Abstract
Purpose
Cutaneous lichen planus (CLP) is an autoinflammatory skin disorder, and it is associated with metabolic syndrome. Wingless-type mouse mammary tumor virus integration site family member 5a (Wnt5a) is a potential factor in metabolic complications and it was shown to be upregulated in CLP lesions. Whether Wnt5a is altered in the circulation of patients with CLP is unclear. This study aimed to measure serum Wnt5a level in patients with CLP and to assess its relationship with body mass index (BMI).
Methods
We included 46 adult patients with CLP and 38 healthy adults as control. Serum Wnt5a was measured using enzyme-linked immunosorbent assay.
Results
The mean serum Wnt5a was significantly higher in patients than controls (all P-value <0.001). The mean serum Wnt5a levels in obese (BMI between 30 and 40) patients were significantly higher than lean (BMI between 20 and 25) patients (P-value <0.001). Compared to lean patients with CLP, the concentration of serum Wnt5a levels was increased gradually with BMI score (all P-value <0.05).
Conclusion
Serum Wnt5a might be a potential biomarker for CLP and it was associated with BMI. An increase in serum Wnt5a may contribute to the development of metabolic comorbidity in CLP patients.
Introduction
Cutaneous lichen planus (CLP) is an idiopathic, chronic and inflammatory skin disease, characterized by purplish, polygonal patches or plaques with a lace-like pattern of white surfaces (classic Wickham striae) on the physical examination, and it often accompanied by severe itching.Citation1 It affects about 1% of the general population, with a slight predominance in middle-aged women adults.Citation2 Although “six P’s” (purple, pruritic, polygonal, planar, papules, and plaques) are able to diagnose CLP in clinical, a biopsy specimen of the skin is helpful to confirm the diagnosis. There are a number of characteristic pathologic findings, including hyperkeratosis without parakeratosis, “wedge-shaped” hypergranulosis, liquefaction deformation of basal cells and apoptotic keratinocytes (Civatte bodies), dense inflammatory cells infiltrated in the superficial dermis.Citation3 At present, it is considered that CLP is an inflammatory disease regulated by T cells, but the specific pathogenesis is unclear. Gene polymorphisms of human leukocyte antigen (HLA) markers are related to the presence of lichen planus (LP). Environmental factors, such as infections (hepatitis C virus infection, Epstein–Barr virus), allergies, drugs, stress/anxiety and internal malignancies are also contribute to the development of LP.Citation4 It is well known that T cell regulated immune response is the core of LP pathogenesis, both CD4+ and CD8+ in the center and mediated immune responses against keratinocytes.Citation5 The perforin/granzyme pathway or the Fas/FasL ligand system seems to be involved in the main pathways of cytotoxicity mediated by CD4+ and CD8+ T cells.Citation6 Besides, non-specific mechanisms including mast cell degranulation and protease activation are involved in the pathogenesis of LP.Citation7 All these mechanisms may combine to induce the keratinocyte apoptosis. Despite the extensive investigation, the mechanism of the inflammatory response in LP has not been clarified.
The Wingless-type mouse mammary tumor virus (MMTV) integration site (Wnt) signaling is a large family of cysteine-rich secreted glycoproteins, and it plays an important role in the development and physiological processes of many tissues.Citation8 As a key factor in the non-classical Wnt pathways, wingless-type MMTV integration site family member 5a (Wnt5a) regulates the inflammatory response and is important in linking inflammation to metabolism.Citation9,Citation10
In CLP, our group and other research findings showed that Wnt5a is upregulated in skin lesions.Citation11,Citation12 Besides, the risk of dyslipidemia and metabolic syndrome is higher in CLP patients.Citation13,Citation14 Based on the above research findings, we speculate that Wnt5a may increase in circulation of patients with CLP. The present study was planned to evaluate whether Wnt5a is altered in the serum of patients with CLP.
