Abstract
Background and aims
Immunoregulatory cytokines influence the persistence of hepatitis C virus (HCV) chronic infection and the extent of liver damage. Interleukin-1 (IL-1) plays an important role in the inflammatory process. Some studies have demonstrated that IL-1α production was impaired in patients with chronic infections of HCV, implying that IL-1α may play a role in viral clearance. The aim of this study was to evaluate the serum level of proinflammatory cytokine IL-1α in patients with chronic hepatitis C (CHC).
Methods
This study was performed on 20 CHC patients with cirrhosis in (Group I), 20 CHC patients without cirrhosis in (Group II), 20 hepatocellular carcinoma (HCC) patients with positive anti-HCV in (Group III), and 10 healthy subjects as a control group. Serum levels of IL-1α were measured by enzyme-linked immunoassay technique.
Results
IL-1α had the highest mean concentration in the HCC group and then in the group of CHC with cirrhosis compared to the group of CHC without cirrhosis. Also, it was higher in all studied groups than in the control group (P<0.001). Statistical analysis showed that IL-1α was positively correlated with bilirubin (P≤0.001), alanine aminotransferase (P=0.006), aspartate aminotransferase (P=0.001), and viral load (P=0.001) but it was negatively correlated with albumin (P≤0.001) and Hb (P≤0.001), and was not significantly correlated with other parameters (age, international normalized ratio, urea, creatinine, white blood cells, and platelet count).
Conclusion
Serum level of IL-1α was elevated in patients with CHC and its related liver diseases (liver cirrhosis and HCC) and can be used as an important parameter of inflammatory activity and for fibrosis evaluation in patients with chronic liver disease.
Introduction
Hepatitis C virus (HCV) infection is one of the most prevalent chronic infectious diseases throughout the world. Approximately 170 million people are chronically infected. Chronic HCV infection is one of the major causes of liver cirrhosis, and hepatocellular carcinoma (HCC). The rate of spontaneous recovery from acute HCV is about 15%–50%, occurring within 3 months. This recovery is characterized by symptom improvement, normalized liver function tests, disappearance of HCV-RNA from serum, and appearance of HCV antibody.Citation1–Citation3
In Egypt, HCV is considered the major cause of chronic hepatitis, liver cirrhosis, and HCC. The prevalence of HCV is estimated to be 14.7%. HCV genotype 4 is the most prevalent genotype accounting about 90% among Egyptian patients with chronic HCV infection.Citation4–Citation9
Liver fibrosis occurs in most types of chronic liver diseases. It results from both liver cell damage and deposition of extracellular matrix protein.Citation10 Liver cirrhosis is a chronic progressive disease affecting the entire organ. It consists of nodular regeneration of liver parenchyma, deposition of fibrous septa, and, in addition, loss of hepatic architecture.Citation11 Cirrhosis can be considered as an end-stage disease that may lead to death unless liver transplantation is done. The only preventive measures in cirrhotic patients are screening for esophageal varices and HCC.Citation12
HCC is the fifth most common cancer throughout the world. It causes approximately 500,000 deaths annually.Citation13 HCC is the second most common cancer in Egyptian men and the sixth most common one in Egyptian women.Citation14,Citation15 The incidence of HCC increases in proportion to the age, reaching its peak in patients over the age of 75.Citation13
The immune response is a key component in the activation and maintenance of antiviral immunity. Defects in the host’s immune response can lead to viral persistence.Citation16 Patients with viral replication have weak HCV antigen-specific T-cell responses. However, strong responses may associate with a more benign course of disease.Citation17 Immune response also includes induction of cytokines and initiation of the adaptive immune response. Early, specific, and sustained CD4+ and CD8+ responses directed against different HCV epitopes have been found to be associated with clearance of chronic HCV.Citation18
