Abstract
Background
The incidence of recurrent cardiovascular events from the progression of nontarget lesions (NTLs) is high for percutaneous coronary intervention-treated patients. However, the underlying mechanisms have not been thoroughly elucidated.
Methods
In this study, ten atherosclerotic rabbits with multiple plaques in the upper and lower segments of abdominal aorta (group A) were randomly divided into two subgroups: group A1 underwent intravascular ultrasound examination and stent implantation in the lower segments of the abdominal aorta (n=5), whereas group A2 was without stenting (n=5). Group B was a control group without balloon injury. The serum levels of high-sensitivity CRP, interleukin-6 (IL-6), oxidized low-density lipoprotein, and CD36 were assessed via ELISA at five time points between the 10th and 18th weeks. The upper abdominal aorta was examined via the immunohistochemical stain and Western blotting of matrix metallopeptidase 9 (MMP-9), CD36, IL-6, and tumor necrosis factor α.
Results
As a result, we found that stent implantation aggravated serum levels of CD36, oxidative stress, and inflammatory cytokines. Meanwhile, the upper abdominal arterial plaque burden significantly increased after stenting by intravascular ultrasound. Immunohistochemistry and Western blotting showed that the local NTLs’ matrix metallopeptidase 9, CD36, IL-6, and tumor necrosis factor α expressions in group A1 were significantly higher than those in groups A2 and B (P<0.05–0.01). More importantly, a strong correlation was identified between CD36 expression and NTLs’ plaque burden before the rabbits were killed.
Conclusion
Taken together, stent implantation accelerated inflammation, induced oxidative stress, and increased the NTLs’ progression, which were associated with the upregulated CD36 expression.
Introduction
Coronary heart disease (CHD) is the leading cause of morbidity and mortality worldwide, particularly in developing countries such as China. To date, percutaneous coronary intervention (PCI) with stent deployment is the most common revascularization procedure for CHD.Citation1,Citation2 It is reported that there are over 4,000,000 CHD patients undergoing PCI every year for the previous three decades.Citation3 Of note, the increased use of PCI does not improve CHD patients’ prognosis of recurrent cardiovascular events. It is verified that clinical events after PCI resulted from not only the in-stent restenosis but also the progression of nontarget lesions (NTLs), remote from the site of stent deployment. NTLs’ events contributed to 46.4% of the overall events over the 5-year follow-up.Citation4 These results are consistent with Stone’s study,Citation5 which indicate that PCI may accelerate the progression of atherosclerosis. However, there is limited evidence from the basic research to confirm this effect and elucidate the underlying mechanisms.
Vascular injury after the cardiovascular intervention is associated with local and systemic inflammation, which depends on the type and complexity of coronary artery disease, particularly in patients with acute coronary syndromes.Citation6 By measuring the temperature difference between the NTLs and their proximal normal vessel wall, Toutouzas et al reported that the systemic inflammatory response triggered by PCI could aggravate remote NTLs’ surface heat production and local inflammatory activation.Citation7 Apart from inflammation, oxidative stress contributes to NTLs’ progression after intervention injury. Reactive oxygen species increased from 24 hours after intervention injury and lasted up to 14 days,Citation6 thus providing the conditions for the formation of oxidized low-density lipoproteins (ox-LDL).Citation8
CD36, a class B scavenger receptor, is a high-affinity receptor for ox-LDL, which mediates the entry of ox-LDL into the subvascular endothelium, thus leading to macrophage activation, foam cell formation, and pro-inflammatory cytokines release.Citation9 In addition, ox-LDL via CD36 inhibits macrophage migration resulting in macrophage trapping in atherosclerotic lesions.Citation10 Our previous clinical study investigated the function of CD36 in atherosclerosis and revealed that CD36 regulated atherosclerotic lesion progression in both the early and late stages.Citation11 However, till now there are few studies probing the relationship between CD36 expression and NTLs’ progression after vascular intervention injury.
The aim of the study was to investigate whether stent-induced inflammation and oxidative stress could affect nonintervened NTLs’ progression and its relationship with CD36 expression in atherosclerotic rabbit models.
