Abstract
Irritable bowel syndrome (IBS) is a complex, functional gastrointestinal disorder characterized by chronic abdominal pain or discomfort and altered bowel habits. Despite the global prevalence and disease burden of IBS, its underlying pathophysiology remains unclear. Inflammation may play a pathogenic role in IBS. Studies have highlighted the persistence of mucosal inflammation at the microscopic and molecular level in IBS, with increased recruitment of enteroendocrine cells. Substantial overlaps between IBS and inflammatory bowel disease have also been reported. This review thus aimed to discuss the body of evidence pertaining to the presence of mucosal inflammation in IBS, its putative role in the disease process of IBS, and its clinical relevance. Increased mast cell density and activity in the gut may correlate with symptoms of visceral hypersensitivity. As evidenced by patients who develop postinfectious IBS, infective gastroenteritis could cause systemic inflammation and altered microbiome diversity, which in turn perpetuates a cycle of chronic, low-grade, subclinical inflammation. Apart from mucosal inflammation, neuroinflammation is probably involved in the pathophysiology of IBS via the “gut–brain” axis, resulting in altered neuroendocrine pathways and glucocorticoid receptor genes. This gives rise to an overall proinflammatory phenotype and dysregulated hypothalamic–pituitary–adrenal axis and serotonergic (5-HT) functioning, which could, at least in part, account for the symptoms of IBS. Although a definite and reproducible pattern of immune response has yet to be recognized, further research into anti-inflammatories may be of clinical value.
Introduction
Irritable bowel syndrome (IBS) is a prevalent functional gastrointestinal disorder characterized by chronic abdominal pain or discomfort and altered bowel habits.Citation1 The Rome IV criteria is frequently used for the clinical diagnosis of IBS.Citation1 The criteria requires recurrent abdominal pain, on average, at least 1 d/wk in the last 3 months, associated with two or more of the following: 1) related to defecation; 2) associated with a change in frequency of stool; and 3) associated with a change in appearance of stools. IBS affects some 12% of the global population and has a significant disease burden in terms of increased absenteeism from school or work and reduced health-related quality of life.Citation2,Citation3
Despite its prevalence and often chronic, relapsing nature, the underlying pathophysiology of IBS remains incompletely understood.Citation4 The etiology is likely multifactorial, involving dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, neuroendocrine alterations, and visceral hypersensitivity, which results in the hallmark symptoms of abdominal pain and disordered gut motility.Citation5 Chronic, low-grade, subclinical inflammation has been implicated in the disease process and is thought to perpetuate the symptoms of IBS.Citation6 Previously, in most patients with IBS, routine histologic examinations did not reveal significant colonic mucosal abnormalities; however, with modern sequencing techniques, immunohistochemical assays, and ultrastructural analyses, subtle microscopic and molecular alterations have been reported.Citation7
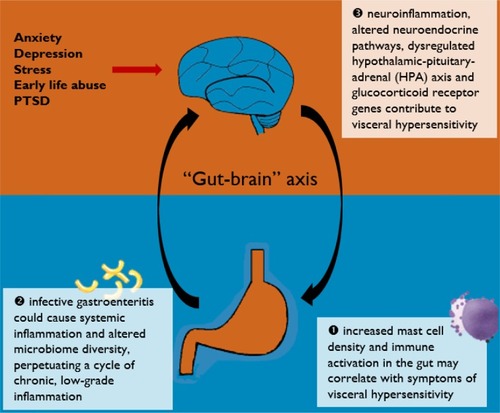
This review aimed to discuss the body of evidence pertaining to the presence of mucosal inflammation in IBS, its putative role in the disease process of IBS, and its clinical relevance. Evidence based on mucosal immunohistochemical examinations, patients with postinfectious IBS, human microbiota analyses, and neuroendocrine functioning would be examined and discussed. A better understanding of the role of inflammation in IBS would help advance current therapeutics and improve clinical outcomes of this difficult-to-treat condition.