Cytokines are low-molecular weight mediators of cellular communication. They are secreted by multiple cell types in the liver, particularly Kupffer cells. Proinflammatory cytokines such as interleukin-1 (IL-1), TNF, and IL-8 are known to be acute-phase cytokines. They play a role in the liver injury of acute and chronic liver diseases.Citation19 Th1 cytokines correlate positively with liver inflammation in HCV infection.Citation20 Functional impairment, suppression, or deletion of antigen-specific T-cells appears to be the most important determinant factor behind the progression to chronic liver disease.Citation21
IL-1 is one of the most prominent proinflammatory cytokines.Citation22 It has a key role in viral clearance and the host immune response. IL-1 has 2 isoforms, IL-1α and IL-1β. Both have similar biological functions. IL-1 can exert numerous biological effects on multiple cell types.Citation23 Changes in IL-1 expression in HCV-infected patients have been well documented. Some studies suggested that patients with HCV had increased levels of serum IL-1.Citation24 The biological roles of IL-1 in HCV infection cannot be denied. It can help viral clearance by regulating immune response, particularly antigen presentation. In addition, it can enhance interferon-stimulated target gene expression, which has a role in antiviral activity.Citation25
As regard to IL1α and IL1β, inactive IL1β precursor is cleaved by caspase-1 into an active cytokine. However, IL1α precursor is cleaved into a mature form and N-terminal propiece by calpain. IL1α can also be expressed on the cell membrane, possibly via a mannose-like receptor.Citation26,Citation27 Studies have suggested that membrane IL1α is immunostimulatory and can promote antitumor immune responses including HCC through activation of T- and natural killer cells.Citation28
Some studies have shown that IL-1β production might be impaired in chronic hepatitis C (CHC) from both peripheral blood and sinusoidal cells due to viral infection of monocytes/macrophages.Citation23 However, CHC is found to be accompanied by augmented expression of other cytokines such as TNF-α, IL-1α, and IL-2 in groups of children and of adult patients. IL-1 could here possibly find its application, which is the subject of further research.Citation29 This study aimed to evaluate the serum level of proinflammatory cytokine IL-1α in patients with CHC.
Methods
This study was performed on 60 CHC patients and 10 healthy subjects as a control group at the Department of Tropical Medicine, Tanta University Hospital, during the period from October 2015 to October 2016.
The subjects in this study were classified into 4 groups: Group I included 20 patients with CHC with cirrhosis; Group II included 20 patients with CHC without cirrhosis; Group III included 20 patients with CHC with HCC; Group IV: included 10 healthy persons as control group.
Written informed consent was obtained from all participants in the research. All participants’ names were hidden and were replaced by code numbers to maintain privacy. Institutional ethical committee approval was obtained before the start of the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Tanta University Faculty of Medicine Research Ethical Committee.
Patients with CHC who were consistently positive for anti-HCV antibodies (Matrix-ELISA; third-generation assay) with detectable HCV-RNA by real-time PCR for at least 6 months were included in the study. HCV-RNA quantitation was done by real-time PCR using Cobas Ampli Prep/Cobas TaqMan HCV-RNA assay (Roche Diagnostics; Pleasanton, CA, USA) with a threshold of detection of 15 IU/mL. Patients with liver disease caused by NAFLD/NASH, patients with cardiac cirrhosis or primary biliary cirrhosis or hereditary conditions of the liver, patients with acute hepatitis, patients with evidence of acute or chronic inflammatory syndrome of other origin, or patients with immunodeficiency states were excluded from the study.
All patients included in the study were subjected to full history taking, complete clinical examination, routine laboratory investigations including total and direct serum bilirubin, serum ALT and AST, serum albumin, prothrombin time, INR, complete blood picture, serum urea and creatinine level, HCV-Ab by enzyme-linked immunosorbent assay (ELISA), and specific laboratory investigation as measurement of serum levels of cytokine IL-1α ELISA. These tests were performed in the clinical pathology laboratory in Tanta University Hospital.
