Abstract
Pannexins belong to a family of ATP-release channels expressed in almost all cell types. An increasing body of literature on pannexins suggests that these channels play dual and sometimes contradictory roles, contributing to normal cell function, as well as to the pathological progression of disease. In this review, we summarize our understanding of pannexin “protective” and “harmful” functions in inflammation, regeneration and mechanical signaling. We also suggest a possible basis for pannexin’s dual roles, related to extracellular ATP and K+ levels and the activation of various types of P2 receptors that are associated with pannexin. Finally, we speculate upon therapeutic strategies related to pannexin using eyes, lacrimal glands, and peripheral nerves as examples of interesting therapeutic targets.
Introduction
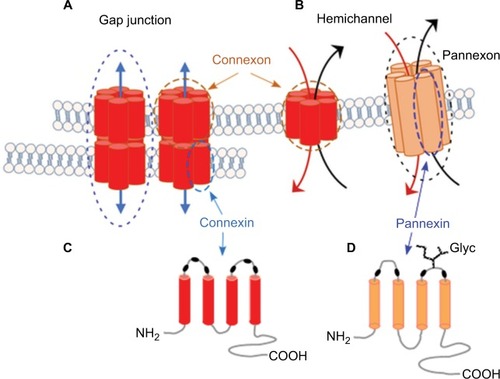
Cell–cell and cell–matrix interactions are fundamental properties of multicellular organisms. Gap junctions, formed by connexins and innexins in vertebrate and invertebrate animals, respectively, allow direct passage of ions and small molecules (<2,000 Da) from cell to cell ().Citation1–Citation3 In addition to gap-junction channels, connexins may form hemichannels (HCs), termed “connexons” (),Citation4,Citation5 which are hexamers of connexin monomers (), each containing four transmembrane domains, two extracellular loops, and cytoplasmic N and C termini ().Citation6 The vertebrate homologues of innexins, called “pannexins”, form mostly HCs, or pannexons (), due to the high level of glycosylation in their extracellular domains ().Citation7–Citation12 Similar to connexins (), pannexins have a cytosolic N-terminal domain, four transmembrane domains with two extracellular loops, and a cytosolic C-terminal domain ().Citation13 However, pannexins have no homology to the vertebrate connexin gap-junction protein,Citation8 and unlike connexins, which have multiple cysteine residues in both extracellular loops, pannexins have only two cysteine residues per loop (, black ovals).Citation13
Figure 1 Connexins and pannexins.
Notes: (A, B) Connexin and pannexin share a similar structure, despite the absence of sequence homology. Connexin and pannexin form functional connexon and pannexon hemichannels, respectively. (C, D) Connexins and pannexins are transmembrane proteins with four transmembrane domains, two extracellular loops, one cytoplasmic loop, and cytoplasmic N- and C-terminal domains. Connexin channels can assemble into a gap junction (A) that mediates intercellular communication, while pannexin’s extracellular loop has a high level of glycosylation in mammalian cells (D), which prevent the formation of gap junctions.
Abbreviation: Glyc, glycosylation.
The pannexin family consists of three proteins, Panx1, Panx2, and Panx3, all of which have been shown to form a single-membrane channel.Citation14,Citation15 Panx1 is ubiquitously expressed in almost all cell types, including those in the nervous and immune systems, eye, muscle, olfactory epithelium, blood vessels, exocrine glands (eg, lacrimal and salivary glands), thyroid, prostate, kidney, and liver ().Citation16–Citation20 Panx2 transcripts are highly expressed in the central nervous system (CNS).Citation21 Lower levels of Panx2 transcripts have been detected in nonneural tissues, including the testis, kidney, retina, and gastrointestinal tract, while Panx3 mainly localizes in the skin, osteoblasts, and chondrocytes ().Citation15,Citation20–Citation24 Panx3 has also been found in skeletal muscle,Citation25 lactating mammary glands, sebaceous glands, and the small intestine.Citation15 Interestingly, Panx2 protein appears to be more ubiquitously expressed than initially predicted by mRNA expressionCitation21 ().
Table 1 Expression and localization of pannexin mRNA and protein
Endogenous Panx1 and Panx3 proteins are localized primarily at the plasma membrane,Citation13,Citation26 while Panx2 is highly expressed in the cytoplasmic compartment,Citation21 suggesting a unique intracellular function for Panx2. However, several studies have reported the cytoplasmic localization of Panx1 and Panx3 proteins when these proteins were overexpressed in cells.Citation21,Citation27 For example, Abeele et alCitation27 demonstrated cytoplasmic localization of Panx1 transiently expressed in LNCaP cells, where it formed Ca2+-permeable channels in the endoplasmic reticulum (). It is quite possible that high levels of pannexin protein expression could lead to both membrane and endoplasmic reticulum-channel formation, thus contributing to sustained increases in intracellular Ca2+.
Pannexins are ATP-release channels that can be activated by caspase cleavage of their pore-associated C-terminal tail, the autoregulatory region controlling channel permeability. The regulated ATP (nucleotide) release through pannexin HCs is implicated in a number of normal physiological functions and in response to stressors or pathological states in cells and tissue.Citation25,Citation28,Citation29 Well-characterized functions of pannexins include regulation of cell differentiation and migration, tissue development and regeneration, inflammation, wound healing and cell death.Citation28 However, mechanistic explanations of how these proteins perform sometimes contradictory roles remain unclear.
In this review, we attempt to clarify existing controversies in the literature on the “protective” and “harmful” roles of pannexin HCs by addressing a question: How do pannexins acquire these different and often opposing roles? We seek to obtain deeper understanding of pannexin signaling, participants in which represent a potential source of novel and promising therapeutic targets in a variety of pathologies. Our focus is entirely on pannexins, with a full understanding that pannexin and connexin HCs have both distinct and complementary but often overlapping functions, particularly in ATP release and inflammation; therefore, we refer the reader to several excellent reviews comparing the roles of these channels.Citation30–Citation33
Pannexins, inflammation, and inflammasome activation
The involvement of pannexins in the induction of inflammation has been reported in multiple publications.Citation28,Citation34–Citation36 Inflammation is the major protective function maintained by the evolutionarily conserved innate immune system in response to harmful stimuli, such as pathogens, stress, injury, or cell death. Acute (short-term) inflammation stimulates a regenerative response, while persistent (chronic) inflammation can cause systemic inflammatory diseases.Citation37 Activation of inflammasomes, facilitating the release of interleukin-1β (IL1β) and IL18 in response to pathogens and tissue injury, is a key function of the innate immune system. The inflammasomes, first characterized in monocytes in 2002Citation38 and in neural cells in 2008,Citation39 are multi-protein complexes mediating proteolytic maturation of Casp1, Casp11, IL1β, and IL18. Proteolytic cleavage of IL1β and IL18 precursors is executed by active Casp1 ();Citation40,Citation41 and the release of the mature cytokines occurs via megapores, formed by N-terminal domains of the Casp1/11-processed recently identified pore forming protein gasdermin D.Citation42–Citation46 A large body of experimental evidence identifies Panx1 and its associated P2X receptors as essential upstream regulators of inflammasomes and proteolytic activation of Casp1 and Casp11.Citation47–Citation51 Panx1 has been reported to activate inflammasomes in many cell types, including macrophages,Citation52,Citation53 microglia,Citation54 neurons, and astrocytes;Citation49 however, the data on particular cell and inflammasome types remain controversial.Citation55,Citation56 Currently, the bulk of published data support a pivotal role for Panx1 in CNS/ retinal inflammasome regulation.Citation55–Citation58 As such, strong suppression of its major components, including Casp1, Casp11, IL1β, and apoptosis-associate speck-like protein containing a caspase recruitment domain (ASC), is observed in both Panx1−/− mice and wild-type retinas after Panx1 blockade by probenecid.Citation16,Citation54,Citation59
Figure 2 The two signaling arms of the inflammasome-activation cascade.
Notes: Signal 1 pathways sense environmental signals via surface Tumor necrosis factor (TNF), Toll-like (TLR) and IL-1 receptors and facilitates transcriptional priming of inflammasome components via the NFκB pathway and upregulates the expression of precursor proteins of IL1β, caspases 1/11(also known as caspase 4), and pro-Nod-like receptors (NLR). Signal 2 facilitates activation of the complex via proteolytic processing and assembly. This arm responds to mechanical stress, activation of a ligand-sensing system within the cytosol or extracellular ATP sensing via Panx1–P2X receptor signalosomes. Upon activation, protease activity of caspases regulates the maturation and release of IL1β and IL18. Recent studies showed that Gasdermin D (GSDMD) is a novel membrane pore-forming protein. Cleaved by inflammatory caspases Casp1 or Casp11(4), GSDMD binds to phosphoinositides in the plasma membrane and oligomerizes to generate membrane pores of ~10–14 nm in diameter.Citation222 This pore size can allow the passage of mature IL1β, IL18, and caspase 1. The formation of the GSDMD pores also disrupts osmotic potential, resulting in an inflammatory form of cell death known as pyroptosis.
Abbreviation: ASC, apoptosis-associate speck-like protein containing a caspase recruitment domain.
