Abstract
The nucleotide-binding oligomerization domain (NOD) protein, NOD2, belonging to the intracellular NOD-like receptor family, detects conserved motifs in bacterial peptidoglycan and promotes their clearance through activation of a proinflammatory transcriptional program and other innate immune pathways, including autophagy and endoplasmic reticulum stress. An inactive form due to mutations or a constitutive high expression of NOD2 is associated with several inflammatory diseases, suggesting that balanced NOD2 signaling is critical for the maintenance of immune homeostasis. In this review, we discuss recent developments about the pathway and mechanisms of regulation of NOD2 and illustrate the principal functions of the gene, with particular emphasis on its central role in maintaining the equilibrium between intestinal microbiota and host immune responses to control inflammation. Furthermore, we survey recent studies illustrating the role of NOD2 in several inflammatory diseases, in particular, inflammatory bowel disease, of which it is the main susceptibility gene.
Introduction
The human body is constantly in contact with a myriad of microorganisms, either pathogens or commensals. Innate immune system, which provides a first line of defense against many common microbes, is essential for an appropriate tissue homoeostasis as well as for common bacterial infections, and its dysfunction leads to infectious, inflammatory and autoimmune diseases.
Innate immune response relies on recognition of evolutionarily conserved structures on the microorganisms, termed pathogen-associated molecular patterns (PAMPs), through a limited number of germ line-encoded pattern recognition receptors (PRRs) present on the host cell surface or in the intracellular compartments.Citation1 Among the latter, nucleotide-binding and oligomerization domain containing protein 2 (NOD2) is a cytosolic receptor belonging to the nucleotide-binding oligomerization (NOD)-like receptor (NLR) family.Citation2 NOD2 is able to detect intracellular muramyl dipeptide (MDP), a component of the bacterial wall that is ubiquitously present in bacterial peptidoglycan.Citation3 Upon activation by ligand, NOD2 mediates innate immune response triggering proinflammatory responses. NOD2 mutation or altered expression has been found in patients with chronic inflammatory disorders such as Crohn’s disease (CD), Blau syndrome (BS) and early-onset sarcoidosis (EOS).Citation4–Citation9 In this review, we summarize the current knowledge about NOD2 functions and regulation, as well as its involvement in chronic inflammatory diseases.
The NLR family
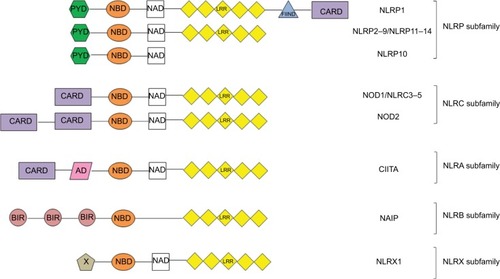
The 23 NLR family members, intracellular sensors of PAMPs, share a common organization consisting of a C-terminal leucine-rich repeat (LRR) domain with regulatory and ligand recognition functions, a central nucleotide-binding and oligomerization domain (NBD) and an N-terminal effector-binding domain. The type of effector domain results in the division of NLR proteins into five subfamilies: acidic transactivation domain (NLRA); baculovirus inhibitor repeat, BIR (NLRB); caspase recruitment domain, CARD (NLRC); pyrin domain (NLRP) and NLRX1 that localizes to the mitochondria and has no homology to any known N-terminal domain ().Citation10,Citation11 NOD2 belongs to the NLRC subfamily of NLRs and, after NOD1, has been the second member of the family to be identified.Citation12 NOD2 receptor, encoded by the NOD2/CARD15 gene, mapping on chromosome 16q12.1 in humans, consists of 1040 amino acids and has a molecular weight of 110 kDa.Citation12 It is expressed in monocytes, macrophages, dendritic cells, hepatocytes, preadipocytes, epithelial cells of oral cavity, lung and intestine, with higher expression in ileal Paneth cells and in intestinal stem cells.Citation13,Citation14 NOD2, like NOD1, is a cytoplasmic protein, although it is recruited in the plasmatic membrane where it detects bacterial invasion at the point of entry.Citation15
Figure 1 Structure of the NLR subfamilies.
Abbreviations: AD, atopic dermatitis; BIR, baculovirus inhibitor repeat; CARD, caspase recruitment domain; CIITA, class II major histocompatibility complex transactivator; FIIND, function to find domain; LRR, leucine-rich repeat; NAD, NBD-associated domain; NBD, nucleotide-binding domain; NLR, NOD-like receptor; NLRA, acidic transactivation domain; NLRB, baculovirus inhibitor repeat; NLRC, caspase recruitment domain; NLRP, NLR family pyrin domain; NOD, nucleotide-binding oligomerization domain; PYD, pyrin domain; X, unknown effector domain.
NOD2 signaling
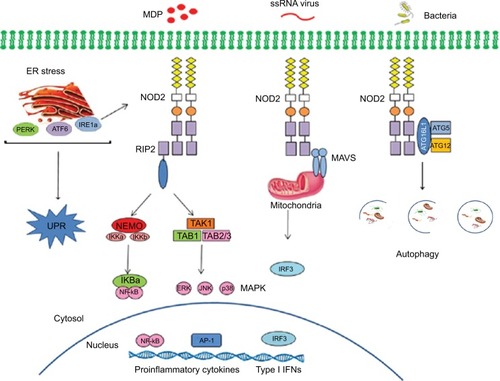
The innate immune system is critical for clearing infection and averting excessive tissue damage. NOD2, an intracellular receptor of microbial components derived from bacterial peptidoglycan, contributes to the maintenance of mucosal homeostasis and the induction of mucosal inflammation. Structurally, NOD2 protein is composed of two tandem N-terminal CARDs that function as effector domain and mediate specific homophilic interaction with downstream CARD-containing molecules.Citation16 On activation by MDP, a cell wall component of both Gram-positive and Gram-negative bacteria, through LRR domain, NOD2 undergoes self-oligomerization and recruitment of the downstream adaptor molecule, the kinase receptor interacting protein 2 (RIP2 also known as RICK, CARDIAK, CCK and Ripk2), via homophilic CARD–CARD interaction.Citation17–Citation19 Active RIP2 leads to ubiquitination of nuclear factor-kappa B (NF-κB) essential modulator, resulting in activation of IκB kinase (IKK) complex that phosphorylates NF-κB inhibitor-α (IKBα), the inhibitor of transcription factor NF-κB, which translocates to the nucleus and starts transcription of proinflammatory genes, including cytokines, growth factors and factors responsible for stimulation of immune cells.Citation20,Citation21 RIP2 targets transforming growth factor-β-activated kinase 1, which, through IKK complex, activates MAP kinases and transcription factor Activator Protein 1 involved in cell proliferation, differentiation and apoptosis.Citation18,Citation22 NOD2 is also known to bind and activate caspase-1, through its CARD domain, and starts interleukin (IL)-1β processing.Citation23 Moreover, MDP challenge promotes the formation of NOD2–NLR family pyrin domain containing 1 complex that induces caspase-1–dependent IL-1β secretion in response to Bacillus anthracis or Yersinia pseudotuberculosis infection.Citation23,Citation24 Recently, NOD2 has been suggested to have a role in the autophagic process due to its interaction with the autophagy protein Autophagy Related 16 Like 1 (ATG16L1), which has been described below in-depth ().Citation25
Figure 2 Signaling pathways triggered by NOD2.