Six mL venous blood samples were collected under aseptic conditions and subdivided in to 3 tubes. Two mL in EDTA tube for complete blood count, 2 mL in citrate tube for prothrombin time, and 2 mL in a plain tube for liver functions and ELISA technique. The samples were centrifuged to separate serum and were kept in sterile containers in the fridge at −20°C till the assay was done.
Measurement of IL-1α was done by quantitative sandwich enzyme immunoassay technique. The Assay Max Human Interleukin-1α ELISA kit (Abcam, Cambridge, UK) was designed for detection of human IL-1α in plasma, serum, and cell culture samples. This assay employs a quantitative sandwich enzyme immunoassay technique which measures IL-1α in >5 hours.
Radiological investigation was performed for all patients enrolled in the study. Abdominal ultrasonography was performed on all patients to detect the liver cirrhosis and had a tumor detection limit of 1 cm. Small HCC (3 cm) appeared hypoechogenic in most cases relative to the surrounding nontumorous liver.Citation30
Triphasic spiral computed tomography, abdominal and pelvic, was performed for patients with HCC. During the arterial phase, tumors appear enhanced against the relatively unenhanced liver parenchyma. HCC is characterized by arterial hypervascularization followed by a rapid wash out, appearing hypovascular in the portal phase.Citation31
Statistical analysis
All patient data were tabulated and processed using SPSS 19.0 (V.19 SPSS Inc, Chicago, IL, USA). Quantitative data are presented by mean (X) ± SD. Tests of normality were done for all groups to detect that data was normally distributed. Statistical methods used in this study were analysis of variance and Tukey’s test. Fisher’s exact test was performed for qualitative data. Pearson correlation test was done between serum IL-1α and other parameters. In all tests, the level of significance was P<0.05.
Results
Group I included 20 patients with chronic HCV with liver cirrhosis; their age ranged from 38 to 65 years. Twenty patients with chronic HCV without cirrhosis were enrolled in Group II, and they were aged between 31 and 60 years. 20 patients with HCC were enrolled in Group III, aged from 31 to 65. 10 normal subjects were included in the study as control group, aged 31–60 years. There was no significant difference as regard to the age and sex between the different groups ( for complete demographic and laboratory data). There was no significant deference between the 3 studied groups regarding the viral load level ().
Table 1 Demographic and laboratory data of the studied groups
Table 2 Mean viral load among 4 studied groups
Regarding other laboratory findings, hemoglobin concentration and platelet count were significantly higher in the control group than in patients in other studied groups, and in noncirrhotic patients than cirrhotic ones. They were significantly low in HCC group. However, the difference detected between studied groups regarding white blood cell count was not significant; in spite of the fact that IL-1α affects neutrophils. This can be explained due to the total leucocytic count only being measured, which might be affected in chronic hepatitis and development of cirrhosis or HCC. All groups showed significant statistical difference as regard to liver function tests. Serum bilirubin and INR were higher in Group I (). However, liver enzymes were higher in Group III. Serum albumin was also lower in Group III.
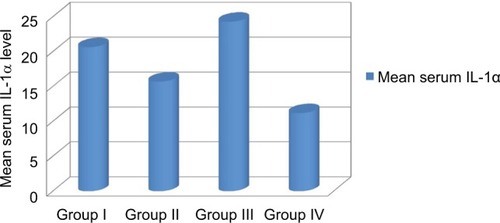
Serum IL-1α level was significantly higher in group HCC than in group chronic HCV with cirrhosis. Also, it was higher in group chronic HCV with cirrhosis compared to chronic HCV without cirrhosis group. Also, it was higher in all groups compared to the control group (, ).
Table 3 Serum IL-1α levels in the studied groups
Figure 1 Comparison of serum IL-1α levels in the studied groups.
Note: Group I included 20 patients with CHC with cirrhosis; Group II included 20 patients with CHC without cirrhosis; Group III included 20 patients with CHC with HCC; Group IV: included 10 healthy persons as control group.
Abbreviation: IL-1α, interleukin-1α.