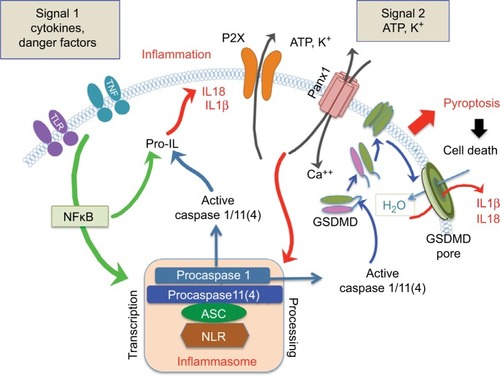
There are two major regulatory arms for inflammasome activation (): signal 1 pathways sense environmental signals via surface TNF, Toll-like, and IL1 receptors and facilitate inflammasome “priming”, ie, transcriptional activation via MyD88–NFκB-mediated pathways;Citation60,Citation61 and signal 2 pathways regulate inflammasome assembly and processing of Casp1/11, IL1β, and IL18 precursors. This arm is regulated via the Panx1–P2X signalosome to facilitate ATP and K+ release, as well as uptake of extracellular Ca2+ and danger/pathogen-signaling patterns.Citation62,Citation63
Though a role for Panx1 in the inflammasome regulatory cascade appears to be generally conserved across cell types, Qu et alCitation55 suggested that pannexin is “dispensable” for inflammasome formation. In particular, LPS-primed bone marrow-derived macrophages were successfully able to activate Casp1 and secrete its associated inflammatory cytokines (IL1β and IL18) in response to a number of stimuli in the absence of Panx1. Moreover, the authors also concluded that P2X7 and Panx1 can function independently and may be involved in distinct signaling pathways.Citation55 These controversial views on Panx1 function could be explained by cell-type-specific differences and potential variation in culture conditions, and need to be resolved.
Mechanisms of pannexin-channel activation
Several diverse mechanisms regulating pannexin-channel function have been proposed to date. Pannexin channels have been posited to be activated by caspase-mediated channel cleavage in apoptotic immune cells, G-protein-coupled receptors in vascular smooth muscle,Citation64,Citation65 low oxygen tension in erythrocytes and neurons,Citation66 high extracellular K+ in various cell types,Citation49,Citation67 and mechanical stretch.Citation68,Citation69 Progressive Panx1-channel opening is directly linked to ion- and large-molecule transport, and occurs during both irreversible (caspase-mediated cleavage)Citation70 and reversible G-protein-coupled receptor (including α1-adrenoceptor-mediated) forms of channel activation.Citation71 Panx1 activation by caspase-mediated cleavage enables the release of ATP as a “find me” signal that recruits phagocytizing macrophages to apoptotic T lymphocytes.Citation65,Citation70 This mechanism is critical for the fast clearance of apoptotic and dead cells during acute inflammation.Citation28,Citation55,Citation65,Citation72 Cleavage activation of Panx1 is also involved in pyroptotic cell death ().Citation73 A recent study employing electron microscopy and single-channel recordings of full-length and caspase-cleaved pannexin concatemers with defined numbers (0–6) of intact and truncated C termini revealed that Panx1 activation was increased in a sequential manner by stepwise removal of the autoinhibitory C termini. This also resulted in a graded increase in current and ATP/dye permeation.Citation71 On the other hand, the reversible G-protein-coupled receptor-mediated mechanism is independent of caspase-mediated pannexin cleavage.Citation74 Comparison of α1-adrenoceptor-activated with cleavage-activated Panx1 channels indicated that α1-adrenoceptor-activated Panx1 channels had a shorter mean open time, but progressively increasing conductance, suggesting that despite differences in gating kinetics, activation of Panx1 channels by both signaling mechanisms involves cumulative changes in open-channel properties.Citation71
Pannexin signaling via ATP release
Panx1 channels can release ATP under physiological conditions and play critical roles in many pathological processes. ATP is a prominent extracellular signaling molecule in both physiological and pathological conditions. For example, ATP release is important for muscle differentiation and function,Citation75–Citation78 and ATP-receptor activation plays a role in regulation of cell proliferation, DNA synthesis, cell differentiation, and cell survival during the course of CNS development.Citation79,Citation80 At the same time, ATP may also serve as a major danger signal for cells,Citation50 despite it having a very short half-life due to rapid degradation by surface ecto-ATPases.Citation81 ATP is released from apoptotic, injured, and viable cells that are challenged by assorted cytokines, as well as mechanical or ischemic stress in the presence of elevated K+.Citation82
ATP-mediated activation of Panx1, the ATP-release channel, typically ramps up in a vicious cycle only to a certain level, due to a retrograde feedback mechanism regulating activity of Panx1 HCs via a low-affinity ATP binding site.Citation83 Therefore, the permeant (ATP) can inhibit the permeating channel when high extracellular ATP concentration is reached. Importantly, however, this inhibition is abrogated by an increased extracellular concentration of potassium ions (K+),Citation82 suggesting a mechanism of toxicity of extracellular ATP in Panx1-expressing cells. In agreement with this mechanism are the findings that massive activation by Casp3/7 cleavage or expression of constitutively active Panx1 HCs results in cell death.Citation65,Citation84 Therefore, the balance between physiological and pathological activities of Panx1 depends on the open-state probability of the channel, which in turn is influenced by the increase in intracellular Ca2+ and extracellular ATP and K+. An additional level of Panx1-channel regulation is achieved via interactions with purinergic P2 (eg, P2X and P2Y) receptors, which are activated by binding extracellular ATP at the plasma membrane.Citation85 Several salient aspects of Panx–P2 interactions, including the mechanisms and significance of such interactions, as well as their sensitivity and specificity, are detailed in the following sections.
Functional interactions of Panx HCs with purinergic P2X and P2Y receptors
There are two major families of purinergic P2 receptors: ionotropic P2X and metabotropic P2Y receptors. Reciprocal interactions, whereby P2 receptors directly activate Panx1 channels,Citation86,Citation87 suggest that these proteins can form a signaling complex at the cell surfaceCitation34,Citation88 that mediates both paracrine and autocrine purinergic communication.
The P2X-receptor family contains 7 isoforms (P2X1–7), and P2X receptors are classified as ligand-gated channels whose activation regulates cellular membrane potential and intracellular Ca2+ levels.Citation89,Citation90 More precisely, though, P2X family members are ATP-gated cation channels, selective for Na+, K+, and Ca2+ ions.Citation91 In the nervous system, P2X receptors are pivotal transducers of ATP-mediated paracrine signals and have been implicated in physiological functions, such as chemotactic cell migration, intercellular calcium-wave propagation, as well as in nervous system dysfunction, leading to neuropathic pain or cell death.Citation92 Five isoforms – P2X1, P2X2, P2X3, P2X4, and P2X7 – have been shown to interact with Panx1,Citation93,Citation94 among which P2X4 and P2X7 are the most common interaction partners in different cell types.Citation51,Citation95,Citation96 Both P2X4 and P2X7 isoforms are calcium channels known to dilate into larger pores upon activation.Citation97,Citation98 P2X7-receptor activation results in the appearance of HC-like currents, reflecting the channel permeability for molecules up to 1 kDa and identified as Panx1 HC.Citation52 Under pathological conditions, overactivation of the Panx1–P2X7 signalosome complex has been implicated in inflammation, cell death, and neuropathic pain.Citation34,Citation96,Citation99,Citation100
The P2Y-receptor family contains eight isoforms (P2Y1,2,4,6,11–14). P2Y receptors are metabotropic G-protein-coupled receptors that couple to Gq, Gs, or Gi in an isoform-specific manner, and their activation modulates intracellular inositol triphosphate, Ca2+, and cAMP levels.Citation101,Citation102 Different isoforms of purinergic P2Y receptors are activated by ATP and its degradation products ADP and UTP, and couple to distinct G proteins to induce cAMP production, activation of phospholipase C, or intracellular Ca2+ via inositol triphosphate second-messenger systems.Citation103,Citation104 Recent publications have implicated P2Y receptors and Panx1 signaling in the regulation of endothelial cell activation in vascular inflammationCitation105 and cell-volume regulation.Citation106 ATP release via Panx1 channels activates P2Y2 receptors to amplify signaling in sensing chemotactic gradients in neutrophils.Citation107,Citation108 Polarization of surface expression by translocation of Panx1, P2Y2, adenosine A3 receptors, and ENTPD1 ectonucleotidase to the leading cell edge allows neutrophils to polarize within the gradients.