Abbreviations: AP-1, activator protein-1; ATF6, activating transcription factor 6; ATG, autophagy-related genes; ATG16L1, autophagy related 16 like 1; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; IFNs, interferons; IKB, NF-κB inhibitor; IKK, IκB kinase; IRF3, interferon response factor 3; JNK, c-Jun N-terminal kinase; MAVS, mitochondrial antiviral signaling; MDP, muramyl dipeptide; NEMO, NF-κB essential modulator; NOD, nucleotide-binding oligomerization domain; PERK, protein kinase RNA-like endoplasmic reticulum kinase; RIP2, receptor-interacting protein kinase 2; TAB, TGF-β activated kinase; TAK1, targets transforming growth factor-β-activated kinase 1; UPR, unfolded protein response.
NOD2 regulation
The complexity of NOD2 signaling is underlined by the evidence that many cellular proteins interact with NOD2 directly and regulate positively or negatively its functional activity.Citation26 Among these, Erbin, Centaurin B1, Angio-Associated Migratory Cell Protein, Carbamoyl-Phosphate Synthetase 2, Mitogen-Activated Protein Kinase Binding Protein 1 (JNKBP1) and heat shock protein (HSP) 90 have been shown to interact with NOD2 and regulate downstream signaling events.Citation26–Citation30 Recently, Suppressor of Cytokine Signaling-3 was found to recruit the ubiquitin machinery to NOD2, facilitating its proteasomal degradation.Citation30 Interferon regulatory factor 4 (IRF4) is another negative regulator of NOD2-dependent NF-κB signaling through inhibition of RIP2 polyubiquitination in human dendritic cells.Citation31 On the contrary, HSP70 and FERM and PDZ domain-containing protein 2 act as positive regulators of NOD2 signaling: the first one, after binding with NOD2, leads to increase in NF-κB activity and the second one favors NOD2 localization at the plasma membrane.Citation29,Citation32
Furthermore, important posttranslational modifications are required to control NOD2 signaling.Citation33 The E3 ubiquitin ligases Pellino 3 and X-linked inhibitor of apoptosis protein, by ubiquitinylating RIP2, are important mediators in the NOD2 pathway and regulators of intestinal inflammation.Citation34,Citation35 Interestingly, loss of Pellino 3 decreases RIP2 ubiquitination and activation of NF-κB and mitogen-activated protein kinases (MAPKs), while genetic X-linked inhibitor of apoptosis protein loss causes a blunted NOD2 response.Citation34−Citation36 Tripartite Motif Containing 27 negatively regulates NOD2 by ubiquitination, while the E3-ubiquitin ligase ZNRF4, degrading RIP2, is a negative regulator of NOD2-induced NF-κB activity as well.Citation37,Citation38 Differently, the ovarian tumor family deubiquitinase OTULIN was shown to dampen NOD2 signaling by increasing NF-κB transcription.Citation39
A recent study shows that leucine-rich repeat kinase 2, whose polymorphisms have been associated with CD, leprosy and familiar Parkinson’s disease, is a new RIP2-positive regulator by enhancing RIP2 phosphorylation upon NOD2 activation.Citation40
NOD2 signaling is known to be regulated by cytoskeleton elements also: the intermediate filament protein vimentin has been recently shown to interact with NOD2, in response to MDP, and affect NF-κB induction.Citation41
Large evidence supports the role of several microRNAs, including mir-320, miR-192, miR-122, miR-512, miR-671 and miR-495, as new important NOD2 regulators.Citation42,Citation43
NOD2 genetics
Hereditary polymorphisms in the gene encoding NOD2 have been associated with an increasing number of chronic inflammatory diseases, such as CD, BS and EOS.Citation6−Citation8
In particular, the three main NOD2 polymorphisms, R702W (Arg702Trp), G908R (Gly908Arg) and L1007fsinsC, are highly associated with susceptibility to CD.Citation6,Citation7,Citation44 R702W and G908R mutations are single amino acid changes within the LRR domain, whereas the L1007fsinsC mutation is caused by a deletion producing a reading frameshift that leads to the loss of 33 amino acids.Citation6,Citation7 CD-associated mutations result in NOD2 loss of functions, with a reduced responsiveness to MDP, enabling invasion of bacteria and abnormal mucosal immune response, which culminates in chronic intestinal inflammation.Citation5−Citation7
The variants in the NBD and in between the NBD and LRR are associated to BS, EOS and NOD2-associated auto-inflammatory disease, respectively.Citation8,Citation9,Citation45,Citation46
At least 17 different mutations have been identified in the NBD domain of NOD2,Citation45 of which the following four missense mutations are the most abundant: Arg334Glu (R334Q), Arg334Trp (R334W) and Leu469Phe (L469F), which together account for 80% of the cases, and Glu383Lys (E383K) (5% of cases).Citation47 Other NOD2 mutations, like the Arg314Glu (R314Q) polymorphism that codes for a truncated form of the protein, have been described most rarely.Citation8 These mutations are supposed to be gain of function, causing excessive NF-κB and MAPK activation.Citation9
NOD2 functions
NOD2 and intestinal microbiota
Humans are colonized by a collection of microbes, the largest numbers of which reside in the distal gut. The constant exposure of the intestinal tissue to gut microorganisms maintains the mucosa in a state of physiological inflammation, which balances tolerogenic and proinflammatory type responses to maintain homeostasis.Citation48 Several studies highlight the essential role that NOD2 plays in maintaining the equilibrium between microbiome and host immune responses.Citation49−Citation51 An imbalance in this relationship results in dysbiosis, whereby pathogenic bacteria prevail on commensals, causing damage in the intestinal epithelial barrier as well as allowing bacterial invasion and inflammation.Citation52,Citation53 A negative feedback loop between NOD2 and commensal bacteria function has been described, whereby commensal bacteria promote NOD2 expression, which in turn prevents their over-expansion.Citation52 Since NOD2 is an intracellular microbial sensor for gram-positive and gram-negative bacteria, it has been proposed that its deficiency or mutations can contribute to the modification of microbial composition and disease development in animal models.Citation18,Citation51,Citation52
NOD2-deficient mice show a reduced number of intestinal intraepithelial lymphocytes impairing the epithelium integrity and leading to altered immune response to the resident microbiota.Citation54 Moreover, NOD2- and RIP2-deficient mice show increased sensitivity to dextran sulfate sodium-induced colitis and colonic adenocarcinoma due to dysbiosis, which is transmitted to wild-type (WT) mice through the microbiota.Citation55 NOD2 is also a critical regulator of microbiota in the small intestine.Citation13 Indeed, NOD2 is highly expressed in Paneth cells, specialized cells located at the base of the intestinal crypts of Lieberkuhn, which are responsible for the ileal microbiota by secreting antimicrobial compounds, in response to bacterial products, including MDP, the agonist of NOD2.Citation13,Citation18,Citation56