Intestinal mucosa and the enteric immune system
The intestinal mucosa is part of an intricate enteric immune system and is endowed with a large variety of immune cells.Citation8 Exposure to food, bacteria, parasites, and viruses may contribute to sensitization of the enteric immune system and activation of the inflammatory cascade. Compared to healthy controls, patients with prototypical IBS symptoms have been reported to have increased lamina propria immune cells in the colonic mucosaCitation9 and significantly reduced levels of oleoylethanolamide, an endogenous PPAR-α agonist and fatty acid amide with anti-inflammatory properties.Citation10 These are suggestive of chronic, low-grade inflammation at the microscopic level. Increased infiltration of mucosal mast cells have also been reported in the cecum of patients with IBS, compared to healthy controls.Citation11 As mast cells mediate numerous inflammatory responses in the human body via activation and degranulation, it is thought that the observed increased volume density of mast cells may be the result of a persistent inflammatory response in patients with IBS. A randomized, controlled trial has found promising beneficial effects of the mast cell stabilizer ketotifen, providing further evidence for the role of mast cells and immune activation in IBS.Citation12
Mucosal T- and B-type lymphocytes also constitute part of the gastrointestinal adaptive immune system. Colonic biopsies taken from patients with IBS have found increased densityCitation13 and activationCitation14 of T-cell, again consistent with the hypothesis of underlying low-grade immune activation in the disease process of IBS. Increased immune activation has also been demonstrated in duodenal biopsies.Citation15 Blood samples taken from patients with IBS also showed an activated phenotype, with an increased frequency of CD4+ and CD8+ T-cells expressing the gut homing integrin β7.Citation16 These findings are suggestive of increased gut immune activation in IBS.
Postinfectious IBS and altered microbiome
Studies on postinfectious IBS have provided etiological insights into the pathogenesis of IBS. It is well documented that following infective gastroenteritis, more than 10% of affected individuals go on to develop postinfectious IBS.Citation17 The risk of postinfectious IBS appears greater with bacterial gastroenteritisCitation18 compared to viral gastroenteritis.Citation19 Upregulated expression of IL-1β mRNA has been reported in rectal biopsies taken 3 months postinfection in patients with postinfectious IBS.Citation20 This was not observed in patients who did not develop postinfectious IBS. IL-1β is a proinflammatory cytokine which drives cellular inflammation.
Serial rectal biopsies of patients who develop postinfectious IBS also revealed persistently increased T-cells and other enteroendocrine cells, compared to individuals whose symptoms eventually abate.Citation21 Patients who developed postinfectious IBS also had significantly higher CD3+, CD4+, and CD8+ T-cell counts in the gastrointestinal mucosa.Citation21 These cells are involved in the gut’s adaptive immunity response. Taken together, these findings support an inflammation–immunological etiopathogenesis of IBS.
Another consequence of infective gastroenteritis is the disruption of normal gut flora, although the gut microbiota during parasitic infection remains unstudied.Citation17 Through the use of 16S rRNA sequencing, studies have found reduced microbial diversity in patients who develop postinfectious IBS.Citation22 Though the gut microbiota is known to have interindividual variations, relative increase in Bacteroides and Prevotella bacteria have been frequently reported in patients with IBS compared to healthy controls.Citation23 The microbiome is thought to modulate inflammation and act either directly or indirectly through microbial metabolites. Microbial dysbiosis has been shown to promote inflammationCitation23 and impair normal lymphocyte function,Citation24 ultimately perpetuating chronic, low-grade inflammation. This is supported by clinical evidence that probiotic supplementation can relieve IBS symptomsCitation25 and have sustained effects on gut microbiota.Citation26 Further research into methods of restoring normal gut flora, eg, via the eubiotic effects of rifaximin,Citation27 should be encouraged. There are also existing gaps in knowledge regarding the interaction between the microbiome and the host in vivo – and the pathway of its metabolites – and how their metabolites influence the microenvironment. Metabolomic profilingCitation28 might help answer these questions.
Overlaps between IBS and IBD
Inflammatory bowel disease (IBD) is a gastrointestinal condition characterized by chronic inflammation of the gut and colon. Although it is clear that the extent of inflammation in IBD is significantly greater than that of patients with IBS, a recent meta-analysis of 13 studies have reported a high prevalence of IBS symptoms in patients with IBD (up to 40%), even in those with quiescent disease and under remissionCitation29 (usually defined using a fecal calprotectin cutoff of 250 mg/g).Citation30 The chronic mucosal inflammation in patients with IBD is thought to be responsible for the lasting alterations in intestinal permeability and gut function,Citation31 thereby generating symptoms typical of IBS. When comparing mucosal biopsies taken from patients with IBS without IBD and those with IBD and IBS-like symptoms, altered intestinal permeability (specifically, decreased expression of the tight junction proteins ZO-1 and α-cathenin) was found to be a common characteristic.Citation31 The presence of IBS-like symptoms even in patients deemed to have quiescent IBD supports the hypothesis that undetected, low-grade inflammation could be the cause of an excess of symptoms in this unique clinical population.