Sensitivity and specificity of Panx1–P2X signaling during normal tissue function and chronic inflammation
The activity and downstream consequences of several P2X- and P2Y-receptor isoforms are dependent on ATP binding, with varying sensitivity. P2X7, expressed by microglia, astrocytes, and neurons, is the most studied isoform, and is implicated in cyto- and neurotoxicity via direct interaction with Panx1 channels.Citation34,Citation87,Citation88 Due to its relatively low affinity to ATP (EC50 936 µM), it can only be activated by nonphysiological (≥1 mM) increases in extracellular ATP, which is locally achievable only at sites of injury or proximal to activated Panx1/Cx43 HCs. Conversely, the activation at low to medium (<900 µM) extracellular ATP levels can be mediated via an interaction between the high-affinity P2X4 receptor (P2X4; EC50 2.3 µM)Citation109 and Panx1Citation49,Citation110 (). This synergistic interaction was shown to coactivate P2X7, resulting in massive local efflux of ATP via the Panx1 channel, a forward-feeding autocrine amplification loop ().Citation111,Citation112 Functional synergy between P2X4 and cytotoxic P2X7Citation110,Citation113–Citation115 is known to be pivotal for Panx1-dependent extracellular ATP-induced cell death,Citation116 which can be suppressed by a blockade of either component in the Panx1–P2X4/7 complex or by extracellular ATP removal with apyrase.Citation51,Citation96 Similarly, in the retina, genetic ablation or pharmacological inhibition of Panx1,Citation117 P2X7, or P2X4Citation118–Citation120 has protected retinal ganglion cells (RGCs) in both acute and chronic ocular hypertension (OHT)-injury models. In other studies, a similar blockade was shown to protect neurons and other cell types from death via a rise in ionized Ca2+ and the induction of the inflammasome in various injury paradigms.Citation34,Citation121,Citation122
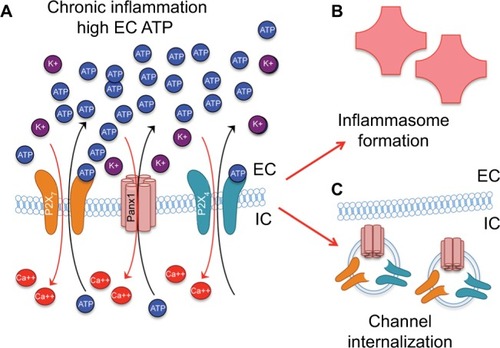
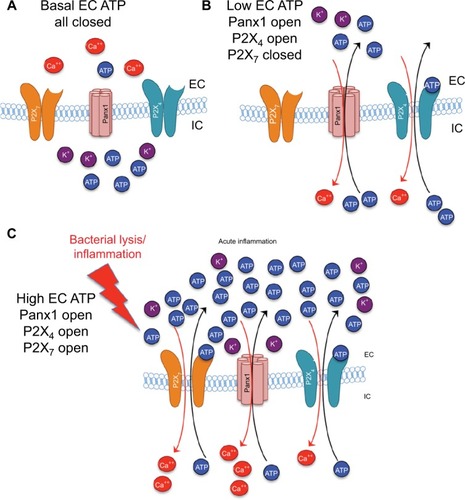
Figure 3 Differential ATP and ion movement depending on Panx1, P2X4, and P2X7 activity.
Notes: (A) Basal levels of EC ATP and normal concentration gradients of ATP and ions. (B) Panx1 opening with low levels of EC ATP results in P2X4 activation, but no P2X7 activity. (C) Higher levels of EC ATP, such as those resulting from bacterial lysis or chronic inflammation, result in opening of P2X4 and P2X7 channels and substantial movement of ATP and ions along their concentration gradients.
Abbreviations: EC, extracellular; IC, intracellular.
In contrast to P2X7, interactions between P2X4 receptor and Panx1 and their link to RGC loss and inflammasome in the retina still require exploration. However, strong evidence of a key cellular role of P2X4 in response to sublethal levels of ATP has been suggested in experiments on channel blockade with the 5-BDBD antagonist in macrophages,Citation116 as well as on inflammasome activation in various tissue types.Citation51,Citation123 In contrast to P2X7, P2X4 blockade has been shown selectively to suppress IL1β but not IL18 cytokine levels,Citation110 which was reported as potentially neuroprotective.Citation124–Citation126
More recently, an interesting phenomenon in acute-wound healing following the use of intracellular ATP delivery was described. In this study, ATP application was accompanied by a massive increase in macrophage trafficking, in situ proliferation, and direct collagen production within the wound.Citation127 Although the signaling mechanism of this phenomenon has not been determined, other researchCitation128 has demonstrated that the recognition and clearance of dying cells and debris from focal points of inflammation is critical in both the induction and resolution of inflammation.Citation28 Moreover, Panx1-mediated vesicular nucleotide transporters (responsible for ATP accumulation in secretory vesicles)-mediated ATP release have been shown to recruit neutrophils/macrophages to injury sites.Citation129–Citation131 It is quite possible that in some types of acute injury, an increase in or acceleration of postinjury inflammation may lead to more rapid resolution of inflammation through ADP or other signaling mechanisms.
Other mediators of Panx signaling and inflammation
In addition to Panx HCs, significant amounts of ATP can be released by bacteria, which trigger Panx1/P2X activity ().Citation132 Bacterial ATP may affect different types of cells and lead to the production of proinflammatory cytokines and growth factors. A recent study showed that commensal bacteria-derived ATP activates CD70highCD11clow cells in the intestinal lamina propria, induce IL6 and IL23 production, as well as TGF-β pathway activation. This then led to local differentiation of IL17-producing CD4+ T lymphocytes (T-helper TH-17, cells involved in host defense and several immune disorders).Citation133 Moreover, systemic or rectal administration of ATP into germ-free mice resulted in a marked increase in the number of lamina propria TH17 cells. The specific effect of ATP on TH17 differentiation was mediated by P2X and P2Y receptors, and ATP-induced TH17 differentiation was inhibited by P2X- and P2Y-receptor blockade. Interestingly, this mechanism commonly operates during the differentiation of both “naturally occurring” and “pathogenic” TH17 cells.Citation127,Citation133
Although ATP-gated unselective cation P2X channels are induced mainly by ATP, some studies report that they also may be activated by other molecules.Citation134 β-Toxin produced by Clostridium perfringens is a key virulence factor in fatal hemorrhagic enterocolitis and enterotoxemia. This toxin belongs to a family of β-pore-forming toxins. The results of a recent study suggested that Panx1 opening is achieved through the interaction of β-toxin with the P2X7 receptor. Then, ATP released by Panx1-channel opening promotes oligomer formation of the toxin, leading to cell death.Citation134 These studies suggest that Panx1 HC is an important contributor to P2X7-receptor signaling and provides a mechanistic link among bacterial stimuli, P2X7–Panx1 signaling, and inflammation.
Pannexins, mechanical signaling, and the cytoskeleton
A mechanosensitive role for connexin HCs in the propagation of intracellular calcium, initiated by the extracellular binding of ATP, was first noted in 1990.Citation135 Numerous reports since then have demonstrated the sensitivity of connexin HCs to extracellular Ca2+, which are believed to keep connexin HCs in a closed state at physiological Ca2+ levels.Citation136–Citation138 In contrast, Panx HCs are not gated by external Ca2+,Citation139 and the mechanical sensitivity of pannexin HCs was not noted until 2004, when single-channel currents were elicited by changes in pressure imposed pneumatically upon membrane patches of Xenopus oocytes expressing Panx1.Citation140 Since then, mechanosensitive purinergic signaling pathways, including pannexin-mediated ATP release, have been demonstrated in many cell types in response to mechanical stimuli. For example, inhibition of pannexin function suppressed hypertonic stress-induced ATP release and reduced downstream transcriptional activation induced by hypertonicity,Citation95,Citation141 and inhibition of Panx1 and several P2X receptors reduced downstream transcriptional activation induced by hypertonicity. Similarly, pannexin- and/or P2-receptor-dependent ATP release has been observed in RGCs,Citation69 lens epithelial cells,Citation142 fibrosarcoma cells,Citation143 urothelial cells,Citation144 and astrocytesCitation130 that were subjected to hypoosmotic conditions. In addition to altered tonicity, shear stress has been shown to activate mechanosensitive pannexin channels. Indeed, bone cells and red blood cells have demonstrated robust pannexin-mediated ATP release in response to oscillatory fluid shear stress.Citation145,Citation146 Consistent with this function, it was recently suggested that pannexin activity induced by transient fluid shear during media changes and manipulation of tissue-culture containers could confound the interpretation of cell-culture experiments.Citation147
In contrast to the bulk of the literature, only one study in HEK293 cells subjected to hypotonic media has suggested that pannexin HCs are not directly mechanosensitive.Citation148 It is likely that this controversial observation may have reflected a unique feature of the examined cell type and/or methodological differences. The authors’ choice to use ethidium bromide internalization as an indicator of pannexin HC activity may have led to different outcomes compared with more conventional and commonly used indicators of pannexin activity, such as dye uptake or ATP release.