Methods
Animals
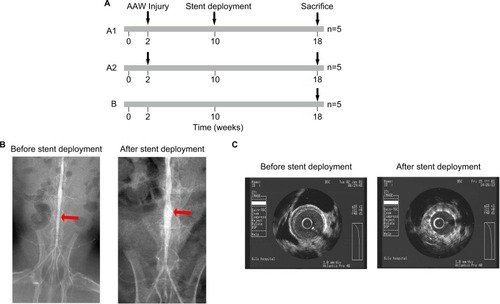
Sixteen male New Zealand rabbits (1.5–2.5 kg) were obtained from the Shandong Provincial Academy of Agricultural Sciences (Jinan, China) and were fed with 18 weeks of a cholesterol-rich diet (1% cholesterol). The animals were randomly divided into two groups: group A (n=11) and group B (n=5). Rabbits in group A underwent the balloon-induced abdominal aortic wall injury at the second week. Balloon-induced aortic wall injury was performed with a 4-Fr balloon catheter (balloon diameter and length 3.5×15 mm2, EMERGE™ PTCA Dilatation Catheter, Boston Scientific Corporation, Shanghai, China), which was introduced through the right femoral artery to the thoracic aorta after anesthetization with an intravenous injection of pentobarbital sodium (30 mg/kg). The balloon was inflated with saline to obtain 8 atmospheres, and the catheter was retracted down to the iliofemoral artery. This process was repeated three times in each rabbit to ensure denudation of the endothelium of the abdominal aorta and one rabbit died in this process. According to the abdominal aortic angiography at the 10th week, ten rabbits in group A with multiple plaques in the upper and lower segments of the abdominal aorta were randomly divided into two subgroups: group A1 underwent stent deployment in the lower segment according to clinical methods (n=5, rapamycin eluting stent, LEPU Medical, Beijing, China), whereas group A2 underwent angiography without a stent (n=5). As for group B, the rabbits were fed a diet of 1% cholesterol, without balloon injury or stent deployment. All rabbits were sacrificed at the end of the 18th week. NTLs were defined as the upper segment of the abdominal aorta remote from the stent at a distance of at least 5 mm. The animal protocols were performed under the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Shandong University.Citation12
Intravascular ultrasound examination
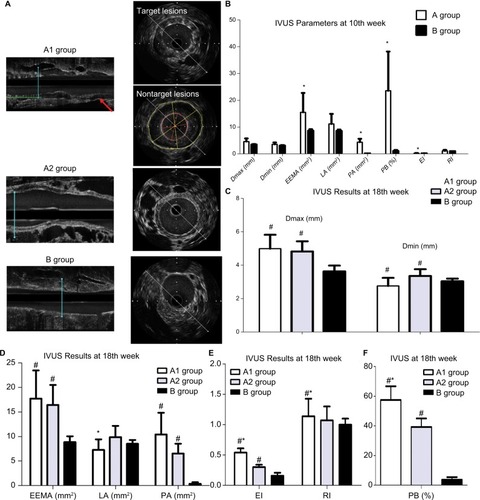
Intravascular ultrasound (IVUS) studies were performed using a 3.2 F catheter, which contained a single rotating element transducer of 40 MHz connected to an IVUS system (iLab, Boston Scientific Corp., Fremont, CA, USA). The catheter was pulled back from the aortic arch to the abdominal aorta by a motorized withdrawal device at a constant speed of 0.5 mm/second. The following parameters were measured from the cross-sectional images: maximum diameter (Dmax), minimum diameter (Dmin), plaque area (PA), eccentricity index (EI), remodeling index (RI), the external elastic membrane area (EEMA), lumen area (LA), plaque area (PA = EEMA−LA), and plaque burden (PB% = PA/EEMA ×100%). The rabbits in groups A1 and A2 underwent IVUS at least twice (at the 10th and 18th weeks), whereas group B underwent the IVUS examination at the 18th week prior to euthanasia.
Biochemical assays
The serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C) were measured by enzymatic assays at the 10th, 11th, and 18th weeks. With the aim to investigate the trend over time, 4 mL of blood was taken from the middle ear artery at different time points (before, 1 hour, 24 hours, 7 days, and 8 weeks after stent). The serum levels of high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), ox-LDL, and CD36 were quantified by the ELISA. Plasma glucose concentration was also measured with the Glucose Analyzer (Yellow Spring Instrument, Yellow Springs, OH, USA).