There was positive significant correlation between serum IL-1αand bilirubin (P≤0.001), ALT (P=0.006), AST (P=0.001), and viral load (P=0.001), but there was negative significant correlation between this and albumin (P<0.001*), Hb (P≤0.001), and white blood cells. Also, there was no significant correlation with other parameters (age, INR, urea, creatinine, platelet) as shown in .
Table 4 Correlation between serum IL-1α with different parameters in patients in the various groups
Discussion
Cytokines, as the products of host response to inflammation, play an important role in the defense against viral infections. However, in HCV infection they may play a prominent role in liver damage.Citation32
Proinflammatory cytokines such as IL-1, TNF, and IL-8 play a role in liver injury during acute and chronic liver disease.Citation22
In the present study, we measured serum levels of IL-1α. Levels were high among all groups compared to the control group. It was higher in cirrhotic cases compared to noncirrhotic ones and higher in HCC patients when compared to cases with or without cirrhosis.
This is in agreement with Lecube et al’sCitation33 study which showed that all the proinflammatory cytokines were significantly higher in anti-HCV+ cirrhotic patients than in anti-HCV – cirrhotic cases.Citation33
In Wang et al’sCitation34 study, the proinflammatory cytokines, the IL-1 family (IL-1 and IL-1ra), and TNF were studied in 274 Japanese patients with chronic HCV infection and 55 healthy individuals using standard polymerase chain reaction-based genotyping techniques. There was elevation in cytokine levels in the chronic HCV group.Citation34
Elevation of cytokine level in chronic HCV patients was also found in the study done by Vanis et al,Citation35 who found that IL-1α had the highest mean concentration in the group with viral hepatitis C, with PCR-positive test, and then in chronic viral hepatitis C with PCR-negative test than IL-1α in the healthy group. This may be explained by the fact that IL-1α has an important role as a marker of both inflammation and hepatic injury, particularly in the course of hepatitis C.
The present study agrees also with Huang et al’s resultsCitation36 which found that cytokines (IL1, IL 2r IL-6, and TNF-α) levels were significantly elevated in CHC with cirrhosis, CHC, and HCC compared with controls. These levels reflect hepatic dysfunction better than liver inflammation parameters, which might explain the higher serum concentrations of cytokines in cirrhosis.
Budhu and WangCitation37 study showed elevation of the serum levels of proinflammatory cytokines in HCC patients.
Lin et alCitation38 stated that IL-1α is one of the most important inflammatory cytokines involved in inflammation and tumor development. However, the role of membrane IL-1α in HCC tumorigenesis is still not clear. They found that membrane IL-1α potently inhibited HCC tumor growth. Further in vitro studies demonstrated that membrane IL-1α could directly activate T-cells and NK cells in a cell contact-dependent manner. Their studies demonstrated that membrane IL-1α could promote antitumor immune responses through activation of T- and NK cells.Citation38
Correlation of IL-1α with various parameters of the study showed a positive association with serum bilirubin, ALT, and viral load. There was a significant negative correlation with albumin and hemoglobin. The increase in ALT and bilirubin reflects hepatic inflammation, which gets worse with progress of HCV.
It is well known that IL-1α level mirrors inflammatory process in the liver. Olteanu et alCitation39 found that selective deficiency of IL-1α in Kupffer cells reduces liver inflammation and expression of inflammatory cytokines, which may implicate Kupffer cell-derived IL-1α in steatohepatitis development. Other IL-1 family ligands are critical for the development of diverse diseases, including inflammatory and allergic diseases.Citation39 Tseilikman et alCitation40 found that IL-1β treatment causes liver inflammation, consisting of infiltrating monocytes and the appearance of necrosis by increasing lipid peroxidation and protein carbonylation. Blocking IL-1 receptors with an antagonist significantly rescues stress-induced liver injury, suggesting that IL-1 might be involved in the cascade of liver injury initiated by sustained stress. There was a significant negative correlation with albumin which reflects the synthetic function of the liver. However, we failed to find a significant correlation between IL-1α and INR.Citation40 However, we failed to find a significant correlation between IL-1α and INR.