While mechanical activation of pannexin HCs has been studied primarily in a general context, there is increasing evidence that mechanical signaling can facilitate pathological states, such as edema that stretches the plasma membrane. Mechanical strain was recently reported to trigger a robust inflammatory response, transcriptional priming of NLRP3 inflammasome formation, and IL1β production via activation of Panx1–P2X7 signaling.Citation54
The eye has emerged as an important model in understanding pathological mechanotransductive roles for Panx1. When the retina is exposed to mechanical stress, ATP is released physiologically by glia and neurons via Panx1 channels.Citation29,Citation69,Citation130 In OHT-injured retina, synergistic effects of mechanical stress induced by elevated pressure and massive ATP release facilitate sustained extracellular ATP elevation and prolonged activation of the Panx1–P2X pathway, a combination that is particularly toxic to RGCs, which are highly enriched in Panx1.Citation119,Citation130,Citation149 Experimental data generated in the murine eye indicate that RGC loss and axonal damage strongly correlate with mechanical deformationCitation150 and repetitive intraocular pressure spikes.Citation29,Citation130,Citation151 An increase in extracellular ATP has been reported in eyes exposed to acute or chronic OHT in animal models, as well as in human primary open-angle glaucoma.Citation69,Citation111,Citation149,Citation151,Citation152 Conversely, strain-activated, pannexin-regulated release of cytokines may also serve a protective role, as demonstrated by increased IL3 and IL6 expression in RGCs subjected to a 4% chronic strain in vitro or increased intraocular pressure in vivo.Citation153 Furthermore, the release of IL18 via inflammasome activation has also been reported to be neuroprotective.Citation125,Citation154
Concurrent with Panx1-mediated ATP release, Cx43 HCs have been demonstrated as another key pathway of ATP release. Although the contribution of Cx43 vs Panx1 to ATP release has been heavily debated recently,Citation155–Citation157 the current consensus indicates that Panx1 channels initiate and Cx43 HCs facilitate the bulk of ATP release from macroglia, especially in the presence of TNFα and IL1β.Citation158–Citation160 Consistent with this view, upon their exposure to TNFα and IL1β cytokines, glial cells become activated and release ATP via Cx43 HCs.Citation161
Given its structural role, the cytoskeleton is a leading candidate to participate in mechanical signaling. Actin dynamics, particularly those mediated by ARP2/3, have been reported to be regulated by pannexin activity,Citation17 and pannexin interacts physically with actin (through the C terminus of Panx1), but not tubulin/microtubules.Citation24,Citation162 In a recent publication, it was reported that the Panx1–P2X7 autocrine loop induced by ATP increased the migration speed of dendritic cells by promoting reorganization of the actin cytoskeleton.Citation163 In addition, pannexin influences a number of cellular changes that require cytoskeletal plasticity, including migration, differentiation, and proliferation.Citation162,Citation163 A role for actin is emphasized by evidence that Rho-kinase pathways, which have long been implicated in regulating actin dynamics, are dependent on pannexin-channel activity.Citation164 The importance of pannexins in regulating cytoskeletal dynamics has also been suggested by the localization of pannexin to the actin-rich filopodia of directionally migrating or path-finding cells.Citation17 We have shown that loss or inhibition of Panx1 increases neurite extension and branching in sensory neurons ex vivo.Citation165 This finding suggests that pannexins may play a suppressive role in neuronal growth. Finally, pannexin channels may also somehow play a role in the mechanical sensitivity of other mechanosensitive channels, such as TRPV4 and TRPV1.Citation166–Citation168 It is not yet clear how the activity of these channels is coupled to pannexin activity. Such regulation may also be mediated by the cytoskeleton, the rigidity of which likely influences mechanosensitive-channel response and the stability of which may be regulated by pannexin–P2-mediated pathways, such as those already noted. Therefore, mechanosignaling through Panx HCs may play a role in normal cell migration, growth, and differentiation. However, persistent exposure to mechanical stress may facilitate sustained activation of a Panx1–P2X-signaling loop, contributing to chronic inflammation ().
Pannexin and receptor plasticity
The magnitude and persistence of an activating signal have been noted to influence the functional plasticity of pannexin and pannexin-associated purinergic receptors (). In addition to the low-affinity binding already mentioned, another negative-feedback mechanism response to increased extracellular ATP involves rapid internalization of Panx1 into endosomes () in as little as 15 minutes, possibly through signaling initiated by P2X receptors.Citation169 Positive feedback mechanisms include coregulation of pannexins with purinergic signaling proteins, detected in experiments with hypertonic saline treatment, which triggered both pannexin-channel activity and expression levels of P2X receptors in Jurkat T cells.Citation95 Similar coregulation was observed in chronic mechanical strain in astrocytes that resulted in an increased expression of Panx1, -2, and -3 both in vitro and in vivo.Citation130
Therapeutic implications of Panx1 inhibition
Pannexin has been implicated in regulating normal and pathological cellular function in a wide range of tissue types. In normal physiology, pannexin has been shown to modulate vascular tone,Citation170 brain development,Citation9 memory, sleep,Citation166,Citation171 skeletal muscle homeostasis,Citation172,Citation173 red blood-cell biomechanics,Citation146 retinal signaling, response to ischemia,Citation117,Citation174 and leukocyte emigration.Citation105 In pathology, pannexin-mediated signaling has been implicated in brain ischemia,Citation64,Citation175 ischemic stroke,Citation176 pain,Citation177,Citation178 cardiomyocyte fibrosis,Citation179 microbial infection,Citation59,Citation180 cancer,Citation181 brain inflammation (autoimmune encephalomyelitis/multiple sclerosis),Citation182–Citation185 and immunogenic cell-death-inducing antineoplastic agents.Citation96,Citation186 On one hand, this potential breadth of functions renders pannexin a powerful and widely applicable therapeutic target. On other hand, this same breadth suggests potentially significant side effects of anti-pannexin therapy, manifested within the same cell type, within the same tissue, or systemically. In addition, as demonstrated by varying results of different commonly used pharmacological inhibitors of pannexin or P2 receptors, including probenecid, Panx1-blocking peptide (Citation10Panx),Citation105 carbenoxolone, P2-receptor-inhibiting peptides, and the extracellular ATP scavenger apyrase, the specificity of inhibition can appreciably impact a phenotypic response. In the following sections, we briefly describe possible outcomes related to the authors’ expertise, in which the diverse functions of Panx1 must be considered within translational therapeutic strategies.
Panx1 inhibition in the eye
Panx1 forms an ATP-, K+-, and Ca2+-permeable membrane channel that is highly expressed in the retina, making this easily accessible neural tissue a good model system for delineation of Panx1 function. In the retina, Panx1 has been shown to be activated by mechanical stress,Citation29,Citation69,Citation111,Citation130 intracellular Ca2+,Citation117 extracellular K+,Citation187 interactions with transient-receptor-potential channels,Citation142,Citation188 N-methyl-D-aspartate receptors,Citation189 activation of C2+-dependent caspases 1/11 and NLRP1/3 inflammasomes,Citation29,Citation117,Citation190,Citation191 and purinergic receptors upon binding extracellular ATP.Citation88 Several of these stressors and agonists are activated in the retina challenged by ischemia, OHT stress, or glaucoma, which can synergize to sustain prolonged Panx1 opening. Consistently with this observation, therapeutic Panx1 blockade protects RGCs and other neurons against mechanical stress and ischemia.Citation29,Citation174 However, due to the physiological significance of Panx1, only transient blockade and partial suppression represent therapeutically feasible options, as they are sufficient to block inflammasome and ionized Ca2+ influx without affecting global retina functionality.
Panx1 inhibition increases lacrimal-gland repair
Recent studies have proposed distinct roles for both Panx1 and P2X7 receptors in the control of inflammasome activation, leading to the release of mature IL1α and IL1β. These data support the model in which Panx1–P2X7 signaling is the key regulator of inflammatory response.Citation52,Citation192 Probenecid, a well-studied inhibitor of Panx1 and P2X7 receptorsCitation193,Citation194 and organic anion transporters, has been traditionally used to treat an inflammatory gout disease.Citation195 Treatment with probenecid has been found to affect ATP releaseCitation195 and suppress neuronal death in ischemic strokeCitation176,Citation196 and cerebral edema.Citation197 This suggests that modulation of Panx1 signaling may prevent inflammatory damage of brain tissue. Another study reports that in vivo administration of the P2X7R antagonist A438079 in the mouse model of salivary gland exocrinopathy could ameliorate salivary gland inflammation and enhance saliva secretion.Citation198
Panx1 and P2 receptors are strongly upregulated during acute and chronic inflammation of the lacrimal gland,Citation18 the primary contributor to the aqueous layer of tear film in humans. Moreover, lacrimal-gland injury due to inflammation leads to aqueous tear-deficiency dry eye. Most current therapies to treat lacrimal-gland disorders suggest topical treatments, including usage of artificial tears and autologous serum eye-drops, but they do not treat the cause of the disease and lead to limited success. Cell-based regenerative therapies may provide better and longer relief to dry-eye patients; however, survival of transplanted cells strictly depends on the degree of inflammation.Citation199,Citation200 We recently have shown that the best cell engraftment is observed when Panx1 has been blocked with specific Panx1 inhibitors, including Citation10Panx and self-deliverable RNAi (sdRNAi) specific to Panx1.Citation200 Moreover, lacrimal-gland treatment with Panx1 sdRNAi resulted in significant reduction in IL1β and Nlrp3 expression in TSP1−/− mice, a mouse model of aqueous tear-deficiency dry eye.Citation200 These findings have implications for therapeutic strategies targeting Panx1-signaling pathways for suppression of inflammation and/or increasing donor lacrimal-gland progenitor-cell engraftment.