Immunohistochemistry
At the 18th week, the rabbits were sacrificed, and the upper abdominal aorta was collected and fixed in 4% formaldehyde. Tissue samples embedded in paraffin were reacted with mouse anti-rabbit matrix metallopeptidase 9 (MMP-9) monoclonal antibody (Abcam, Cambridge, MA, USA), mouse anti-rabbit CD-36 polyclonal antibody (Bioss ANTIBODIES, Beijing, China), mouse anti-rabbit IL-6 polyclonal antibody (Bioss ANTIBODIES), and mouse anti-rabbit tumor necrosis factor α (TNF-α) polyclonal antibody (Bioss ANTIBODIES). The sections were incubated with a goat anti-mouse peroxidase-labeled anti-body (ZSGB-BIO) as the secondary antibody at room temperature for 15 minutes. Histopathological slides were analyzed using a computer-assisted morphometric analysis system (Image-Pro Plus 5.0, Media Cybernetics, Rockville, MD, USA). The positive staining of MMP-9, CD-36, IL-6, and TNF-α was counted in five images of every slice under high-power fields (×400), and three individuals independently reported the results.
Western blots
We examined the protein expression of MMP-9, CD36, IL-6, and TNF-α in the upper abdominal aorta by Western blot analysis. In brief, 30 µg of tissue lysates were prepared as the standard protocol and separated on a 12% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis. The protein bands were subsequently transferred to a polyvinylidene fluoride membrane for 1 hour and were blocked with 5% skimmed milk for 1 hour at room temperature. The membranes were then incubated with rat anti-MMP-9 (Abcam) and rat anti-CD36/IL-6/TNF-α (Bioss ANTIBODIES) at 4°C overnight. The membranes were subsequently incubated with human, anti-rabbit secondary antibodies (563-2; 1:1,000 dilution Zemai Biotech Corporation, Shanghai, China) for 1 hour at room temperature after three washes. Finally, the reaction was visualized using an enhanced chemiluminescence detection system (LAS MINI 4000, GE Healthcare Life Sciences, Little Chalfont, UK).
Statistical analysis
Continuous variables were reported as the mean ± standard deviation (SD) and were analyzed using unpaired Student’s t-tests and ANOVAs, whereas categorical variables were analyzed via chi-square tests. The relationship between variables was estimated by Spearman analysis. For all analyses, a P-value <0.05 was considered significant, using SPSS 17.0.
Results
Stent implantation increased upper abdominal arterial plaque burden in atherosclerotic models
Five animals per group were analyzed following our protocol (). shows that stent implantation effectively restored the narrowed abdominal aorta. shows the morphological difference of the NTLs before sacrifice by IVUS. At the 10th week, IVUS showed that Dmax (4.60±1.14 mm vs 3.99±0.15 mm, P<0.05), EEMA (15.5±7.24 mm2 vs 8.66±0.54 mm2, P<0.05), PA (4.34±1.28 mm2 vs 0.09±0.04 mm2, P<0.05), and PB (23.57±14.63% vs 1.07±0.37%, P<0.05) were significantly increased in group A compared with those in group B (, ). Furthermore, the above parameters were much higher in groups A1 and A2 compared with group B at the 18th week (, –). Of note, an obvious increase in EI, RI, and PB was observed in group A1 compared with those in group A2 (, ).
Table 1 Values of IVUS for nontarget lesions
Figure 1 The establishment of an atherosclerotic rabbit model.
Figure 2 IVUS results at 10th week and 18th week.
Notes: (A) Representative IVUS images from three groups at the 18th week. (B) IVUS assessed the differences between groups a and B at 10th week. *P<0.05 vs group B. (C) IVUS demonstrated increased Dmax and Dmin in groups A1 and A2 at the 18th week. #P<0.05 vs group B. (D) IVUS demonstrated the change of EEMA, LA, and PA among three groups at the 18th week. #P<0.05 vs group B; *P<0.05 vs group A2. (E) Increased EI and RI in groups A1 and A2 at the 18th week by IVUS. #P<0.05 vs group B; *P<0.05 vs group A2. (F) Increased PB in groups A1 and A2 at the 18th week by IVUS. #P<0.05 vs group B; *P<0.05 vs group A2.