Correlation of IL-1α with viral load agrees with the results of Vanis et al,Citation35 who found that analysis of serum level of IL-1α showed a high degree of correlation with active replication of genetic material (HCV-RNA PCR positive).
The limitations of this study were the small number of patients and the dependence on laboratory data and radiological criteria for diagnosis of cirrhosis and HCC rather than liver biopsy in many patients. IL-1β and TGF-β were not evaluated here. However, it can be said that IL-1β may decrease in CHC, and TGF-β is responsible mainly for fibro-genesis. This study also included an HCC group. The cost is also a limitation here. Another limitation was that the study was directed toward presence of cirrhosis or HCC without focusing on the stage of HCC or presence or absence of hepatic decompensation.
Conclusion
The serum level of IL-1α is elevated in patients with CHC. In addition, the levels correlate well with deterioration of liver function in chronic HCV patients, and also with the development of HCC. It can be considered as a promising parameter of inflammatory activity and fibrosis evaluation in chronic liver disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZaltronSSpinettiABiasiLBaigueraCCastelliFChronic HCV infection: epidemiological and clinical relevanceBMC Infect Dis201212Suppl 2S223173556
- PerzJFArmstrongGLFarringtonLAHutinYJBellBPThe contributions of hepatitis B virus and hepatitis C virus to cirrhosis and primary liver cancer worldwideJ Hepatol200645452953816879891
- ThomasEGhanyMGLiangTJThe application and mechanism of action of ribavirin in therapy of hepatitis CAntivir Chem Chemother2013231112
- MohamoudYAMumtazGRRiomeSMillerDAbu-RaddadLJThe epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesisBMC Infect Dis20131328823799878
- ElwanNElfertAAbd-ElsalamSStudy of hepatic steatosis index in patients with chronic HCV infectionInt J Curr Microbiol App Sci201655266274
- Abd-ElsalamSSharaf-EldinMSolimanSElfertABadawiRAhmadYKEfficacy and safety of sofosbuvir plus ribavirin for treatment of cirrhotic patients with genotype 4 hepatitis C virus in real-life clinical practiceArch Virol20181631515628983675
- AhmedOAKaisarHHHawashNEfficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in treatment of a cohort of Egyptian patients with hepatitis C virus infectionInfect Disord Drug Targets20171729510028413993
- AhmedOAElsebaeyMAFouadMHAOutcomes and predictors of treatment response with sofosbuvir plus daclatasvir with or without ribavirin in Egyptian patients with genotype 4 hepatitis C virus infectionInfect Drug Resist20181144144529628768
- AhmedOAKaisarHHBadawiREfficacy and safety of sofosbuvir-ledipasvir for treatment of a cohort of Egyptian patients with chronic hepatitis C genotype 4 infectionInfect Drug Resist20181129529829535545
- BatallerRBrennerDALiver fibrosisJ Clin Invest2005115220921815690074
- GalambosJTAlcoholic liver disease, fatty liver and cirrhosisBerkJEHaubrichWSKalserMHRothJLASchaffnerFBockus Gastroenterology4th edPhiladelphiaWB Saunders198529853048
- TsochatzisEAGermaniGBurroughsAKTransarterial chemoembolization, transarterial chemotherapy, and intra-arterial chemotherapy for hepatocellular carcinoma treatmentSemin Oncol2010372899320494700
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- ZiadaDHEl SadanySSolimanHPrevalence of hepatocellular carcinoma in chronic hepatitis C patients in Mid Delta, Egypt: a single center studyJ Egypt Natl Canc Inst201628425726227378258
- ShetaEEl-KallaFEl-GharibMComparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled studyEur J Gastroenterol Hepatol201628101198120327362551