Materials and Methods
Subject
We recruited CLP patients, who attended the outpatient Department of Dermatology. Patients with CLP accompanied by typical clinical manifestations and confirmed diagnosed by histopathology. Normal healthy adults (from the Medical Examination Center) as control group. Inclusion criteria including: 1. Both patients and normal healthy adults (men or women) aged older than 18 years. 2. There were no history of hypertension, diabetes and other chronic diseases when patients and healthy adults were included. 3. All recruited as research patients or healthy adults were not administered with any systemic drugs within 2 months, and topical drugs in the previous 2 weeks. Exclusion criteria including: 1. Patients with CLP who received systematic treatment and were not confirmed by histopathology and healthy volunteers who refused to participate in the study were not included. 2. Other subtypes of CLP (vesiculobullous, actinic, atrophic, pigmented, follicular subtypes). 3. Age under 18 years old. 4. Other related inflammatory or immune mediated conditions that may affect serum Wnt5a concentration (such as hypertension, diabetes, psoriasis, alopecia areata or rheumatoid arthritis). Age, sex, height and weight were recorded using a standardized questionnaire. Body mass index (BMI) was calculated as weight divided by the square of height and expressed in kg/m2. For patients and controls, BMI between 20 and 25 was defined as lean, BMI between >25 and <30 defined as overweight and BMI between 30 and 40 was represented as obese. Written informed consent was obtained from all participants. All experimental protocols were approved by the Institutional Review Board of the Xi’an Jiaotong University, and performed according to guidelines governing ethics’ care in China. This study was performed in accordance with the rules laid down in the Declaration of Helsinki.
Analysis the Serum Concentration of Wnt5a Using Enzyme-Linked Immunosorbent Assays (ELISA)
A total of 5mL peripheral blood was extracted from all patients and controls and the serum was separated. The separated serum was stored at −80°C until all samples were collected. The level of Wnt5a was measured by using ELISA-based (Enzyme-linked Immunosorbent Assay) assays kit (Wnt5a: Uscn Life Science Inc., Wuhan, PRC; number SEP549Hu). The assay procedures were carried out according to the instructions of the kit. The results were detected using Microplate Reader at a wavelength of 450 nm.
Statistical Analysis
Data were stored and analyzed using SPSS (version 18.0, SPSS Inc., Chicago, IL, USA) software. Unpaired T-test and Chi-square test were used to analyze the differences. The results were presented as mean±standard deviation, P<0.05 was regarded as statistically significant.
Results
The Characteristics of the Participants
A total of 46 patients with CLP were enrolled (15 males and 31 females, age range 21–63 years, mean age was 48.95±9.43 years). As shown in , stratified for BMI, there were 18 patients and 20 healthy volunteers defined as lean, with a BMI between 20 and 25, 18 patients and 18 healthy volunteers represented as obese with a BMI between 30 and 40. There was no significant difference between the patients and the control groups on gender distribution, mean age and BMI (all P-value >0.05).
Table 1 Demographic Data of Patients with CLP and Healthy Volunteers
The Expression of Serum Wnt5a in Patients with CLP
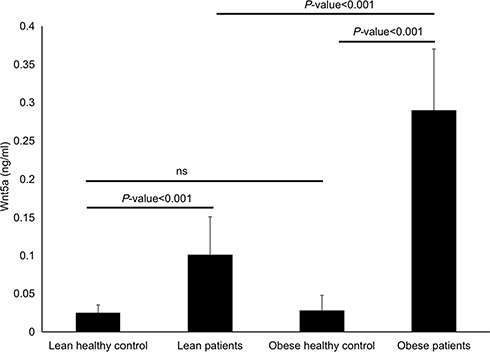
As shown in , serum Wnt5a was significantly higher in lean patients with CLP compared to lean healthy volunteers (0.101±0.05ng/mL vs 0.025±0.01ng/mL, P-value <0.001), also higher in obese patients with CLP compared to obese control group (0.291±0.08 ng/mL vs 0.028±0.012 ng/mL, P-value <0.001). Furthermore, obese patients with CLP had significantly higher serum levels of Wnt5a compared with the lean CLP patients (P-value <0.001). However, the difference between lean and obese healthy volunteers was not statistically significant (P-value >0.05).
The Expression of Serum Wnt5a in Patients with CLP Based on BMI Stratification
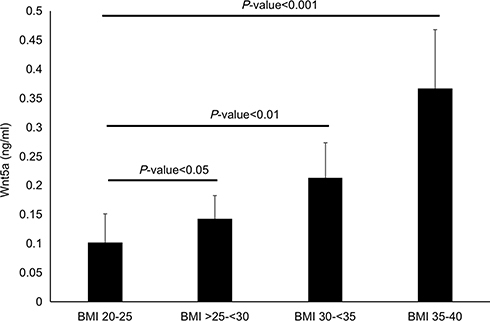
Based on the BMI, 46 patients was divided into lean group (BMI 20–25, n = 18), overweight group (BMI >25–<30, n = 10), obese grade I (BMI 30–<35, n = 10) and obese grade II (BMI 35–40, n = 8). As shown in , with the increase of BMI score, the serum concentration of Wnt5a also increased gradually (all P-value <0.05). The Wnt5a serum concentration of obese grade II patients was highest, which was statistically different compared with the lean group (0.367±0.101 ng/mL vs 0.101±0.05 ng/mL, P-value <0.001).