The role of Panx1 in peripheral nerve disease and repair
Pannexins have been implicated in a number of pain-sensitization pathways, in both the peripheral nervous system and the CNS, through activity within and communication between neurons and their supporting cells.Citation99,Citation100,Citation201,Citation202 Zhang et al provided initial evidence that cell bodies of sensory neurons release ATP in response to electrical stimulation. Further, this release stimulated activation of P2X7 receptors and subsequent release of the inflammatory cytokine TNFα in neighboring glial cells.Citation203 Pannexin interactions with various isoforms of P2 receptors may be localization-specific, as P2X4 receptors have also been implicated in purinergic pain pathways: in this case, through activity at sensory endings in the skin.Citation204 Interestingly, P2X3 receptors have also been noted to play a prominent role in pannexin-mediated signaling in DRG neurons.Citation203,Citation205,Citation206 Consistently with a role for pannexin-P2X3 activity in pain pathways, the neurotoxin BomoTx, from the Brazilian lancehead pit viper, activates ATP release through pannexin HCs and downstream P2X3-receptor activation, resulting in inflammatory pain, thermal hyperalgesia, and mechanical allodynia. Further, nerve injury results in increased Panx1 gene and protein expression due to epigenetic mechanisms.Citation178 In contrast, a recent study revealed that Panx1 inhibition, genetically or through pharmacological reagents, reduced hypersensitivity induced by nerve injury.Citation207
Although studied less comprehensively, pannexins also likely play an important role in peripheral nerve development and regeneration. For example, a role in myelination has been suggested for both P2X7 receptors and pannexin,Citation208 likely due to communication between stimulated neurons and their flanking Schwann cells.Citation209 In addition, a recent study by our research team indicated that pannexin negatively regulated developmental and regenerative growth of peripheral neurons, as suggested by the increased caliber of axons in the sciatic nerves of Panx1−/− mice and increased regenerative outgrowth and branching of cultured DRG explants harvested from Panx1−/− mice, as well as in wild-type DRG explants treated with inhibitors of the Panx1-signaling pathway, including apyrase, probenecid, and Citation10Panx.Citation210 Based on its physiological properties and roles in inflammasome activation, it is feasible to suggest that pannexin activity can modulate the neuroregenerative environment, which is enriched in inflammatory cytokines, as well as immune and activated glial cells that respond to inflammatory cues. As such, pannexin reduction may be an effective strategy to reduce pain and promote regeneration after nerve injury.
Conclusion
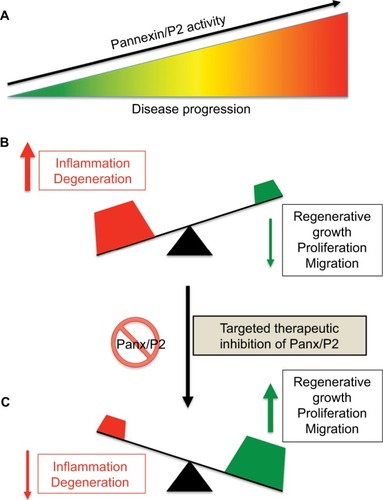
The switch between normal (minor) and pathological (massive) ATP release from Panx HCs and downstream P2 receptors and other channels can determine whether an outcome will be “good” or “bad”. Low levels of extracellular ATP and K+ produced by physiological pannexin activity is required for homeostatic cell function (). Conversely, high levels of extracellular ATP and K+ and overload of the intracellular compartment with Ca2+ synergistically lead to sustained activation of Panx1–P2X7 signaling and inflammasome pathways, inducing Casp1/11-dependent pyroptotic cell death (). Therefore, therapeutic modulation of Panx1 channels represents a feasible new strategy to reduce inflammation and promote regeneration ().
Figure 5 Effect of therapeutic suppression of pannexin function.
Notes: (A) Disease progression often correlates with increased Panx/P2-receptor activity and chronic inflammation (B). We hypothesize that therapeutic suppression of pannexin function may tilt the balance of a progressing disease from that of inflammation and degeneration to one of enhanced cellular growth, proliferation, and/or migration (C).
Acknowledgments
This work was supported by National Institutes of Health National Eye Institute grants 1R01EY026202 (to HPM) and R01EY021517 (to VIS), Russian Science Foundation grant N17-15-01433 (to VIS), and the Department of Veterans Affairs (Merit Award 1 I01 RX001471 to SBS).
Disclosure
The authors report no conflicts of interest in this work.
References
- PhelanPBaconJPDaviesJAInnexins: a family of invertebrate gap-junction proteinsTrends Genet19981493483499769729
- PhelanPStarichTAInnexins get into the gapBioessays200123538839611340620
- HerveJCPhelanPBruzzoneRWhiteTWConnexins, innexins and pannexins: bridging the communication gapBiochim Biophys Acta200517191–23516359939
- MakowskiLCasparDLPhillipsWCBakerTSGoodenoughDAGap junction structures – VI: variation and conservation in connexon conformation and packingBiophys J19844512082186324904
- SáezJCRetamalMABasilioDBukauskasFFBennettMVConnexin-based gap junction hemichannels: gating mechanismsBiochim Biophys Acta20051711221522415955306
- SöhlGWilleckeKGap junctions and the connexin protein familyCardiovasc Res200462222823215094343
- d’HondtCPonsaertsRde SmedtHBultynckGHimpensBPannexins, distant relatives of the connexin family with specific cellular functions?Bioessays200931995397419644918
- BarbeMTMonyerHBruzzoneRCell-cell communication beyond connexins: the pannexin channelsPhysiology (Bethesda)20062110311416565476
- BruzzoneRHormuzdiSGBarbeMTHerbAMonyerHPannexins, a family of gap junction proteins expressed in brainProc Natl Acad Sci U S A200310023136441364914597722
- ShestopalovVIPanchinYPannexins and gap junction protein diversityCell Mol Life Sci200865337639417982731
- GiaumeCLeybaertLNausCCSáezJCConnexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and rolesFront Pharmacol201348823882216
- Rodriguez-SinovasASanchezJAFernandez-SanzCRuiz-MeanaMGarcia-DoradoDConnexin and pannexin as modulators of myocardial injuryBiochim Biophys Acta2012181881962197021839721
- PenuelaSBhallaRGongXQPannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteinsJ Cell Sci2007120Pt 213772378317925379
- BaranovaAIvanovDPetrashNThe mammalian pannexin family is homologous to the invertebrate innexin gap junction proteinsGenomics200483470671615028292
- BondSRLauAPenuelaSPannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytesJ Bone Miner Res201126122911292221915903
- PenuelaSKellyJJChurkoJMBarrKJBergerACLairdDWPanx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healingJ Invest Dermatol201413472026203524522432
- Wicki-StordeurLESwayneLAPanx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elementsCell Commun Signal2013116223964896
- BasovaLVTangXUmasumeTManipulation of Panx1 Activity increases the engraftment of transplanted lacrimal gland epithelial progenitor cellsInvest Ophthalmol Vis Sci201758135654566529098296
- KurtenbachSWhyte-FagundesPGelisLInvestigation of olfactory function in a Panx1 knock out mouse modelFront Cell Neurosci2014826625309319
- LohmanAWBillaudMStraubACExpression of pannexin isoforms in the systemic murine arterial networkJ Vasc Res201249540541622739252
- le VasseurMLelowskiJBechbergerJFSinWCNausCCPannexin 2 protein expression is not restricted to the CNSFront Cell Neurosci2014839225505382
- SwayneLASorbaraCDBennettSAPannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitmentJ Biol Chem201028532249772498620529862
- ZhangHChenYZhangCPatterns of heterogeneous expression of pannexin 1 and pannexin 2 transcripts in the olfactory epithelium and olfactory bulbJ Mol Histol201243665166022945868
- Bhalla-GehiRPenuelaSChurkoJMShaoQLairdDWPannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactionsJ Biol Chem2010285129147916020086016
- LangloisSXiangXYoungKCowanBJPenuelaSCowanKNPannexin 1 and pannexin 3 channels regulate skeletal muscle myoblast proliferation and differentiationJ Biol Chem201428944307173073125239622
- PenuelaSBhallaRNagKLairdDWGlycosylation regulates pannexin intermixing and cellular localizationMol Biol Cell200920204313432319692571
- AbeeleFVBidauxGGordienkoDFunctional implications of calcium permeability of the channel formed by pannexin 1J Cell Biol2006174453554616908669
- MakarenkovaHPShestopalovVIThe role of pannexin hemichannels in inflammation and regenerationFront Physiol201456324616702
- KrižajDRyskampDATianNFrom mechanosensitivity to inflammatory responses: new players in the pathology of glaucomaCurr Eye Res201439210511924144321
- RetamalMARiquelmeMAStehbergJAlcayagaJConnexin43 hemichannels in satellite glial cells, can they influence sensory neuron activity?Front Mol Neurosci20171037429200997
- LapatoASTiwari-WoodruffSKConnexins and pannexins: at the junction of neuro-glial homeostasis and diseaseJ Neurosci Res2018961314428580666
- SarrouilheDDejeanCMesnilMConnexin43- and pannexin-based channels in neuroinflammation and cerebral neuropathiesFront Mol Neurosci20171032029066951