There is evidence that NOD2 mutations increase the susceptibility to abnormal ileal inflammation. NOD2-deficient mice display Paneth and goblet cell dysfunction that promotes larger loads of bacteria in the ileum, including Bacteroides vulgatus, and excessive interferon-γ (IFNγ) production.Citation53,Citation57
However, several studies do not support the evidence of a relationship between NOD2 mutations and altered microbiota composition, suggesting the contribution of other factors independent of the genotype, such as maternal microbiota transmission or environmental causes, but results are still controversial.Citation58–Citation60 NOD2 participates in the recognition of a subset of pathogenic microorganisms that are able to invade and multiply intracellularly, such as Campylobacter, Citrobacter, Escherichia, Helicobacter, Listeria, Mycobacteria, Pseudomonas, Staphylococcus, Yersinia and other species.Citation51,Citation61,Citation62 In a murine skin Staphylococcus aureus infection model, Nod2−/− mice show skin lesions and increased bacterial numbers in the skin, compared to WT mice.Citation63 Moreover, NOD2-deficient mice show a higher fecal bacterial load after Citrobacter rodentium infection, besides increased susceptibility to enteric Salmonella spp. and an impaired activation of Th17 cells after C. rodentium or Salmonella spp. exposure.Citation64–Citation66 Interestingly, NOD2L1007fsinsC mutants show a lower reactive oxygen species production and a reduced protection against bacterial invasion.Citation67
NOD2 upregulation following vitamin D treatment results in increased killing of pneumococci in patients with frequent respiratory tract infections.Citation68 Bacterial lipoprotein-tolerogenic macrophages show improved NOD1/NOD2-dependent bactericidal activity to Sta. aureus and Salmonella typhimurium.Citation69
NOD2 cooperates with other proteins for defense against pathogens, such as the autophagic protein ATG16L1 and the oxidase dual oxidase 2 that represents a major source of NOD2-dependent bactericidal reactive oxygen species generation.Citation70
NOD2 in innate and adaptive immunity
NOD2 drives the innate inflammatory response to bacteria and viruses through the activation of NF-κB, MAPK and caspase-1 pathways, which results in increased expression of proinflammatory factors, including IL-1β, tumor necrosis factor-alpha (TNFα), IL-6, IL-12p40, CC-chemokine ligand 2, the neutrophil chemoattractants CXC-chemokine ligand 8 (also known as IL-8), CXC-chemokine ligand 2 as well as various antimicrobial agents, as defensins, and in recruitment and priming of neutrophils, inflammatory monocytes and dendritic cells.Citation64,Citation71–Citation73
NOD2 also activates other signaling pathways. Upon binding the bacterial ligand, NOD2 induces recruitment of RIP2, which binds TNF receptor-associated factor (TRAF) 3 that activates the TANK binding kinase 1 and IKKε, which phosphorylates and activates regulatory interferon response factor 3 (IRF3). Activated IRF3 passes into the nucleus, where it binds with the molecules of IFN-stimulated response elements inducing the IFN gene expression of type I.Citation74,Citation75
Moreover, NOD2 triggers Notch1 signaling response. Crosstalk between NOD2 and its novel downstream effector Notch1-PI3K in the macrophages contributes to macrophage survival, reducing TNFα/IFNγ-induced apoptosis, and modulates the expression of IL-10 and a battery of genes associated with anti-inflammatory functions.Citation76
In addition to its main role in innate immunity, NOD2 is capable of activating the adaptive immune system; indeed, it is a key driver of T helper (Th) 2-type immunity resulting in IL-4 and IL-5 production.Citation77 Full Th2 induction upon Nod2 activation is dependent on both thymic stromal lymphopoietin production by the stromal cells and the upregulation of the co-stimulatory molecule, OX40 ligand, on the dendritic cells.Citation77 Several studies have shown that co-stimulation with NOD2 and toll-like receptor (TLR) agonists induces a synergistic production of Th1-associated cytokines in different types of cells, although the mechanism is still unclear.Citation78,Citation79
Finally, MDP-induced NOD2 activation has been shown to promote the development of Th17 cells and the consequent production of IL-17A, IL-17F, IL-21 and IL-22.Citation80,Citation81
NOD2 and antiviral response
The innate immune system detects viral infections through the recognition of virus-associated PAMPs, such as genomic DNA, RNA or dsRNA produced in infected cells, which are recognized by the PRRs expressed in innate immune cells.Citation82 After recognition of the viral components, PRRs activate an antiviral response that results in the production of type I IFNs, proinflammatory cytokines, eicosanoids and chemokines and subsequent induction of adaptive immune response.Citation82 Current literature evidences that NOD2 is also able to control virus infections.Citation83,Citation84 Indeed, the antiviral SB 9200, a novel first-in-class oral modulator of innate immunity with a broad-spectrum activity in vitro and in vivo against RNA viruses including hepatitis C virus, norovirus, respiratory syncytial virus and hepatitis B virus, is also believed to act via NOD2 pathway.Citation85 Furthermore, infection with human cytomegalovirus results in significant induction of NOD2 expression, activating downstream NF-κB and IFN pathways.Citation84 Besides, following recognition of a viral ssRNA genome, NOD2 uses the adaptor mitochondrial antiviral signaling protein to activate IRF3; interestingly, NOD2-deficient mice fail to produce IFN efficiently. Similar roles for NOD2 are observed in response to influenza A and parainfluenza viruses.Citation75
NOD2-induced triggering of NF-κB and IRF3 in response to viral infection is also interesting since it has been shown that a coordinated activation of NF-κB and IRF3 pathways synergistically promotes optimal IFN expression, including IFN-β, and hence the antiviral resistance.Citation86 Moreover, a regulatory role of NOD2 in enhancing the function of RNase-L through the binding with 2′-5′-oligoadenylate synthetase type 2, a dsRNA binding protein, has been described as an additional strategy to induce an innate immune response to control bacteria and viruses.Citation87
NOD2 and autophagy
Autophagy represents a cellular stress response that plays key roles in physiological processes, such as innate and adaptive immunity, adaptation to starvation, degradation of misfolded or aggregated proteins or damaged organelles and elimination of intracellular pathogens, in order to promote cellular survival. The role of NOD1 and NOD2 in autophagy is a recent discovery and still under debate.