Abbreviations: Dmax, maximum diameter; Dmin, minimum diameter; EEMA, external elastic membrane area; EI, eccentricity index; IVUS, intravascular ultrasound; LA, lumen area; PA, plaque area; PB, plaque burden; RI, remodeling index.
Notes: (A) Schematic showing that animals were separated into A1, A2, and B groups with different treatments. (B) Angiography showed a narrowing abdominal artery before (left) and after (right) stent deployment. Red arrows indicate the narrowing artery. (C) Intravascular ultrasonography showed that the stent was placed in the narrowing artery.
Abbreviation: AAW, abdominal aortic wall.

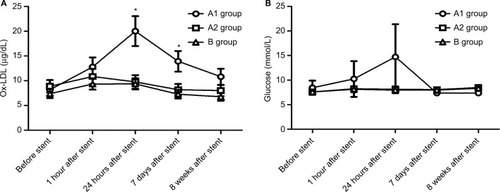
Stent implantation aggravated the serum ox-LDL and glucose levels, not including TC, TG, and LDL-C
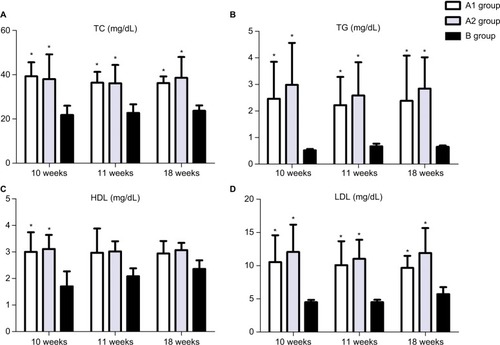
Both and show that TC, TG, and LDL-C in group A were significantly higher than those in group B (P<0.01), while there was no significant difference between groups A1 and A2 (P>0.05). Ox-LDL level was more significantly increased in group A1 compared with group A2 (P<0.05), keeping high levels till 8 weeks after stenting (). Also, blood glucose levels were significantly higher in group A1 than those in both groups A2 and B at 24 hours after stenting ().
Table 2 Changes in serum cholesterol (mmol/L) and triglycerides (mmol/L) in rabbits over time
Figure 3 ELISA showed upregulated TC (A), TG (B), HDL-C (C), and LDL-C (D) in the blood at the 10th, 11th, and 18th weeks. *P<0.05 vs group B.
Figure 4 Stent implantation aggravated the serum ox-LDL and glucose levels.
Notes: (A) ELISA showed the blood levels of ox-LDL at five time points; *P<0.05 vs ox-LDL levels before stent. (B) Glucose analyzer was utilized to measure the plasma glucose over time.
Abbreviation: ox-LDL, oxidized low-density lipoprotein.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.

CD36 protein level was associated with upregulated inflammation, increased oxidative stress, and abdominal arterial PB
Immunohistochemistry showed that the local NTLs’ expressions of MMP-9, IL-6, and TNF-α in groups A1 and A2 were significantly higher than those in group B (all P<0.05, ). Furthermore, the expressions of MMP-9, IL-6, and TNF-α in group A1 were substantially higher than those in group A2 (all P<0.05, ). Western blotting showed similar results in which the protein expression levels of MMP-9, IL-6, and TNF-α in group A1 were substantially higher than the levels in groups A2 and B (P<0.05, ).
Figure 5 Effect of stent implantation on the nontarget lesions’ inflammation determined by immunostaining and Western blot.
Notes: (A) Representative immunostaining images of IL-6 are shown in the left panel and quantification was achieved by normalizing IL-6 positive areas to the plaque sizes. (B) Representative immunostaining images of MMP-9 in the left panel and quantification was achieved by normalizing MMP-9 positive areas to the plaque sizes. (C) Representative immunostaining images of TNF-α and analysis data. (D) Western blot was used to measure the protein level changes of IL-6, MMP-9, and TNF-α in the upper segment of abdominal aorta. (E) Image J was used to quantify the results in . *P<0.05 vs group B; #P<0.05 vs group A2.
Abbreviations: IL-6, interleukin-6; MMP-9, matrix metallopeptidase 9; TNF-α, tumor necrosis factor α.
