 2022;42(12):2830–2842. doi:10.1111/liv.15464
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
- European Association for the Study of the Liver. Electronic address eee, European Association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
- Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
- Yu SC, Hui JW, Hui EP, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology. 2014;270(2):607–620. doi:10.1148/radiol.13130498
- D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–1012. doi:10.1002/hep.32468
- Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a Phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3):600–609. doi:10.1016/j.jhep.2021.04.047
- Berardi G, Morise Z, Sposito C, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72(1):75–84. doi:10.1016/j.jhep.2019.08.032
- Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74(4):931–943. doi:10.1016/j.jhep.2020.11.026
- Zhong BY, Yan ZP, Sun JH, et al. Prognostic performance of albumin-bilirubin grade with artificial intelligence for hepatocellular carcinoma treated with transarterial chemoembolization combined with sorafenib. Front Oncol. 2020;10:525461. doi:10.3389/fonc.2020.525461
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
- Lu J, Zhao M, Arai Y, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO). Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi:10.21037/hbsn-21-260
- Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi:10.1053/jhep.2003.50047
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi:10.1016/S0140-6736(02)08649-X
- Piscaglia F, Terzi E, Cucchetti A, et al. Treatment of hepatocellular carcinoma in Child-Pugh B patients. Dig Liver Dis. 2013;45(10):852–858. doi:10.1016/j.dld.2013.03.002
- Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27(6):1578–1583. doi:10.1002/hep.510270617
- Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453

![Figure 3 Kaplan–Meier survival curves stratified by the Child-Pugh class [Child-Pugh (A) vs Child-Pugh (B) (score of 8–9)] after propensity score matching.](/cms/asset/43e078e3-7ea8-42cc-9fde-10af995b91a3/djhc_a_12295834_f0003_c.jpg)
![Figure 4 Kaplan–Meier survival curves stratified by the Child-Pugh class [Child-Pugh (A) vs Child-Pugh (B) (score of 7)] after propensity score matching.](/cms/asset/12c1b288-2b6c-4c92-95d9-9802b50820c1/djhc_a_12295834_f0004_c.jpg)