Travassos et al suggested that, by a mechanism independent of the adaptor RIP2 and transcription factor NF-κB, NOD1 and NOD2 recruited the autophagy protein ATG16L1, an essential component of the autophagic machinery, to the plasma membrane at the bacterial entry site.Citation25 Moreover, in cells homozygous for the CD-associated NOD2 frameshift mutation, mutant NOD2 failed to recruit ATG16L1 to the membrane and wrapping of invading bacteria by autophagosomes was impaired.Citation25 Furthermore, knockdown of ATG16L1 resulted in specific upregulation of NOD2 response, establishing a role of ATG16L1 as a negative regulator of the NOD2/RIP2 pathway.Citation88 NOD2-mediated autophagy is crucial to hold intramucosal bacterial burden and limit intestinal inflammation.Citation89–Citation91
Interestingly, ATG16L1 and NOD2 single nucleotide polymorphisms are both implicated in increased susceptibility to CD.Citation91−Citation94
More recently, other authors have provided evidence of an alternative mechanism of NOD2-dependent autophagy activation which requires RIP2. Cooney et al have shown that NOD2 triggering by MDP induces autophagy in dendritic cells and this effect requires RIP2.Citation95 Anand et al demonstrated that macrophages deficient in the TLR2 and NOD/RIP2 pathway display defective autophagy induction in response to Listeria monocytogenes.Citation96 Homer et al have shown a dual role for RIP2 tyrosine kinase activity in NOD2-dependent autophagy: RIP2 both sends a positive autophagy signal through activation of p38 MAPK and relieves repression of autophagy mediated by the phosphatase PP2A.Citation97
NOD2 and endoplasmic reticulum (ER) stress
The ER stress, activated by accumulation of unfolded or misfolded proteins and microbial infections, triggers a host response known as the unfolded protein response (UPR), which involves the activation of three transmembrane receptors: activating transcription factor 6, protein kinase RNA-like ER kinase and inositol-requiring enzyme 1α (IRE1α). Once activated, IRE1α recruits TRAF2 to the ER membrane to initiate inflammatory responses via c-Jun N-terminal kinase (JNK) pathway and NF-κB.Citation98 NOD2 was found to transduce ER stress signals to elicit inflammation.Citation99,Citation100 Indeed, TRAF2 has been shown to interact with NOD1 and NOD2 to orchestrate this inflammatory branch of UPR, which also requires the adaptor protein RIP2.Citation98 However, the mechanism by which ER stress, and more specifically IRE1α, activates NOD1/NOD2 signaling is still unclear. Recent work provides evidence that the ER stress inducers thapsigargin and dithiothreitol trigger production of the proinflammatory cytokine IL-6 in a NOD1/2-dependent fashion.Citation100,Citation101 In vivo experiments confirmed the in vitro observations by showing that systemic proinflammatory responses induced by thapsigargin administration were blunted in NOD1−/−NOD2−/− mice.Citation100
Several UPR-related genes have been identified as inflammatory bowel disease (IBD) risk loci.Citation102,Citation103 In particular, new evidence has linked the UPR and autophagy in Paneth cells to the development of CD-like transmural ileitis.Citation104 The genetic convergence of genetic polymorphisms on innate immune pathways, such as NOD2, autophagy and ER stress, may open novel therapeutic options for the treatment of intestinal inflammation.Citation105,Citation106 The involvement of ER stress and NOD2 in chronic inflammatory diseases, including IBD and type 2 diabetes, has important implications for understanding the pathogenesis and for the management of these diseases.Citation107,Citation108
NOD2 and IBD
IBD is a group of chronic multifactorial disorders that includes CD, characterized by transmural inflammation that can affect any region of the gastrointestinal tract and ulcerative colitis that results in inflammation and ulcers of the colon and rectum. The etiology of IBD has yet to be fully elucidated; however, it is postulated that it is the result of an unbalanced crosstalk between gut luminal content and the mucosal immune system in genetically susceptible hosts.Citation109
Recent genome-wide association studies have revealed 163 susceptibility loci for IBD.Citation110 NOD2 was the first gene identified as a risk factor for ileal CD.Citation6,Citation7
Three NOD2 polymorphisms in the LRR region are directly associated with CD, of which the most known is the frameshift mutation (L1007fs), whereas the other two are missense mutations (R702W and G908R).Citation6,Citation7 It is postulated that the LRR domain of CD-associated variants is likely to be impaired, possibly to various degrees, in recognizing microbial components and/or in physiologically inhibiting NOD2 dimerization, thus resulting in the inappropriate activation of NF-κB in monocytes, bacterial clearance and impairment of intestinal permeability.Citation6,Citation111,Citation112 Thus, individuals who are heterozygous for NOD2 variants have a 2–4-fold increased risk of developing CD, whereas homozygous variants have an additional risk of 20–40-fold.Citation6,Citation7,Citation113 However, many NOD2 variant carriers do not develop the disease, suggesting that other factors are involved in disease onset.Citation111,Citation112 Similarly, NOD2-knockout mice lack symptoms associated with spontaneous intestinal inflammation.Citation114 Nevertheless, NOD2-deficient mice demonstrate defects in mediating antibacterial responses, such as increased systemic Listeria colonization, suggesting decreased antimicrobial peptides in their intestine.Citation18 Moreover, NOD2-deficient mice show altered gut permeability.Citation49 This evidence is also supported by Amendola et al who attributed the absence of spontaneous colonic inflammation in NOD2-deficient mice to permeability changes: the latter may increase the exposure of dendritic cells to factors, such as TLR ligands, that influence T-reg cell development and subsequent changes in the microbiota.Citation115
Homozygous/compound heterozygous carriage of the CD-associated NOD2 variants has been reported to be significantly associated with ileal disease involvement, stricturing/stenosing disease behavior and 2–3 years earlier age of disease onset compared to NOD2 WT CD patients.Citation116,Citation117 Ileal CD patients with NOD2 L1007fs mutation showed a reduced release of α-defensin from Paneth cells.Citation118,Citation119
Interestingly, the reduction of α-defensin release seems to be a secondary effect of the NOD2 knockdown, causing excessive inflammation and loss of Paneth cell. However, the question remains controversial.Citation120
Notably, CD-associated NOD2 variants are defective in ATG16L1 recruitment and exhibit an altered autophagy in a cell type-specific manner.Citation91 Furthermore, in patients with ileal CD, mutations in autophagy genes other than in NOD2 lead to an impaired secretion of Paneth cell-derived α-defensins with a deficiency in clearance of internalized bacteria.Citation119–Citation122 Therefore, the combination of defective innate immune responses by NOD2 and ineffective bacterial clearance by autophagy could together be responsible for CD development and progression.