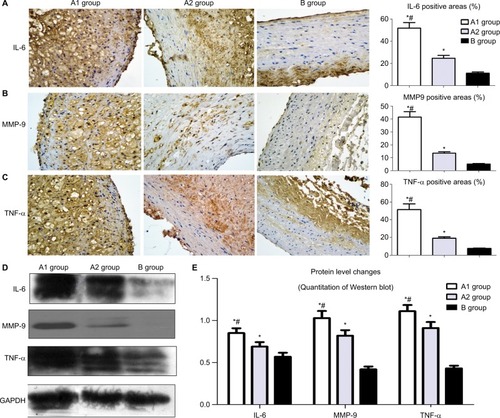
Western blot showed CD36 levels in group A1 were significantly higher than those in group A2 and B (P<0.05–0.01, ). Similar results were obtained by immunostaining of CD36. The local NTLs’ expressions of CD36 in group A1 were much higher than those in groups A2 and B (P<0.05, ).
Figure 6 CD36 protein expression of the upper segment of abdominal aorta among the three groups by Western blot (A) and quantitative analysis (B). *P<0.05 vs group B. (C) Representative immunostaining images of CD36 expression in the upper segment of abdominal aorta. (D) Quantitative analysis of CD36 in . *P<0.05 vs group B. ELISA was performed to assess the serum IL-6 protein levels (E), hs-CRP protein levels (F), CD36 protein levels (G) at five time points. (H) CD36 levels in blood were associated with ox-LDL, glucose, and IL-6 at 24 hours after stenting. (I) CD36 levels in blood were associated with PB by IVUS at 18th week.
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IVUS, intravascular ultrasound; ox-LDL, oxidized low-density lipoprotein; PB, plaque burden.
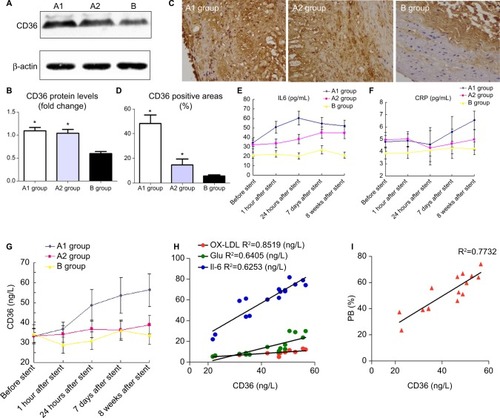
With the aim to elucidate the trend of inflammation over time, serum IL-6, hs-CRP, and CD36 were detected at five time points (before, 1 hour, 24 hours, 7 days, and 8 weeks) after stenting. IL-6 (not hs-CRP) increased at 1 hour after stenting, reached a peak at 24 hours post procedure, then declined gradually at 7 days till 8 weeks after stent (). However, hs-CRP increased at 7 days after stenting and continued rising until post-stent 8 weeks (). CD36 levels slightly increased at 1 hour after stenting, were significantly raised at 24 hours, and kept increasing until the rabbits were sacrificed ().
More importantly, only CD36 levels were positively correlated with elevated inflammation and oxidative stress at 24 hours after stent deployment (). In addition, a strong correlation was identified between CD36 expression and upper abdominal arterial PB at the 18th week ().
Discussion
In this study, we established an atherosclerotic model with multiple artery plaques by using the large balloon injury, which is more similar to the atherosclerotic distribution in CHD patients. The present study confirmed that stent implantation accelerated NTLs’ vulnerability and progress, which can be predicted by the serum CD36 level.
IVUS, the most often used methodology to evaluate the coronary arteries in CHD patients, provided high-quality, cross-sectional imaging of the entire artery wall and important information about the build up of plaque.Citation13 In our study, NTLs after stenting presented with greater PBs, less luminal area, and an increase in eccentric index by IVUS. Immunohistochemistry and Western blot confirmed the increased expressions of the inflammatory cytokines IL-6, TNF-α, and MMP-9 in local NTLs after stenting, which all potentially increased NTLs’ vulnerability. These results suggested that stent implantation accelerated NTLs’ vulnerability and progression by inflammation activation.