- SpenglerULechmannMIrrgangBDumoulinFLSauerbruchTImmune responses in hepatitis C virus infectionJ Hepatol199624Suppl 220258836885
- LechmannMIhlenfeldHGBraunschweigerIT and B cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and healthy seropositivesHepatology19962447907958855177
- ThimmteROldachDChangKMSteigerCRaySCChisariFVDeterminants of viral clearance and persistence during acute hepatitis C virus infectionJ Exp Med2001194101395140611714747
- McClainCJBarveSDeaciucIKugelmasMHillDCytokines in alcoholic liver diseaseSemin Liver Dis199919220521910422201
- FalascaKUcciferriCDalessandroMCytokine patterns correlate with liver damage in patients with chronic hepatitis B and CAnn Clin Lab Sci200636214415016682509
- SchmidtJBlumHEThimmeRT-cell responses in hepatitis B and C virus infection: similarities and differencesEmerg Microbes Infect201323e1526038456
- DinarelloCAProinflammatory cytokinesChest2000118250350810936147
- DumoulinFLLeifeldLHoneckerUSauerbruchTSpenglerUIntrahepatic expression of interleukin-1 beta and tumor necrosis factor alpha in chronic hepatitis CJ Infect Dis199918051704170810515837
- KaplanskiGFarnarierCPayanMJBongrandPDurandJMIncreased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosisDig Dis Sci19974211227722849398806
- IchikawaTNakaoKNakataKInvolvement of IL-1β and IL-10 in IFN-α-mediated antiviral gene induction in human hepatoma cellsBiochem Biophys Res Commun2002294241442212051728
- BrodyDTDurumSKMembrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interactionJ Immunol19891434118311872787357
- Kurt-JonesEABellerDIMizelSBUnanueERIdentification of a membrane-associated interleukin 1 in macrophagesProc Natl Acad Sci U S A1985824120412083919388
- LinDLeiLLiuYMembrane IL1α inhibits the development of hepatocellular carcinoma via promoting T- and NK-cell activationCancer Res201676113179318827206848
- KasprzakAZabelZBiczyskoWExpression of cytokines (TNF-α, IL-1α, and IL-2) in chronic hepatitis C: comparative hybridocytochemical and immunocytochemical study in children and adult patientsJ Histochem Cytochem2004521293814688215
- YuASKeeffeEBManagement of hepatocellular carcinomaRev Gastroenterol Disord200331924
- MurakamiTKimTTakahashiSNakamuraHHepatocellular carcinoma multidetector row helical CTAbdom Imaging200227213914611847573
- KozielMJCytokines in viral hepatitis CSemin Liver Dis199919215716910422198
- LecubeAHernándezCGenescàJSimóRProinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patientsDiabetes Care20062951096110116644643
- WangYKatoNHoshidaYInterleukin-1β gene polymorphisms associated with hepatocellular carcinoma in hepatitis Cvirus infectionHepatology2003371657112500190
- VanisNMehmedovicAMesihovicRUse of serum levels of pro-inflammatory cytokine IL-1α in chronic hepatitis CColl Antropol2015391757926040073
- HuangYSHwangSJChanCYSerum levels of cytokines in hepatitis C-related liver diseaseZhonghua Yi Xue Za Zhi (Taipei)199962632733310389289
- BudhuAWangXWThe role of cytokines in hepatocellular carcinomaJ Leukoc Biol20068061197121316946019
- LinDLeiLLiuYMembrane IL-1α inhibits the development of hepatocellular carcinoma via promoting T and NK cell activationCancer Res201676113179318827206848
- OlteanuSKandel-KfirMShaishALack of interleukin-1α in Kupffer cells attenuates liver inflammation and expression of inflammatory cytokines in hypercholesterolaemic miceDig Liver Dis201446543343924582082
- TseilikmanVKozochkinDSynitskyADoes stress-induced release of interleukin-1 cause liver injury?Cell Mol Neurobiol20123271069107822869351