NOD2 and extraintestinal diseases
A consistent issue in human genetics is that genes implicated in one disorder could potentially increase susceptibility in other related disorders. It has been shown that three major missense mutations in the NBD domain of NOD2, R334Q, R334W and L469F are involved in an extremely rare, monogenic dominant disorder characterized by granulomatous inflammatory arthritis, uveitis and dermatitis, known as BS.Citation45,Citation123,Citation124 The latter is related to gain-of-function mutations as opposed to CD mutations which appear to be recessive and are characterized with respect to NF-κB activation by a loss of function.Citation44 More rarely, the NOD2 R314Q polymorphism, coding for a truncated form of the protein, has also been found to be associated with BS.Citation8
NOD2 mutations, leading to increased activity of NF-κB, have been associated to EOS, a multisystemic granulomatous disease characterized by arthritis, uveitis and cutaneous involvement.Citation125 NOD2 mutations and similar clinical and histologic characteristics suggest a link between EOS and BS.Citation9,Citation47
Increased NOD2 expression has been found in patients with rheumatoid arthritis, a chronic inflammatory disorder that affects the joints, causing structural deformities.Citation126 Upon stimulation with MDP, peripheral blood mononuclear cells isolated from rheumatoid arthritis patients produced high amounts of TNFα, IL-8 and IL-1β, while NOD2 downregulation significantly decreased proinflammatory cytokines, NF-κB, TRAF6 and IKK levels.Citation127
Clinical and experimental studies have recently shown a role of NOD2 in cardiovascular diseases inducing vascular inflammation and severity of atherosclerosis, the most common pathologic process of coronary artery and cerebrovascular disease.Citation128 NOD2 has been localized in inflamed areas of atherosclerotic lesions and is overexpressed in endothelial cells delimiting the lumen of diseased vessels.Citation129 Moreover, NOD2-mediated IL-6, IL-8 and IL-1β production induces vascular inflammation and promotes expansion of the lipid-rich necrotic areas.Citation130
NOD2 mRNA has also been found to be highly expressed in bronchoalveolar lavage and in peripheral blood mono-nuclear cells from patients with Behcet’s disease, a rare disorder with unknown etiology, characterized by a systemic vasculitis and pulmonary manifestations.Citation131,Citation132 A recent study has shown that NOD2 aggravates myocardial ischemia/reperfusion injury by inducing cardiomyocyte apoptosis and inflammation through JNK, p38MAPK and NF-κB signaling in mice and has suggested NOD2 as a potential target for the treatment of the disease.Citation133 Furthermore, NOD2 has been reported to be involved in IL-6, IL-8 and monocyte chemoattractant protein-1 production induced by the invasion of human aortic endothelial cells by Streptococcus mutans, the primary etiologic agent of dental caries, associated with the development of cardiovascular disease.Citation134
NOD2 variants have been associated with the systemic autoinflammatory disease (NOD2-associated autoinflammatory disease), a genetically complex multisystem disorder characterized by periodic fever, dermatitis, arthritis and gastrointestinal and sicca-like symptoms, but with a phenotypic and genotypic profile distinct from CD.Citation135
NOD2 polymorphisms have been also related to atopic dermatitis, a chronically relapsing inflammatory skin disease associated with basophil infiltration and an exacerbation of inflammation by Sta. aureus.Citation136−Citation138
Recent literature describes a central role of NOD2 in susceptibility to obesity and metabolic dysfunction.Citation139 NOD2-deficient mice show increased bacterial adherence to the intestinal mucosa and bacterial infiltration in metabolic tissues, such as hepatic and adipose tissue, exacerbating inflammation and insulin resistance.Citation140 Moreover, NOD2−/− BALB/c mice have shown susceptibility to obesity, hyperlipidemia, hyperglycemia, glucose intolerance, increased adiposity and hepatic steatosis, as compared to WT mice.Citation141
Finally, NOD2-increased expression has been observed in patients with active Vogt–Koyanagi–Harada disease, a rare granulomatous inflammatory disease that affects pigmented structures, promoting proinflammatory cytokine production and Th1 and Th17 cells stimulation.Citation142
NOD2 and cancer
NOD2 has been associated to cancer development. Data from animal models indicate that NOD2 deficiency leads to dysbiosis resulting in increased risk of colitis and colitis associated colorectal cancer.Citation143 A recent study in NOD2- or RIP2-deficient mice shows an increased epithelial dysplasia following dextran sulfate sodium-induced colitis, suggesting a NOD2-protective role in cancer development via regulation of gut bacterial equilibrium.Citation55
Emerging evidence in human studies implies that several NOD2 polymorphisms may influence individual susceptibility to cancer.Citation143,Citation144 However, the results are still inconclusive due to confounding genetic, bacterial and environmental factors that may alter variant allele penetrance or the differences in sample size, geographic variation and genotyping methods. In general, NOD2 gene polymorphisms are associated with altered risk of gastric, colorectal, breast, ovarian, prostate, testicular, lung, laryngeal, liver, gallbladder, biliary tract, pancreatic, small bowel, kidney, urinary bladder cancer, skin cancer, non-thyroid endocrine tumors, lymphoma and leukemia.Citation145 In particular, the three most common NOD2 polymorphisms, rs2066844 C/T (R702W), rs2066845 C/G (G908R) and rs2066847 (3020insC), have been associated with increased risk of gastrointestinal cancer.Citation146 Furthermore, NOD2 has also been involved in the development of gastric cancer induced by Helicobacter pylori.Citation144
Conclusion
The involvement of NOD2 in the pathogenesis of several genetic diseases indicates that this protein is a key regulator of immune and inflammatory responses. Extensive studies have evidenced a fundamental role of NOD2 in maintaining the equilibrium between bacteria, epithelia and innate immune response of the host. This protective function is lost in case of NOD2 mutations, resulting in exacerbated inflammation and the onset of various diseases. On the other hand, inappropriate activation of WT NOD2 is reported in many chronic inflammatory diseases, resulting in continuous production of proinflammatory mediators, but the exact mechanism underlying this NOD2 dysregulation is not yet well established. Recent literature has shifted the interest from genetic to epigenetic control and to interactions with other innate immune pathways such as autophagy and ER stress. Many questions remain unanswered, including the relation between NOD2 mutations and microbiota and the understanding of the processes by which mutations in the NOD2 could be associated with the susceptibility to inflammation and development of diseases. When the exact mechanism of regulation and functions of NOD2 will be unraveled, it could lead to the development of more effective therapies for inflammatory disorders.
Disclosure
The authors report no conflicts of interest in this work.