Previous studies have reported that IL-6 is an upstream inflammatory cytokine that propagates the downstream inflammatory response responsible for the progression of atherosclerosis.Citation14,Citation15 In this study, we determined that only IL-6, not hs-CRP, significantly increased at 1 hour of rabbit abdominal arterial stent implantation. This level peaked at 24 hours and then gradually declined after 7 days for 4 weeks; however, it remained higher than the control and balloon-injury groups. Interestingly, the IL-6 levels at 24 hours post stent deployment were related to the CD36 levels. We confirmed that IL-6 is the earliest released inflammatory factor induced by stent implantation, which was associated with CD36 upregulation. With respect to another inflammation indicator hs-CRP, we surprisingly found that the hs-CRP levels were unchanged within the first 24 hours and began to increase on the seventh day compared with the baseline levels. This trend was inconsistent with previous clinical observations.Citation16,Citation17 One reason for the early upregulation of hs-CRP in CHD patients after PCI may be the perioperative myocardial infarction induced by microthrombosis,Citation18 which rarely occurred in our study because the stent site-abdominal aorta does not locate in terminal vessels. However, elevated levels of hs-CRP from the seventh day to the 18th week suggested that hs-CRP participated in the late progression of vulnerable plaques.
In addition to inflammation, ox-LDL (a marker of lipoprotein-associated oxidative stress) and blood glucose significantly increased after stenting in our study. A positive relationship between the ox-LDL, hyperglycemia, and CD36 levels was observed, suggesting that oxidative stress (represented by ox-LDL and hyperglycemia) played a central role in the initiation of CD36.Citation19–Citation21 CD36 belongs to class B scavenger receptors, which are membrane glycoproteins with a mass of 88 kDa, composed of a single protein chain. CD36 locates on the multiple cell surface, such as adipocytes, monocytes, platelets, and macrophages. The macrophage is a key in atherosclerosis progression, which takes up ox-LDL mainly through scavenger receptors.Citation9 CD36 is the most important scavenger receptor in macrophage recruitment to atherosclerotic lesions and leads to vascular wall inflammation through the combination of ox-LDL in a receptor-type manner.Citation22 Recent studies have reported an additional atherogenic effect by platelet CD36-bound ox-LDL. Platelets interacting with ox-LDL release chemokines and mediate platelet-monocyte aggregate formation, which enhances monocytes, phenotypic changes and foam cell formation.Citation23,Citation24
Our previous study showed that CD36 regulated atherosclerotic lesion progression in both the early and late stages.Citation11 Harb et al showed that the recruitment of radiolabeled macrophages to atherosclerotic lesions was reduced by pretreatment with the CD36-binding peptide EP80317. In contrast, in CD36-deficient mice, the pretreatment had no effects.Citation25 In humans, autopsy studies performed in CHD patients have demonstrated increased expression of the CD36 receptor on macrophages, comprised within atherosclerotic plaques. Taken together, CD36 is an important receptor in atherosclerosis progression.Citation26
In this study, we determined that the local expression of CD36 in NTLs was substantially higher after stenting than the control and vehicle groups. Moreover, the serum CD36 was the only cytokine that significantly increased from the early time and maintained high levels until 8 weeks. In addition, CD36 levels in the blood were positively correlated with elevated inflammation and oxidative stress which are confirmed to accelerate the progression of NTLs in our study. More importantly, the relationship analysis showed that only CD36 levels were significantly related to the progression of PB of NTLs at the 18th week. This observation suggests that CD36 has a great potential to be a novel biomarker to predict the progression of NTLs. Meanwhile, CD36 has high expression levels in the smooth muscle cells by immunostaining. But, whether the circulating CD36 originates from smooth muscle cells or not remains to be further studied. Taken together, CD36 was confirmed as a key receptor in the progression of NTLs, which might have potential implications for clinical practice.
An important limitation was that our study failed to block or interfere with CD36 expression by gene knockout or siRNA. Furthermore, atherosclerotic rabbit abdominal aortic stents were deployed without drug treatment. Moreover, animal models established by balloon injury and high-fat diet do not completely resemble atherosclerotic plaques in humans.
In this study, we confirmed that nonintervened NTLs were accelerated by stenting-induced inflammation and oxidative stress, which were associated with upregulated CD36 expression.
Acknowledgments
This research was supported by China Postdoctoral Science Foundation (ZR2015HM039), Shandong Province Natural Science Foundation (2015M572054), and Shandong Province Medical Science and Technology Development Plan (2013WS0216).