Neuroinflammation and the “Gut–Brain” axis
Psychosocial factors have been strongly implicated in the etiology of IBS.Citation32 Studies have found that childhood abuse and posttraumatic stress disorder are associated with the development IBS in adulthood.Citation33 Stress is thought to potentiate immune activation as it stimulates proinflammatory cytokines and NF-κB.Citation34 Anxiety and mood disorders are also well-established risk factors for developing postinfectious IBS, conferring risk similar to a severe infective gastroenteritis episode.Citation35 Modern research into the etiology of mood disorders have often alluded to the persistence of systemic and neuroinflammation.Citation36
Early-life abuse and posttraumatic stress disorder have been shown to cause sensitized corticotropin-releasing factor systems and dysregulated HPA axis,Citation37,Citation38 thereby promoting a proinflammatory phenotype. Elevated levels of proinflammatory plasma cytokines IL-6 and IL-8 have been implicated in patients with IBS.Citation39 As a result of increased proinflammatory cytokines, the activity of indoamine-2,3-dioxygenase (the rate-limiting enzyme in the degradation pathway of tryptophan) would be upregulated, ultimately affecting tryptophan metabolism and contributing to abnormal serotonergic (5-HT) functioning.Citation40 A hyperresponsive HPA axis could contribute to visceral hypersensitivity,Citation41 which is typically seen in patients with IBS. Abnormal 5-HT functioning is associated with altered gut motility and enhanced nociceptive pain sensitivity,Citation42 both symptoms characteristic of IBS.
Functional magnetic resonance imaging studies have also found heightened visceral stimuli response among IBS patients, with increased activation of the anterior cingulate cortex, prefrontal cortex, and thalamus in response to rectal distention in most instances.Citation43,Citation44 These responses also appeared to be modulated by anxiety and depression.Citation44
Conclusion
Current evidence does lend support to an inflammation–immunological etiopathogenesis of IBS. The various pathways discussed in this review are illustrated in . Although a definite and reproducible pattern of immune response has yet to be recognized, increased mast cell density and activity in the gut may correlate with symptoms of visceral hypersensitivity. As evidenced by patients who develop postinfectious IBS, infective gastroenteritis could cause systemic inflammation and altered microbiome diversity, which in turn perpetuates a cycle of chronic, low-grade, subclinical inflammation. Apart from mucosal inflammation, neuroinflammation is probably involved in the pathophysiology of IBS via the “gut–brain” axis, resulting in altered neuroendocrine pathways and glucocorticoid receptor genes. This gives rise to an overall proinflammatory phenotype and dysregulated HPA axis and serotonergic (5-HT) functioning, which could, at least in part, account for the symptoms of IBS.
Author contributions
Qin Xiang Ng conceived, designed and carried out the study and the relevant data analysis and interpretation. Wee-Song Yeo, Wayren Loke and Alex Yu Sen Soh carried out the study and the relevant data analysis and interpretation. Donovan Yutong Lim contributed to the data analysis and interpretation. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors alone are responsible for the content and writing of the article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr Ng and Dr Loke are employees of MOH Holdings Pte Ltd (MOH Holdings is the holding company for Singapore’s healthcare institutions; MOH Holdings Pte Ltd was not involved in the writing or preparation of this manuscript). The authors report no other conflicts of interest in this work.
References
- DrossmanDAFunctional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IVGastroenterology2016150612621279
- LovellRMFordACGlobal prevalence of and risk factors for irritable bowel syndrome: a meta-analysisClin Gastroenterol Hepatol201210771272122426087
- AkehurstRLBrazierJEMathersNHealth-related quality of life and cost impact of irritable bowel syndrome in a UK primary care settingPharmacoeconomics200220745546212093301
- KimJHLinEPimentelMBiomarkers of Irritable Bowel SyndromeJ Neurogastroenterol Motil2017231202627817184
- BarbaraGCremonCde GiorgioRMechanisms underlying visceral hypersensitivity in irritable bowel syndromeCurr Gastroenterol Rep201113430831521537962
- BercikPVerduEFCollinsSMIs irritable bowel syndrome a low-grade inflammatory bowel disease?Gastroenterol Clin North Am200534223524515862932
- KirschRKirschRHRiddellRHRiddellRHistopathological alterations in irritable bowel syndromeMod Pathol200619121638164517013373
- CastroGAGut immunophysiology: Regulatory pathways within a common mucosal immune systemPhysiology1989425964
- SalzmannJLPeltier-KochFBlochFPetiteJPCamilleriJPMorphometric study of colonic biopsies: a new method of estimating inflammatory diseasesLaboratory investigation; a journal of technical methods and pathology19896068478512733385