- LohmanAWIsaksonBEDifferentiating connexin hemichannels and pannexin channels in cellular ATP releaseFEBS Lett201458881379138824548565
- GulbransenBDBashashatiMHirotaSAActivation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitisNat Med201218460060422426419
- PelegrinPTargeting interleukin-1 signaling in chronic inflammation: focus on P2X7 receptor and pannexin-1Drug News Perspect200821842443319034348
- KannegantiTDLamkanfiMKimYGPannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signalingImmunity200726443344317433728
- MartinonFTschoppJInflammatory caspases and inflammasomes: master switches of inflammationCell Death Differ2007141102216977329
- MartinonFBurnsKTschoppJThe inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-βMol Cell200210241742612191486
- VaccariJPLotockiGMarcilloAEDietrichWDKeaneRWA molecular platform in neurons regulates inflammation after spinal cord injuryJ Neurosci200828133404341418367607
- KesavardhanaSKannegantiTDMechanisms governing inflammasome activation, assembly and pyroptosis inductionInt Immunol201729520121028531279
- MalikAKannegantiTDInflammasome activation and assembly at a glanceJ Cell Sci2017130233955396329196474
- LeiXZhangZXiaoXQiJHeBWangJEnterovirus 71 inhibits pyroptosis through cleavage of gasdermin DJ Virol20179118e010691728679757
- ChenXHeWTHuLPyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosisCell Res20162691007102027573174
- HeWTWanHHuLGasdermin D is an executor of pyroptosis and required for interleukin-1β secretionCell Res201525121285129826611636
- KayagakiNStoweIBLeeBLCaspase-11 cleaves gasdermin D for non-canonical inflammasome signallingNature2015526757566667126375259
- ManSMKannegantiTDGasdermin D: the long-awaited executioner of pyroptosisCell Res201525111183118426482951
- YueNHuangHZhuXActivation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviorsJ Neuroinflammation201714110228486969
- VaccariJPDietrichWDKeaneRWActivation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injuryJ Cereb Blood Flow Metab201434336937524398940
- SilvermanWRVaccariJPLocoveiSThe pannexin 1 channel activates the inflammasome in neurons and astrocytesJ Biol Chem200928427181431815119416975
- RiteauNGassePFauconnierLExtracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosisAm J Respir Crit Care Med2010182677478320522787
- HungSCChoiCHSaid-SadierNP2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activationPLoS One201387e7021023936165
- PelegrinPSurprenantAPannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1β release through a dye uptake-independent pathwayJ Biol Chem200728242386239417121814
- PelegrinPSurprenantAPannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptorEMBO J200625215071508217036048
- AlbalawiFLuWBeckelJMLimJCMcCaugheySAMitchellCHThe P2X7 receptor primes IL-1β and the NLRP3 Inflammasome in astrocytes exposed to mechanical strainFront Cell Neurosci20171122728848393
- QuYMisaghiSNewtonKPannexin-1 is required for ATP release during apoptosis but not for inflammasome activationJ Immunol2011186116553656121508259
- TakahashiMNLRP3 in myocardial ischaemia-reperfusion injury: inflammasome-dependent or -independent role in different cell typesCardiovasc Res20139914523737495
- YanguasSCWillebrordsJJohnstoneSRPannexin1 as mediator of inflammation and cell deathBiochim Biophys Acta201718641516127741412
- BroughDPelegrinPRothwellNJPannexin-1-dependent caspase-1 activation and secretion of IL-1β is regulated by zincEur J Immunol200939235235819130485
- McKuenMJDahlGFieldsKAAssessing a potential role of host pannexin 1 during Chlamydia trachomatis infectionPLoS One201385e6373223700432
- LiuMWuQWangMFuYWangJLactobacillus rhamnosus GR-1 limits Escherichia coli-induced inflammatory responses via attenuating MyD88-dependent and MyD88-independent pathway activation in bovine endometrial epithelial cellsInflammation20163941483149427236308
- Correa-CostaMBragaTTSemedoPPivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritisPLoS One2011612e2900422194975
- WangHXingYMaoLLuoYKangLMengGPannexin-1 influences peritoneal cavity cell population but is not involved in NLRP3 inflammasome activationProtein Cell20134425926523549611
- ParzychKZetterqvistAVWrightWRKirkbyNSMitchellJAPaul-ClarkMJDifferential role of pannexin-1/ATP/P2X7 axis in IL-1β release by human monocytesFASEB J20173162439244528246166
- WeilingerNLLohmanAWRakaiBDMetabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicityNat Neurosci201619343244226854804
- ChekeniFBElliottMRSandilosJKPannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosisNature2010467731786386720944749
- SridharanMAdderleySPBowlesEAPannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytesAm J Physiol Heart Circ Physiol20102994H1146H115220622111
- ScemesESprayDCExtracellular K+ and astrocyte signaling via connexin and pannexin channelsNeurochem Res201237112310231622481627
- SandilosJKBaylissDAPhysiological mechanisms for the modulation of pannexin 1 channel activityJ Physiol2012590Pt 246257626623070703
- XiaJLimJCLuWNeurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptorsJ Physiol2012590102285230422411013
- PenuelaSSimekJThompsonRJRegulation of pannexin channels by post-translational modificationsFEBS Lett201458881411141524486011
- ChiuYHJinXMedinaCBA quantized mechanism for activation of pannexin channelsNat Commun201781432428134257
- SandilosJKChiuYHChekeniFBPannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory regionJ Biol Chem201228714113031131122311983
- YangDHeYMunoz-PlanilloRLiuQNunezGCaspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shockImmunity201543592393226572062
- BillaudMChiuYHLohmanAWA molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cellsSci Signal20158364ra1725690012
- MartinelloTBaldoinMCMorbiatoLExtracellular ATP signaling during differentiation of C2C12 skeletal muscle cells: role in proliferationMol Cell Biochem20113511–218319621308481
- MeyerMPGröschel-StewartURobsonTBurnstockGExpression of two ATP-gated ion channels, P2X5 and P2X6, in developing chick skeletal muscleDev Dyn19992164–544244910633863
- RiquelmeMACeaLAVegaJLThe ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannelsNeuropharmacology20137559460323583931
- BuvinicSAlmarzaGBustamanteMATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscleJ Biol Chem200928450344903450519822518
- MessemerNKunertCGrohmannMP2X7 receptors at adult neural progenitor cells of the mouse subventricular zoneNeuropharmacology20137312213723727220
- Jacques-SilvaMCRodnightRLenzGP2X7 receptors stimulate Akt phosphorylation in astrocytesBr J Pharmacol200414171106111715023862
- PlesnerLEcto-ATPases: identities and functionsInt Rev Cytol19951581412147721538
- JacksonDGWangJKeaneRWScemesEDahlGATP and potassium ions: a deadly combination for astrocytesSci Rep20144457624694658
- QiuFDahlGA permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATPAm J Physiol Cell Physiol20092962C250C25518945939
- McIlwainDRBergerTMakTWCaspase functions in cell death and diseaseCold Spring Harb Perspect Biol201354a00865623545416
- WilkaniecAGassowskaMCzapskiGACieslikMSulkowskiGAdamczykAP2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cellsPurinergic Signal201713334736128516276
- LocoveiSWangJDahlGActivation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calciumFEBS Lett2006580123924416364313
- IglesiasRLocoveiSRoqueAP2X7 receptor-pannexin1 complex: pharmacology and signalingAm J Physiol Cell Physiol20082953C752C76018596211
- LocoveiSScemesEQiuFSprayDCDahlGPannexin1 is part of the pore forming unit of the P2X7 receptor death complexFEBS Lett2007581348348817240370
- RobertsJAVialCDigbyHRMolecular properties of P2X receptorsPflugers Arch2006452548650016607539
- Kaczmarek-HajekKLorincziEHausmannRNickeAMolecular and functional properties of P2X receptors: recent progress and persisting challengesPurinergic Signal20128337541722547202
- NorthRAMolecular physiology of P2X receptorsPhysiol Rev20028241013106712270951
- ChenLLiuYWYueKDifferential expression of ATP-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat modelsPurinergic Signal2016121798726531254
- LiSTomićMStojilkovicSSCharacterization of novel pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channelsGen Comp Endocrinol2011174220221021907716
- WoehrleTYipLElkhalAPannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapseBlood2010116183475348420660288
- WoehrleTYipLManoharMHypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptorsJ Leukoc Biol20108861181118920884646
- DraganovDGopalakrishna-PillaiSChenYRModulation of P2X4/P2X7/pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell deathSci Rep201551622226552848
- KhakhBSProctorWRDunwiddieTVLabarcaCLesterHAAllosteric control of gating and kinetics at P2X4 receptor channelsJ Neurosci199919177289729910460235
- KhakhBSBaoXRLabarcaCLesterHANeuronal P2X transmitter-gated cation channels change their ion selectivity in secondsNat Neurosci19992432233010204538