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkHWYoonCHKangSHEarly- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarctionInt J Cardiol2013169425426124071385
- DengFLvJHWangHLGaoJMZhouZLExpanding public health in China: an empirical analysis of healthcare inputs and outputsPublic Health2017142738428057203
- FengCJiTLiuYRole of depression in secondary prevention of Chinese coronary heart disease patients receiving percutaneous coronary interventionPLoS One20171212e018701629267269
- BodenWEO’RourkeRATeoKKOptimal medical therapy with or without PCI for stable coronary diseaseN Engl J Med20073561503151617387127
- StoneGWMaeharaALanskyAJA prospective natural-history study of coronary atherosclerosisN Engl J Med2011364322623521247313
- RosenbaumMAMiyazakiKGrahamLMHypercholesterolemia and oxidative stress inhibit endothelial cell healing after arterial injuryJ Vasc Surg201255248949622047834
- ToutouzasKDrakopoulouMMarkouVCorrelation of systemic inflammation with local inflammatory activity in non-culprit lesions: beneficial effect of statinsInt J Cardiol2007119336837317258821
- ShiYNiculescuRWangDPatelSDavenpeckKLZalewskiAIncreased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injuryArterioscler Thromb Vasc Biol200121573974511348868
- YamashitaSHiranoKKuwasakoTPhysiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patientsMol Cell Biochem20072991–2192216670819
- ParkYMDrazbaJAVasanjiAEgelhoffTFebbraioMSilversteinRLOxidized LDL/CD36 interaction induces loss of cell polarity and inhibits macrophage locomotionMol Biol Cell201223163057306822718904
- LiRJYangMLiJFXueLChenYGChenWQCirculating CD36 and fractalkine levels are associated with vulnerable plaque progression in patients with unstable angina pectorisClin Exp Pharmacol Physiol2014411186386925224515
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National AcademiesGuide for the Care and Use of Laboratory Animals8th edWashingtonNational Academies Press2011
- MehranRMintzGSHongMKValidation of the in vivo intra-vascular ultrasound measurement of in-stent neointimal hyperplasia volumesJ Am Coll Cardiol1998327947999741529
- AggarwalASchneiderDJTerrienEFGilbertKEDauermanHLIncrease in interleukin-6 in the first hour after coronary stenting: an early marker of the inflammatory responseJ Thromb Thrombolysis2003151253114574073
- SegevAKassamSBullerCEPre-procedural plasma levels of C-reactive protein and interleukin-6 do not predict late coronary angiographic restenosis after elective stentingEur Heart J200425121029103515191773
- SzmitkoPEWangCHWeiselRDde AlmeidaJRAndersonTJVermaSNew markers of inflammation and endothelial cell activation: Part ICirculation2003108161917192314568885
- XuYLLiJJXuBIncreased plasma C-reactive protein level predicts rapid progression of non-target atherosclerotic lesions in patients with stable angina after stentingChin Med J (Engl)2011124193022302922040548
- YunKHJeongMHOhSKResponse of high-sensitivity C-reactive protein to percutaneous coronary intervention in patients with acute coronary syndromeHeart Vessels200924317518019466517
- HolvoetPde KeyzerDJacobsDROxidized LDL and the metabolic syndromeFuture Lipidol20083663764919802339
- FiorentinoTVPriolettaAZuoPFolliFHyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseasesCurr Pharm Des201319325695570323448484
- LuHYaoKHuangDHigh glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cellsCardiovasc Diabetol2013128023718574
- Nergiz-UnalRRademakersTCosemansJMHeemskerkJWCD36 as a multiple-ligand signaling receptor in atherothrombosisCardiovasc Hematol Agents Med Chem201191425520939828
- Siegel-AxelDDaubKSeizerPLindemannSGawazMPlatelet lipoprotein interplay: trigger of foam cell formation and driver of atherosclerosisCardiovasc Res200878181718218686
- BadrnyaSSchrottmaierWCKralJBPlatelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formationArterioscler Thromb Vasc Biol201434357158024371083
- HarbDBujoldKFebbraioMSiroisMGOngHMarleauSThe role of the scavenger receptor CD36 in regulating mononuclear phagocyte trafficking to atherosclerotic lesions and vascular inflammationCardiovasc Res2009831425119264766
- ChoromańskaBMyśliwiecPChoromańskaKDadanJChabowskiAThe role of CD36 receptor in the pathogenesis of atherosclerosisAdv Clin Exp Med201726471772228691408