References
- TakeuchiOAkiraSPattern recognition receptors and inflammationCell201014068052020303872
- ProellMRiedlSJFritzJHRojasAMSchwarzenbacherRThe Nod-like receptor (NLR) family: a tale of similarities and differencesPLoS One200834e211918446235
- KawaiTAkiraSThe roles of TLRs, RLRs and NLRs in pathogen recognitionInt Immunol200921431733719246554
- FeerickCLMcKernanDPUnderstanding the regulation of pattern recognition receptors in inflammatory diseases – a ‘Nod’ in the right directionImmunology2017150323724727706808
- PhilpottDJSorbaraMTRobertsonSJCroitoruKGirardinSENOD proteins: regulators of inflammation in health and diseaseNat Rev Immunol201414192324336102
- HugotJPChamaillardMZoualiHAssociation of NOD2 leucine rich repeat variants with susceptibility to Crohn’s diseaseNature2001411683759960311385576
- OguraYBonenDKInoharaNA frameshift mutation in NOD2 associated with susceptibility to Crohn’s diseaseNature2001411683760360611385577
- DuganJGriffithsESnowPBlau syndrome-associated Nod2 mutation alters expression of full length NOD2 and limits responses to muramyl dipeptide in knock-in miceJ Immunol2015194134935725429073
- KanazawaNOkafujiIKambeNEarly-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndromeBlood200510531195119715459013
- TattoliICarneiroLAJéhannoMNLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species productionEMBO Rep20089329330018219313
- MooreCBBergstralhDTDuncanJANLRX1 is a regulator of mitochondrial antiviral immunityNature2008451717857357718200010
- BoyleJPParkhouseRMonieTPInsights into the molecular basis of the NOD2 signalling pathwayOpen Biol201441214017825520185
- SidiqTYoshihamaSDownsIKobayashiKSNod2: a critical regulator of ileal microbiota and Crohn’s diseaseFront Immunol20167367 eCollection 201627703457
- NigroGRossiRCommerePHJayPSansonettiPJThe cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regenerationCell Host Microbe201415679279824882705
- PhilpottDJGirardinSENod-like receptors: sentinels at host membranesCurr Opin Immunol201022442843420605429
- HofmannKBucherPTschoppJThe CARD domain: a new apoptotic signaling motifTrends Biochem Sci19972251551669175472
- GirardinSEBonecaIGVialaJNod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detectionJ Biol Chem2003278118869887212527755
- KobayashiKSChamaillardMOguraYNod2-dependent regulation of innate and adaptive immunity in the intestinal tractScience2005307571073173415692051
- ParkJHKimYGMcDonaldCRICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRsJ Immunol200717842380238617277144
- RahighiSIkedaFKawasakiMSpecific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activationCell200913661098110919303852
- JiangXChenZJThe role of ubiquitylation in immune defence and pathogen evasionNat Rev Immunol2011121354822158412
- KarinMThe Regulation of AP-1 Activity by Mitogen-activated Protein KinasesJ Biol Chem19952702816483164867622446
- HsuLCAliSRMcGillivraySA NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptideProc Natl Acad Sci U S A2008105227803780818511561
- MeinzerUBarreauFEsmiol-WelterlinSYersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunctionCell Host Microbe201211433735122520462
- TravassosLHCarneiroLARamjeetMNod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entryNat. Immunol2010111556219898471
- WarnerNBurberryAFranchiLA genome-wide siRNA screen reveals positive and negative regulators of the NOD2 and NF-κB signaling pathwaysSci Signal20136258rs323322906
- RichmondALKabiAHomerCRThe nucleotide synthesis enzyme CAD inhibits NOD2 antibacterial function in human intestinal epithelial cellsGastroenterology201214271483149222387394
- LecatADi ValentinESomjaJThe c-Jun N-terminal kinase (JNK)-binding protein (JNKBP1) acts as a negative regulator of NOD2 protein signaling by inhibiting its oligomerization processJ Biol Chem201228735292132922622700971
- LipinskiSGrabeNJacobsGRNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognitionProc Natl Acad Sci USA201210952214262143123213202
- LeeKHBiswasALiuYJKobayashiKSProteasomal degradation of Nod2 protein mediates tolerance to bacterial cell wall componentsJ Biol Chem201228747398003981123019338
- WatanabeTAsanoNMengGNOD2 downregulates colonic inflammation by IRF4-mediated inhibition of K63-linked polyubiquitination of RICK and TRAF6Mucosal Immunol2014761312132524670424
- MohananVGrimesCLThe molecular chaperone HSP70 binds to and stabilizes NOD2, an important protein involved in Crohn diseaseJ Biol Chem201428927189871899824790089
- Tigno-AranjuezJTAbbottDWUbiquitination and phosphorylation in the regulation of NOD2 signaling and NOD2-mediated diseaseBiochim Biophys Acta20121823112022202822522061
- YangSWangBHumphriesFPellino3 ubiquitinates RIP2 and mediates Nod2-induced signaling and protective effects in colitisNat Immunol201314992793623892723
- DamgaardRBNachburUYabalMThe ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunityMol Cell201246674675822607974
- ChirieleisonSMMarshRAKumarPRathkeyJKDubyakGRAbbottDWNucleotide-binding oligomerization domain (NOD) signaling defects and cell death susceptibility cannot be uncoupled in X-linked inhibitor of apoptosis (XIAP)-driven inflammatory diseaseJ Biol Chem2017292239666967928404814
- ZurekBSchoultzINeerincxATRIM27 negatively regulates NOD2 by ubiquitination and proteasomal degradationPLoS One201277e4125522829933
- BistPCheongWSNgAE3 Ubiquitin ligase ZNRF4 negatively regulates NOD2 signalling and induces tolerance to MDPNat Commun201781586528656966
- FiilBKDamgaardRBWagnerSAOTULIN restricts Met1-linked ubiquitination to control innate immune signalingMol Cell201350681883023806334
- YanRLiuZLRRK2 enhances Nod1/2-mediated inflammatory cytokine production by promoting Rip2 phosphorylationProtein Cell201781556627830463
- StevensCHendersonPNimmoERThe intermediate filament protein, vimentin, is a regulator of NOD2 activityGut201362569570722684479
- PierdomenicoMCesiVCucchiaraSNOD2 is regulated by Mir-320 in physiological conditions but this control is altered in inflamed tissues of patients with inflammatory bowel diseaseInflamm Bowel Dis201622231532626752466
- ChuangAYChuangJCZhaiZWuFKwonJHNOD2 expression is regulated by microRNAs in colonic epithelial HCT116 cellsInflamm Bowel Dis20142011263524297055
- StroberWAsanoNFussIKitaniAWatanabeTCellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s diseaseImmunol Rev2014260124926024942694
- Miceli-RichardCLesageSRybojadMCARD15 mutations in Blau syndromeNat Genet2001291192011528384
- YaoQNucleotide-binding oligomerization domain containing 2: structure, function, and diseasesSemin Arthritis Rheum201343112513023352252
- CasoFGalozziPCostaLSfrisoPCantariniLPunziLAutoinflammatory granulomatous diseases: from Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s diseaseRMD Open201511e00009726509073
- BlanderJMLongmanRSIlievIDSonnenbergGFArtisDRegulation of inflammation by microbiota interactions with the hostNat Immunol201718885186028722709
- BalasubramanianIGaoNFrom sensing to shaping microbiota: insights into the role of NOD2 in intestinal homeostasis and progression of Crohn’s diseaseAm J Physiol Gastrointest Liver Physiol20173131G7G1328450278