- BravoDMaturanaCJPelissierTHernandezAConstandilLInteractions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: possible role on chronic painPharmacol Res2015101869326211949
- BravoDIbarraPRetamalJPannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cordPain2014155102108211525102401
- LommenJStahrAIngenwerthMAliAAvon GallCTime-of-day-dependent expression of purinergic receptors in mouse suprachiasmatic nucleusCell Tissue Res2017369357959028547658
- KashfiSPeymaniMGhaediKBaharvandHNasr-EsfahaniMHJavanMPurinergic receptor expression and potential association with human embryonic stem cell-derived oligodendrocyte progenitor cell developmentCell J201719338640228836401
- JacobsonKAIvanovAAde CastroSHardenTKKoHDevelopment of selective agonists and antagonists of P2Y receptorsPurinergic Signal200951758918600475
- BurnstockGPurine and pyrimidine receptorsCell Mol Life Sci200764121471148317375261
- LohmanAWLeskovILButcherJTPannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammationNat Commun20156796526242575
- SandersonJDarttDATrinkaus-RandallVPurines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal glandExp Eye Res201412727027925151301
- BaoYChenYLedderoseCLiLJungerWGPannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophilsJ Biol Chem201328831226502265723798685
- ChenYCorridenRInoueYATP release guides neutrophil chemotaxis via P2Y2 and A3 receptorsScience200631458061792179517170310
- YoungMTPelegrinPSurprenantAAmino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATPMol Pharmacol20077119210017032903
- VaccariJPBastienDYurcisinGP2X4 receptors influence inflammasome activation after spinal cord injuryJ Neurosci20123293058306622378878
- ReigadaDLuWZhangMMitchellCHElevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannelsNeuroscience2008157239640418822352
- XiaJLimJCLuWNeurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptorsJ Physiol2012590Pt 102285230422411013
- BoumechacheMMasinMEdwardsonJMGoreckiDCMurrell-LagnadoRAnalysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cellsJ Biol Chem200928420134461345419304656
- CraigieEBirchREUnwinRJWildmanSSThe relationship between P2X4 and P2X7: a physiologically important interaction?Front Physiol2013421623966951
- Casas-PrunedaGReyesJPPerez-FloresGPerez-CornejoPArreolaJFunctional interactions between P2X4 and P2X7 receptors from mouse salivary epitheliaJ Physiol2009587Pt 122887290119403602
- KawanoATsukimotoMNoguchiTInvolvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophagesBiochem Biophys Res Commun2012419237438022349510
- DvoriantchikovaGIvanovDBarakatDGenetic ablation of pannexin1 protects retinal neurons from ischemic injuryPLoS One201272e3199122384122
- MitchellCHLuWHuHZhangXReigadaDZhangMThe P2X7 receptor in retinal ganglion cells: a neuronal model of pressure-induced damage and protection by a shifting purinergic balancePurinergic Signal20095224124919241145
- ZhangXZhangMLatiesAMMitchellCHStimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cellsInvest Ophthalmol Vis Sci20054662183219115914640
- KakuraiKSugiyamaTKurimotoTOkuHIkedaTInvolvement of P2X7 receptors in retinal ganglion cell death after optic nerve crush injury in ratsNeurosci Lett201353423724123262079
- DomercqMPerez-SamartinAAparicioDAlberdiEPampliegaOMatuteCP2X7 receptors mediate ischemic damage to oligodendrocytesGlia2010673074020029962
- ShojiKFSaezPJHarchaPAAguilaHLSáezJCPannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell deathChannels (Austin)20148214215624590064
- ChenKZhangJZhangWATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathyInt J Biochem Cell Biol201345593294323434541
- ShenJChoyDFYoshidaTInterleukin-18 has antipermeability and antiangiogenic activities in the eye: reciprocal suppression with VEGFJ Cell Physiol2014229897498324515951
- DoyleSLLopezFJCelkovaLIL-18 immunotherapy for neovascular AMD: tolerability and efficacy in nonhuman primatesInvest Ophthalmol Vis Sci20155695424543026284546
- MurphyAJKraakmanMJKammounHLIL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndromeCell Metab201623115516426603191
- SarojiniHBilleterATEichenbergerSRapid tissue regeneration induced by intracellular ATP delivery: a preliminary mechanistic studyPLoS One2017124e017489928380006
- LeeSRGuoSZScannevinRHInduction of matrix metalloproteinase, cytokines and chemokines in rat cortical astrocytes exposed to plasminogen activatorsNeurosci Lett200741711517386975
- AdamsonSELeitingerNThe role of pannexin1 in the induction and resolution of inflammationFEBS Lett201458881416142224642372
- BeckelJMArgallAJLimJCMechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strainGlia20146291486150124839011
- SakakiHTsukimotoMHaradaHMoriyamaYKojimaSAutocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptorPLoS One201384e5977823577075
- YaginumaHKawaiSTabataKVDiversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imagingSci Rep20144652225283467
- AtarashiKNishimuraJShimaTATP drives lamina propria TH17 cell differentiationNature2008455721480881218716618
- SeikeSTakeharaMKobayashiKNagahamaMRole of pannexin 1 in Clostridium perfringens beta-toxin-caused cell deathBiochim Biophys Acta20161858123150315627720686
- SáezJCNairnACCzernikAJPhosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase IIEur J Biochem199019222632732170122
- CarrerALeparuloACrispinoGCx32 hemichannel opening by cytosolic Ca2+ is inhibited by the R220X mutation that causes Charcot-Marie-Tooth diseaseHum Mol Genet2018271809429077882
- DheinSDuerrschmidtNSchollAA new role for extracellular Ca2+ in gap-junction remodeling: studies in humans and ratsNaunyn Schmiedebergs Arch Pharmacol2008377212513818278481
- EbiharaLNew roles for connexonsNews Physiol Sci20031810010312750444
- BruzzoneRBarbeMTJakobNJMonyerHPharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytesJ Neurochem20059251033104315715654
- BaoLLocoveiSDahlGPannexin membrane channels are mechanosensitive conduits for ATPFEBS Lett20045721–3656815304325
- YipLCheungCWCorridenRChenYInselPAJungerWGHypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosineShock200727324225017304104
- ShahidullahMMandalABeimgrabenCDelamereNAHyposmotic stress causes ATP release and stimulates Na, K-ATPase activity in porcine lensJ Cell Physiol201222741428143721618533
- IslamMRUramotoHOkadaTSabirovRZOkadaYMaxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cellsAm J Physiol Cell Physiol20123039C924C93522785119
- NegoroHUrban-MaldonadoMLiouLSSprayDCThiMMSuadicaniSOPannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signalingPLoS One201498e10626925170954
- Seref-FerlengezZMaungSSchafflerMBSprayDCSuadicaniSOThiMMP2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetesPLoS One2016115e015510727159053
- ForsythAMWanJOwrutskyPDAbkarianMStoneHAMultiscale approach to link red blood cell dynamics, shear viscosity, and ATP releaseProc Natl Acad Sci U S A201110827109861099121690355
- BurnstockGKnightGECell culture: complications due to mechanical release of ATP and activation of purinoceptorsCell Tissue Res2017370111128434079
- ReyesJPHernandez-CarballoCYPerez-FloresGPerez-CornejoPArreolaJLack of coupling between membrane stretching and pannexin-1 hemichannelsBiochem Biophys Res Commun20093801505319150332
- LiAZhangXZhengDGeJLatiesAMMitchellCHSustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucomaExp Eye Res201193452853321745471
- ConeFEGelmanSESonJLPeaseMEQuigleyHADifferential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injectionExp Eye Res201091341542420599961
- RestaVNovelliEVozziGAcute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATPEur J Neurosci20072592741275417459106
- LuWHuHSevignyJRat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eyeInvest Ophthalmol Vis Sci20155653075308326024091
- LimJCLuWBeckelJMMitchellCHNeuronal release of cytokine IL-3 triggered by mechanosensitive autostimulation of the P2X7 receptor is neuroprotectiveFront Cell Neurosci20161027027932954
- CampbellMDoyleSLOzakiEAn overview of the involvement of interleukin-18 in degenerative retinopathiesAdv Exp Med Biol201480140941524664725
- IglesiasRDahlGQiuFSprayDCScemesEPannexin 1: the molecular substrate of astrocyte “hemichannels”J Neurosci200929217092709719474335
- KangJKangNLovattDConnexin 43 hemichannels are permeable to ATPJ Neurosci200828184702471118448647
- SuadicaniSOBrosnanCFScemesEP2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signalingJ Neurosci20062651378138516452661
- BennettMVGarréJMOrellanaJABukauskasFFNedergaardMSáezJCConnexin and pannexin hemichannels in inflammatory responses of glia and neuronsBrain Res2012148731522975435
- OrellanaJASaezPJShojiKFModulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegenerationAntioxid Redox Signal200911236939918816186
- GarreJMRetamalMACassinaPFGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannelsProc Natl Acad Sci U S A201010752226592266421148774
- FrogerNOrellanaJACalvoCFInhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotectiveMol Cell Neurosci2010451374620684043