Discussion
In our study, the mean age of CLP patients was 48.95 years and was consistent with the literature, that LP often affected middle age.Citation15 Consistent with previous research finding,Citation15 we found more female (67.39%) cases in this research, and the ratio of men to women was 1:2.1. Although the exact etiology and pathophysiology is unclear, immune dysregulation was considered to involve in the pathogenesis of CLP. Activated T cells, principally cytotoxic CD8+ cells assisted by CD4+ cells, launch proinflammatory and promote cell-mediated cytotoxicity on keratinocytes.Citation16 Apoptotic keratinocytes are an important histopathological feature in LP, which appear as colloids. After binding of antigen to Major Histocompatibility Complex class 1 (MHC-1) in keratinocytes, then activate CD8+ cytotoxic T cell directly to induce the apoptotic of keratinocytes.Citation17 It has been proved that the expression of various factors were up-regulated or down regulated in the process of inflammation in LP.Citation17 In addition to being associated with chronic inflammatory diseases, cytokines such as tumor necrosis factor alpha (TNF-α), Interleukin 6 (IL-6), IL-10 and IL-4 were involved in the pathogenesis of the LP, so LP patients have an increased risk of complications.Citation18 In addition to skin involvement, LP can have some complications, including type I and type II diabetes, metabolic syndrome and coronary heart disease.Citation13,Citation14 Higher risk of dyslipidemia has been proved to be a common complication in patients with LP.Citation13,Citation19
The Wnt5a pathway plays an important role in the pathogenesis of various inflammatory diseases. After treatment with Wnt5a, the expression levels of inflammatory markers, including interferon gamma (IFN-γ), IL-8 and IL-17A were upregulated in keratinocytes.Citation20 Several cytokines, including Wnt5a, IL-17, IFN-γ and TNF-a, play critical roles in the initiation, maintenance of inflammatory in CLP.Citation11,Citation21,Citation22 Wnt5a can increase the activity of nuclear factor kB (NF-κB) and subsequently induce the occurrence of inflammatory diseases.Citation23 NF-κB shown to be upregulated and is considered to play a major role in the recruitment of inflammatory cells in LP.Citation24 After translocation in keratinocytes, NF-κB may induce the production of inflammatory cytokines and chemokines, as well as the expression of adhesion molecules in LP.Citation24
Our previous study found that Wnt5a mRNA and protein were significantly expressed in CLP lesions.Citation11 In the present study, we further confirmed the high expression of Wnt5a in serum of patients with CLP, and the concentration of Wnt5a was increased with the BMI index. This finding is consistent with its expression in the serum of psoriasis patients.Citation25 Both psoriasis and LP have similarities in their immune-pathophysiology, and both are T cell-mediated autoimmune skin diseases.Citation26
There is a strong correlation between BMI and the components of metabolic syndrome.Citation27 Obesity is strongly associated with comorbidities such as insulin resistance, type 2 diabetes and cardiovascular disease.Citation28 Whereas some studies found Wnt5a was increased in obesity, others could not confirm these observations.Citation29 Consistent with the findings of Gerdes,Citation25 our study found that Wnt5a serum concentrations not increased in even severe obesity healthy volunteers. The possible reason may be the number of healthy volunteers in control group was small. What is more, because of the elevated Wnt5a level in CLP patient lesions,Citation11,Citation12 we hypothesized that the expression of Wnt5a in CLP lesions will subsequently lead to an increase in circulating Wnt5a, which partly explains why patients with CLP may develop metabolic diseases. The serum concentration of Wnt5a was increased with BMI. Therefore, obese patients with CLP may have a greater risk of comorbidity. Wnt5a plays an important role in the linking between obesity inflammation and metabolism, and involved in controlling the process of adipose tissue expansion and inflammation, and thereby represents a new target to prevent and/or treat Wnt5a-mediated inflammatory diseases.Citation10 So we suggest paying close attention and applying early protective measures to prevent the development of metabolic comorbidity in patients with CLP.