- RehmanASinaCGavrilovaONod2 is essential for temporal development of intestinal microbial communitiesGut201160101354136221421666
- Al NabhaniZDietrichGHugotJPBarreauFNod2: the intestinal gate keeperPLoS Pathog2017133e100617728253332
- BiswasAPetnicki-OcwiejaTKobayashiKSNod2: a key regulator linking microbiota to intestinal mucosal immunityJ Mol Med (Berl)2012901152421861185
- RamananDTangMSBowcuttRLokePCadwellKBacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatusImmunity201441231132425088769
- JiangWWangXZengBRecognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytesJ Exp Med2013210112465247624062413
- Couturier-MaillardASecherTRehmanANOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancerJ Clin Invest2013123270071123281400
- OguraYLalaSXinWExpression of NOD2 in Paneth cells: a possible link to Crohn’s ileitisGut200352111591159714570728
- Petnicki-OcwiejaTHrncirTLiuYJNod2 is required for the regulation of commensal microbiota in the intestineProc Natl Acad Sci USA200910637158131581819805227
- RobertsonSJZhouJYGeddesKNod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasisGut Microbes20134322223123549220
- RobertsonSJGeddesKMaisonneuveCStreutkerCJPhilpottDJResilience of the intestinal microbiota following pathogenic bacterial infection is independent of innate immunity mediated by NOD1 or NOD2Microbes Infect2016187–846047127083475
- ShanahanMTCarrollIMGrossniklausEMouse Paneth cell antimicrobial function is independent of Nod2Gut201463690391023512834
- BereswillSGrundmannUAlutisMEFischerAHeimesaatMMCampylobacter jejuni infection of conventionally colonized mice lacking nucleotide-oligomerization-domain-2Gut Pathog20179528127403
- CarusoRWarnerNInoharaNNúñezGNOD1 and NOD2: signaling, host defense, and inflammatory diseaseImmunity201441689890825526305
- HruzPZinkernagelbASJenikovaaGNOD2 contributes to cutaneous defense against Staphylococcus aureus through α-toxin-dependent innate immune activationProc Natl Acad Sci USA200910631128731287819541630
- KimYGKamadaNShawMHThe NOD2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytesImmunity201134576978021565531
- GeddesKRubinoSStreutkerCNOD1 and NOD2 regulation of inflammation in the salmonella colitis modelInfect. Immun201078125107511520921147
- GeddesKRubinoSJMagalhaesJGIdentification of an innate T helper type 17 response to intestinal bacterial pathogensNat Med201117783784421666695
- LecatAPietteJLegrand-PoelsSThe protein Nod2: an innate receptor more complex than previously assumedBiochem Pharmacol201080122021203120643110
- SubramanianKBergmanPHenriques-NormarkBVitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophilsJ Innate Immun20179437538628241127
- LiuJXiangJLiXNF-κB activation is critical for bacterial lipoprotein tolerance-enhanced bactericidal activity in macrophages during microbial infectionSci Rep201774041828079153
- LipinskiSTillASinaCDUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responsesCell Sci2009122Pt 1935223530
- BeynonVCotofanaSBrandSNOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytesInflamm Bowel Dis20081481033104018383179
- FritzJHGirardinSEFittingCSynergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonistsEur J Immunol20053582459247016021602
- CoulombeFFiolaSAkiraSCormierYGosselinJMuramyl dipeptide induces NOD2-dependent Ly6Chigh monocyte recruitment to the lungs and protects against influenza virus infectionPLoS One201275e3673422590599
- KapoorAFanYHArav-BogerRBacterial muramyl dipeptide (MDP) restricts human cytomegalovirus replication via an IFN-β-dependent pathwaySci Rep201662029526830977
- SabbahAChangTHHarnackRActivation of innate immune antiviral responses by Nod2Nat. Immunol200910101073108019701189
- BansalKBalajiKNIntracellular pathogen sensor NOD2 programs macrophages to trigger Notch1 activationJ Biol Chem201128675823583521156799
- MagalhaesJGRubinoSJTravassosLHNucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activationProc Natl Acad Sci U S A201110836148961490121856952
- TadaHAibaSShibataKOhtekiTTakadaHSynergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cellsInfect Immun200573127967797616299289
- JeongYJKangMJLeeSJNod2 and Rip2 contribute to innate immune responses in mouse neutrophilsImmunology2014143226927624766550
- BrainOOwensBMPichulikTThe intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 releaseImmunity201339352153624054330
- Van BeelenAJZelinkovaZTaanman-KueterEWStimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T CellsImmunity200727466066917919942
- KawaiTAkiraSInnate immune recognition of viral infectionNat Immunol20067213113716424890
- Keestra-GounderAMTsolisRMNOD1 and NOD2: beyond peptidoglycan sensingTrends Immunol2017381075876728823510
- KapoorAFormanMArav-BogerRActivation of nucleotide oligomerization domain 2 (NOD2) by human cytomegalovirus initiates innate immune responses and restricts virus replicationPLoS One201493e9270424671169
- JonesMCunninghamMEWingPSB 9200, a novel agonist of innate immunity, shows potent antiviral activity against resistant HCV variantsJ Med Virol20178991620162828303593
- WatheletMGLinCHParekhBSRoncoLVHowleyPMManiatisTVirus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivoMol Cell1998145075189660935
- DuganJWAlborADavidLNucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cellsMol Immunol2009472–356056619853919
- SorbaraMTEllisonLKRamjeetMThe protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent mannerImmunity201339585887324238340
- NegroniAColantoniEVitaliRNOD2 induces autophagy to control AIEC bacteria infectiveness in intestinal epithelial cellsInflamm Res2016651080381327335178
- MűzesGTulassayZSiposFInterplay of autophagy and innate immunity in Crohn’s disease: a key immunobiologic featureWorld J Gastroenterol201319284447445423901219
- HomerCRRichmondALRebertNAAchkarJPMcDonaldCATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesisGastroenterology201013951630164120637199
- CadwellKCrohn’s disease susceptibility gene interactions, a NOD to the newcomer ATG16L1Gastroenterology201013951448145020875485
- SalemMAmmitzboellMNysKSeidelinJBNielsenOHATG16L1: a multifunctional susceptibility factor in Crohn diseaseAutophagy201511458559425906181
- NguyenHTLapaquettePBringerMADarfeuille-MichaudAAutophagy and Crohn’s diseaseJ Innate Immun20135543444323328432
- CooneyRBakerJBrainONOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentationNat Med2010161909719966812
- AnandPKTaitSWLamkanfiMTLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activationJ Biol Chem201128650429814299122033934
- HomerCRKabiAMarina-GarcíaNA dual role for receptor-interacting protein kinase 2 (RIP2) kinase activity in nucleotide-binding oligomerization domain 2 (NOD2)-dependent autophagyJ Biol Chem201228730255652557622665475
- UranoFWangXBertolottiACoupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1Science2000287545366466610650002
- ByndlossMXKeestra-GounderAMBäumlerAJTsolisRMNOD1 and NOD2: new functions linking endoplasmic reticulum stress and inflammationDNA Cell Biol201635731131327341284