- CremonCStanghelliniVBarbaroMRRandomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndromeAliment Pharmacol Ther201745790992228164346
- O’SullivanClaytonNBreslinNPIncreased mast cells in the irritable bowel syndromeNeurogastroenterology and Motility200012544945711012945
- KlookerTKBraakBKoopmanKEThe mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndromeGut201059912131221 Jan 1:gut-201020650926
- TörnblomHLindbergGNybergBVeressBFull-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndromeGastroenterology200212361972197912454854
- ÖhmanLIsakssonSLindmarkA-CT-Cell Activation in Patients With Irritable Bowel SyndromeAm J Gastroenterol200910451205121219367268
- FoleySGarsedKSinghGImpaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activationGastroenterology201114051434144321315720
- ÖhmanLIsakssonSLundgrenASimrénMSjövallHA Controlled Study of Colonic Immune Activity and β7 Blood T Lymphocytes in Patients With Irritable Bowel SyndromeClinical Gastroenterology and Hepatology200531098098616234043
- ThabaneMKottachchiDTMarshallJKSystematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndromeAliment Pharmacol Ther200726453554417661757
- MearinFPérez-OliverasMPerellóADyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort studyGastroenterology200512919810416012939
- MarshallJKThabaneMBorgaonkarMRJamesCPostinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogenClinical Gastroenterology and Hepatology20075445746017289440
- GweeKACollinsSMReadNWIncreased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndromeGut200352452352612631663
- SpillerRCJenkinsDThornleyJPIncreased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndromeGut200047680481111076879
- SundinJRangelIFuentesSAltered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distressAliment Pharmacol Ther201541434235125521822
- Jalanka-TuovinenJSalojärviJSalonenAFaecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndromeGut2014631117374524310267
- SundinJRangelIRepsilberDBrummerR-JCytokine response after stimulation with key commensal bacteria differ in post-infectious irritable bowel syndrome (PI-IBS) patients compared to healthy controlsPLoS One2015109e013483626366730
- KajanderKMyllyluomaERajilić-StojanovićMClinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiotaAliment Pharmacol Ther2008271485717919270
- AlanderMSatokariRKorpelaRPersistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosusGG, after oral consumptionApplied and Environmental Microbiology19996513513549872808
- NgQXHoCYXShinDA meta-analysis of the use of rifaximin to prevent travellers’ diarrhoeaJ Travel Med201724515
- ZhangBZhangHDuCMetabolic responses of the growing Daphnia similis to chronic AgNPs exposure as revealed by GC-Q-TOF/MS and LC-Q-TOF/MSWater Res201711413514328237781
- HalpinSJFordACPrevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysisAm J Gastroenterol2012107101474148222929759
- GracieDJWilliamsCJMSoodRNegative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome–type Symptoms in Patients With Inflammatory Bowel DiseaseClinical Gastroenterology and Hepatology201715337638427189912
- Vivinus-NébotMFrin-MathyGBziouecheHFunctional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammationGut201463574475223878165
- NgQXSohAYSLimDYYeoWSAgomelatine, a novel therapeutic option for the management of irritable bowel syndromeJ Clin Pharm Ther201843575275630014556
- NgQXSohAYSLokeWSystematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndromeJ Gastroenterol Hepatol Epub2582018
- PaceTWWMletzkoTCAlagbeOIncreased stress-induced inflammatory responses in male patients with major depression and increased early life stressAm J Psychiatry200616391630163316946190
- WoutersMMVan WanrooySNguyenAPsychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritisGut2016651279128826071133
- BritesDFernandesANeuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulationFront Cell Neurosci2015947626733805
- DinanTGCryanJShanahanFKeelingPWNQuigleyEMMIBS: an epigenetic perspectiveNat Rev Gastroenterol Hepatol20107846547120585338
- NgQXYongBZJHoCYXEarly life sexual abuse is associated with increased suicide attempts: An update meta-analysisJ Psychiatr Res20189912914129454220
- ClarkeGQuigleyEMMCryanJFDinanTGIrritable bowel syndrome: towards biomarker identificationTrends Mol Med2009151047848919811951
- ClarkeGFitzgeraldPCryanJFCassidyEMQuigleyEMDinanTGTryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohortBMC Gastroenterol200991619154614
- ChangLSundareshSElliottJDysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndromeNeurogastroenterology & Motility200921214915918684212
- GroverMCamilleriMEffects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseasesJ Gastroenterol201348217718123254779
- HallGbcKamathMvCollinsSHeightened central affective response to visceral sensations of pain and discomfort in IBSNeurogastroenterology & Motility2010223276e8020003075
- ElsenbruchSRosenbergerCSchedlowskiMForstingMGizewskiERAffective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI studyPPmP - Psychotherapie · Psychosomatik · Medizinische Psychologie20095902