- BoyceAKWicki-StordeurLESwayneLAPowerful partnership: crosstalk between pannexin 1 and the cytoskeletonFront Physiol201452724523699
- SáezPJVargasPShojiKFHarchaPALennon-DuménilAMSáezJCATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptorsSci Signal201710506eaah710729162744
- StankovaVTsikoliaNViebahnCRho kinase activity controls directional cell movements during primitive streak formation in the rabbit embryoDevelopment20151421929825516971
- HortonSMLopezCLBlevinsEPannexin 1 modulates axonal growth in mouse peripheral nervesFront Cell Neurosci20171136529213230
- KovalzonVMMoiseenkoLSAmbaryanAVKurtenbachSShestopalovVIPanchinYVSleep-wakefulness cycle and behavior in pannexin1 knockout miceBehav Brain Res2017318242727769744
- SappingtonRMCalkinsDJContribution of TRPV1 to microglia-derived IL-6 and NFκB translocation with elevated hydrostatic pressureInvest Ophthalmol Vis Sci20084973004301718362111
- SappingtonRMSidorovaTLongDJCalkinsDJTRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressureInvest Ophthalmol Vis Sci200950271772818952924
- BoyceAKKimMSWicki-StordeurLESwayneLAATP stimulates pannexin 1 internalization to endosomal compartmentsBiochem J2015470331933026195825
- BillaudMSandilosJKIsaksonBEPannexin 1 in the regulation of vascular toneTrends Cardiovasc Med2012223687222841835
- ProchnowNAbdulazimAKurtenbachSPannexin1 stabilizes synaptic plasticity and is needed for learningPLoS One2012712e5176723284764
- CeaLARiquelmeMACisternaBAConnexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophyJ Membr Biol2012245842343622850938
- CeaLARiquelmeMAVargasAAUrrutiaCSáezJCPannexin 1 channels in skeletal musclesFront Physiol2014513924782784
- DvoriantchikovaGIvanovDPanchinYShestopalovVIExpression of pannexin family of proteins in the retinaFEBS Lett200658092178218216616526
- WeilingerNLMaslieievaVBialeckiJSridharanSSTangPLThompsonRJIonotropic receptors and ion channels in ischemic neuronal death and dysfunctionActa Pharmacol Sin2013341394822864302
- Wicki-StordeurLESanchez-AriasJCDhaliwalJPannexin 1 differentially affects neural precursor cell maintenance in the ventricular zone and peri-infarct cortexJ Neurosci20163641203121026818508
- MannelliLCMarcoliMMicheliLOxaliplatin evokes P2X7-dependent glutamate release in the cerebral cortex: a pain mechanism mediated by pannexin 1Neuropharmacology20159713314126071109
- ZhangYLaumetGChenSRHittelmanWNPanHLPannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain developmentJ Biol Chem201529023146471465525925949
- DolmatovaESpagnolGBoassaDCardiomyocyte ATP release through pannexin 1 aids in early fibroblast activationAm J Physiol Heart Circ Physiol201230310H1208H121822982782
- WonnenbergBTschernigTVossMProbenecid reduces infection and inflammation in acute Pseudomonas aeruginosa pneumoniaInt J Med Microbiol20143045–672572924938792
- LaiCPBechbergerJFThompsonRJMacVicarBABruzzoneRNausCCTumor-suppressive effects of pannexin 1 in C6 glioma cellsCancer Res20076741545155417308093
- LutzSEGonzalez-FernandezEVenturaJCContribution of pannexin1 to experimental autoimmune encephalomyelitisPLoS One201386e6665723885286
- HainzNWolfSTschernigTMeierCProbenecid application prevents clinical symptoms and inflammation in experimental autoimmune encephalomyelitisInflammation201639112312826276126
- VelasquezSMalikSLutzSEScemesEEugeninEAPannexin1 channels are required for chemokine-mediated migration of CD4+ T lymphocytes: role in inflammation and experimental autoimmune encephalomyelitisJ Immunol2016196104338434727076682
- JinZDingYXueRInvolvement of interstitial cells of Cajal in bladder dysfunction in mice with experimental autoimmune encephalomyelitisInt Urol Nephrol20174981353135928425078
- MartinsIWangYMichaudMMolecular mechanisms of ATP secretion during immunogenic cell deathCell Death Differ2014211799123852373
- BunseSLocoveiSSchmidtMThe potassium channel subunit Kvβ3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentialsFEBS J2009276216258627019780818
- ShibukawaYSatoMKimuraMOdontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transductionPflugers Arch2015467484386324939701
- WeilingerNLTangPLThompsonRJAnoxia-induced NMDA receptor activation opens pannexin channels via Src family kinasesJ Neurosci20123236125791258822956847
- VaccariJPLotockiGAlonsoOFBramlettHMDietrichWDKeaneRWTherapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injuryJ Cereb Blood Flow Metab20092971251126119401709
- MengXFWangXLTianXJNod-like receptor protein 1 inflammasome mediates neuron injury under high glucoseMol Neurobiol201449267368424014157
- PelegrinPBarroso-GutierrezCSurprenantAP2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophageJ Immunol2008180117147715718490713
- SilvermanWLocoveiSDahlGProbenecid, a gout remedy, inhibits pannexin 1 channelsAm J Physiol Cell Physiol20082953C761C76718596212
- BhaskaracharyaADao-UngPJalilianIProbenecid blocks human P2X7 receptor-induced dye uptake via a pannexin-1 independent mechanismPLoS One201493e9305824671093
- XiongXXGuLJShenJProbenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in miceNeurochem Res201439121622424317635
- DahlGKeaneRWPannexin: from discovery to bedside in 11±4 years?Brain Res2012148715015922771709
- OrellanaJAAvendanoBCMonteroTDRole of connexins and pannexins in ischemic strokeCurr Med Chem201421192165218224372216
- KhalafallaMGWoodsLTCamdenJMP2X7 receptor antagonism prevents IL-1beta release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathyJ Biol Chem2017292166261663728798231
- UmazumeTThomasWMCampbellSLacrimal gland inflammation deregulates extracellular matrix remodeling and alters molecular signature of epithelial stem/progenitor cellsInvest Ophthalmol Vis Sci201556138392840226747770
- GromovaAVoronovDAYoshidaMLacrimal gland repair using progenitor cellsStem Cells Transl Med201761889828170196
- SprayDCHananiMGap junctions, pannexins and painNeurosci Lett Epub2017 622
- HuangLYGuYChenYCommunication between neuronal somata and satellite glial cells in sensory gangliaGlia201361101571158123918214
- ZhangXChenYWangCHuangLYNeuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root gangliaProc Natl Acad Sci U S A2007104239864986917525149
- BarrTPHrnjicAKhodorovaASpragueJMStrichartzGRSensitization of cutaneous neuronal purinergic receptors contributes to endothelin-1-induced mechanical hypersensitivityPain201415561091110124569146
- FabbrettiEATP-gated P2X3 receptors are specialised sensors of the extracellular environmentAdv Exp Med Biol2017105171628639246
- FabbrettiEATP P2X3 receptors and neuronal sensitizationFront Cell Neurosci2013723624363643
- WeaverJLArandjelovicSBrownGHematopoietic pannexin 1 function is critical for neuropathic painSci Rep201774255028195232
- FaroniASmithRJProcacciPPurinergic signaling mediated by P2X7 receptors controls myelination in sciatic nervesJ Neurosci Res201492101259126924903685
- InoDSagaraHSuzukiJKanemaruKOkuboYIinoMNeuronal regulation of Schwann cell mitochondrial Ca2+ signaling during myelinationCell Rep201512121951195926365190
- HortonSMLuna LopezCBlevinsEPannexin 1 modulates axonal growth in mouse peripheral nervesFront Cell Neurosci20171136529213230
- PenuelaSGehiRLairdDWThe biochemistry and function of pannexin channelsBiochim Biophys Acta201318281152222305965
- KwonTJKimDBBaeJWMolecular cloning, characterization, and expression of pannexin genes in chickenPoult Sci20149392253226125002553
- FagerbergLHallströmBMOksvoldPAnalysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomicsMol Cell Proteomics201413239740624309898
- IwamotoTNakamuraTDoyleAPannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiationJ Biol Chem201028524189481895820404334
- RayAZoidlGWeickertSWahlePDermietzelRSite-specific and developmental expression of pannexin1 in the mouse nervous systemEur J Neurosci200521123277329016026466
- DiezmosEFSandowSLMarkusIExpression and localization of pannexin-1 hemichannels in human colon in health and diseaseNeurogastroenterol Motil2013256e395e40523594276
- ZoidlGPetrasch-ParwezERayALocalization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampusNeuroscience2007146191617379420
- PenuelaSCelettiSJBhallaRShaoQLairdDWDiverse subcellular distribution profiles of pannexin 1 and pannexin 3Cell Commun Adhes200815113314218649185
- IshikawaMIwamotoTNakamuraTDoyleAFukumotoSYamadaYPannexin 3 functions as an ER Ca2+ channel, hemichannel, and gap junction to promote osteoblast differentiationJ Cell Biol201119371257127421690309
- CowanKNLangloisSPenuelaSCowanBJLairdDWPannexin1 and pannexin3 exhibit distinct localization patterns in human skin appendages and are regulated during keratinocyte differentiation and carcinogenesisCell Commun Adhes2012193–4455322947051
- AmbrosiCGassmannOPranskevichJNPannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each otherJ Biol Chem201028532244202443120516070
- DingJWangKLiuWPore-forming activity and structural autoinhibition of the gasdermin familyNature2016535761011111627281216