- Keestra-GounderAMByndlossMXSeyffertNNOD1 and NOD2 signalling links ER stress with inflammationNature2016532759939439727007849
- CarusoRNúñezGInnate immunity: ER stress recruits NOD1 and NOD2 for delivery of inflammationCurr Biol20162612R508R51127326714
- KaserABlumbergRSEndoplasmic reticulum stress and intestinal inflammationMucosal Immunol201031111619865077
- HosomiSKaserABlumbergRSRole of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel diseaseCurr Opin Gastroenterol2015311818825426970
- AdolphTETomczakMFNiederreiterLPaneth cells as a site of origin for intestinal inflammationNature2013503747527227624089213
- FritzTNiederreiterLAdolphTBlumbergRSKaserACrohn’s disease: NOD2, autophagy and ER stress convergeGut201160111580158821252204
- HoefkensENysKJohnJMGenetic association and functional role of Crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stressAutophagy20139122046205524247223
- KaserALeeAHFrankeAXBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel diseaseCell2008134574375618775308
- MontaneJCadavezLNovialsAStress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetesDiabetes Metab Syndr Obes20147253424520198
- KimDHCheonJHPathogenesis of inflammatory bowel disease and recent advances in biologic therapiesImmune Netw2017171254028261018
- JostinsLRipkeSWeersmaRKHost-microbe interactions have shaped the genetic architecture of inflammatory bowel diseaseNature201249174221192423128233
- KimYGShawMHWarnerNCutting edge: Crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis induced lethalityJ Immunol201118762849285221849681
- SalemMSeidelinJBEickhardtSAlhedeMRoglerGNielsenOHSpecies-specific engagement of human nucleotide oligomerization domain 2 (NOD)2 and Toll-like receptor (TLR) signalling upon intracellular bacterial infection: role of Crohn’s associated NOD2 gene variantsClin Exp Immunol2015179342643425335775
- HugotJPZaccariaICavanaughJIBD International Genetics ConsortiumPrevalence of CARD15/NOD2 mutations in Caucasian healthy peopleAm J Gastroenterol200710261259126717319929
- PauleauALMurrayPJRole of nod2 in the response of macrophages to toll-like receptor agonistsMol Cell Biol200323217531753914560001
- AmendolaAButeraASanchezMStroberWBoirivantMNod2 deficiency is associated with an increased mucosal immune regulatory response to commensal microorganismsMucosal Immunol20147239140423962873
- LesageSZoualiHCézardJPCARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel diseaseAm J Hum Genet200270484585711875755
- HampeJGrebeJNikolausSAssociation of NOD2 (CARD 15) genotype with clinical course of Crohn’s disease: a cohort studyLancet200235993181661166512020527
- WehkampJHarderJWeichenthalMNOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal a-defensin expressionGut200453111658166415479689
- WehkampJSalzmanNHPorterEReduced Paneth cell a-defensins in ileal Crohn’s diseaseProc Natl Acad Sci U S A200510250181291813416330776
- SimmsLADoeckeJDWalshMDHuangNFowlerEVRadford-SmithGLReduced a-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s diseaseGut200857790311018305068
- CadwellKLiuJYBrownSLA key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cellsNature2008456721925926318849966
- WolfkampSCVerseydenCVogelsEWATG16L1 and NOD2 polymorphisms enhance phagocytosis in monocytes of Crohn’s disease patientsWorld J Gastroenterol201420102664267224627602
- TrompGKuivaniemiHRaphaelSGenetic linkage of familial granulomatous inflammatory arthritis, skin rash, and uveitis to chromosome 16Am J Hum Genet1996595109711078900239
- BlauEBFamilial granulomatous arthritis, iritis, and rashJ Pediatr198510756896934056967
- WoutersCHMaesAFoleyKPBertinJRoseCDBlau syndrome, the prototypic auto-inflammatory granulomatous diseasePediatr Rheumatol Online J2014123325136265
- HaslerPGabay C. Rheumatoid arthritis: from basic findings and clinical manifestations to future therapiesSemin Immunopathol201739433934128639062
- KimHWKwonYJParkBWSongJJParkYBParkMCDifferential expressions of NOD-like receptors and their associations with inflammatory responses in rheumatoid arthritisClin Exp Rheumatol201735463063728240593
- YuanHZelkhaSBurkatovskayaMGupteRLeemanSEAmarSPivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone lossProc Natl Acad Sci U S A201311052E5059E506824324141
- LiuHQZhangXYEdfeldtKNOD2-mediated innate immune signaling regulates the eicosanoids in atherosclerosisArterioscler Thromb Vasc Biol20133392193220123868940
- JohanssonMEZhangXYEdfeldtKInnate immune receptor NOD2 promotes vascular inflammation and formation of lipid-rich necrotic cores in hypercholesterolemic miceEur J Immunol201444103081309225042478
- ZeidanMJSaadounDGarridoMKlatzmannDSixACacoubPBehçet’s disease physiopathology: a contemporary reviewAuto Immun Highlights201671426868128
- HamzaouiKAbidHBerraiesAAmmarJHamzaouiANOD2 is highly expressed in Behcet disease with pulmonary manifestationsJ Inflamm (Lond)201291322330585
- LiuYYangHLiuLXNOD2 contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and inflammationLife Sci2016149101726874029
- NagataEOhoTInvasive Streptococcus mutans induces inflammatory cytokine production in human aortic endothelial cells via regulation of intracellular toll-like receptor 2 and nucleotide-binding oligomerization domain 2Mol Oral Microbiol201732213114127004566
- YaoQSuLCTomeckiKJZhouLJayakarBShenBDermatitis as a characteristic phenotype of a new autoinflammatory disease associated with NOD2 mutationsJ Am Acad Dermatol201368462463123102769
- MacalusoFNothnagelMParwezQPolymorphisms in NACHT-LRR (NLR) genes in atopic dermatitisExp Dermatol200716869269817620097
- WongCKChuIMHonKLTsangMSLamCWAberrant expression of bacterial pattern recognition receptor NOD2 of basophils and microbicidal peptides in atopic dermatitisMolecules201621447127077833
- JiaoDWongCKQiuHNNOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammationCell Mol Immunol201613453555026388234
- Rodriguez-NunezICaluagTKirbyKRudickCNDziarskiRGuptaDNod2 and Nod2-regulated microbiota protect BALB/c mice from diet-induced obesity and metabolic dysfunctionSci Rep20177154828373658
- DenouELolmèdeKGaridouLDefective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistanceEMBO Mol Med20157325927425666722
- PrajapatiBJenaPKRajputPPurandharKSeshadriSUnderstanding and modulating the toll like receptors (TLRs) and NOD like receptors (NLRs) cross talk in type 2 diabetesCurr Diabetes Rev201410319020024828062
- DengBYeZLiLHigher expression of NOD1 and NOD2 is associated with Vogt-Koyanagi-Harada (VKH) Syndrome but not Behcet’s disease (BD)Curr Mol Med201616442443526980698
- BranquinhoDFreirePSofiaCNOD2 mutations and colorectal cancer – Where do we stand?World J Gastrointest Surg20168428429327152134
- Castaño-RodríguezNKaakoushNOMitchellHMPattern-recognition receptors and gastric cancerFront Immunol2014533625101079
- KutikhinAGRole of NOD1/CARD4 and NOD2/CARD15 gene polymorphisms in cancer etiologyHum Immunol2011721095596821745515
- LiuJHeCXuQXingCYuanYNOD2 polymorphisms associated with cancer risk: a meta-analysisPLoS One201492e8934024586700