This study also has some limitations: we failed to analyze the relationship between the expression of serum Wnt5a and the disease severity of the CLP. It would be better further experimental to verify that the disease severity of CLP could be correlated positively with the expression of Wnt5a. We did not detect the expression of the anti-inflammatory secreted frizzled-related protein 5 (SFRP5), a soluble Wnt5a receptor, always secreted by adipocytes from healthy subjects, and together with Wnt5a involved in the occurrence and development of obesity and inflammatory diseases.Citation29,Citation30
Conclusion
In conclusion, serum Wnt5a was elevated in patients with CLP and was associated with BMI. Our study suggests that Wnt5a may play a role in the pathophysiology of CLP and may contribute to the development of metabolic comorbidity in CLP patients. Further studies are needed to confirm our results, and to better understand the interaction between Wnt5a signaling pathway and obesity in the pathogenesis of CLP.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79(5):789–804. doi:10.1016/j.jaad.2018.02.010
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366(8):723–732. doi:10.1056/NEJMcp1103641
- Weedon D. The lichenoid reaction pattern (interface dermatitis). In: Weedon’s Skin Pathology. 3rd ed. Edinburgh: Elsevier Limited; 2010:35.
- Boch K, Langan EA, Kridin K, et al. Lichen Planus. Front Med. 2021;8:737813. doi:10.3389/fmed.2021.737813
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142(3):449–456. doi:10.1046/j.1365-2133.2000.03355.x
- Kastelan M, Prpić Massari L, Gruber F, et al. The role of perforin-mediated apoptosis in lichen planus lesions. Arch Dermatol Res. 2004;296(5):226–230. doi:10.1007/s00403-004-0512-1
- Kitkhajornkiat A, Rungsiyanont S, Talungchit S, et al. The expression of Cathepsin L in oral lichen planus. J Oral Biol Craniofac Res. 2020;10(3):281–286. doi:10.1016/j.jobcr.2020.06.003
- Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
- Kumawat K, Gosens R. WNT-5A: signaling and functions in health and disease. Cell Mol Life Sci. 2016;73(3):567–587. doi:10.1007/s00018-015-2076-y
- Relling I, Akcay G, Fangmann D, et al. Role of wnt5a in metabolic inflammation in humans. J Clin Endocrinol Metab. 2018;103(11):4253–4264. doi:10.1210/jc.2018-01007
- Zhang Y, Zhang D, Tu C, et al. Wnt5a is involved in the pathogenesis of cutaneous lichen planus. Clin Exp Dermatol. 2015;40(6):659–664. doi:10.1111/ced.12561
- Kumaran MS, Bishnoi A, Srivastava N, et al. Significant reduction in the expression of interleukins-17A, 22 and 23A, forkhead box p3 and interferon gamma delineates lichen planus pigmentosus from lichen planus. Arch Dermatol Res. 2019;311(7):519–527. doi:10.1007/s00403-019-01926-9
- Lai YC, Yew YW, Schwartz RA. Lichen planus and dyslipidemia: a systematic review and meta-analysis of observational studies. Int J Dermatol. 2016;55(5):295–304. doi:10.1111/ijd.13234
- Daye M, Temiz SA, Isık B. The relationship between lichen planus and metabolic syndrome. J Cosmet Dermatol. 2021;20(8):2635–2639. doi:10.1111/jocd.13905
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48(7):682–694. doi:10.1111/j.1365-4632.2009.04062.x
- Sezer E, Ozugurlu F, Ozyurt H, et al. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32(4):430–434. doi:10.1111/j.1365-2230.2007.02436.x
- Atas H, Cemil BC, Kurmus GI, et al. Assessment of systemic inflammation with neutrophil-lymphocyte ratio in lichen planus. Postepy Dermatol Alergol. 2016;33(3):188–192. doi:10.5114/pdia.2016.56930
- Arias-Santiago S, Buendia-Eisman A, Aneiros-Fernandez J, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. 2011;25(12):1398–1401. doi:10.1111/j.1468-3083.2011.03983.x
- Ozbagcivan O, Akarsu S, Semiz F, et al. Comparison of serum lipid parameters between patients with classic cutaneous lichen planus and oral lichen planus. Clin Oral Investig. 2020;24(2):719–725. doi:10.1007/s00784-019-02961-6
- Wang W, Yu X, Wu C, et al. Differential effects of Wnt5a on the proliferation, differentiation and inflammatory response of keratinocytes. Mol Med Rep. 2018;17(3):4043–4048. doi:10.3892/mmr.2017.8358
- Żychowska M, Batycka-Baran A, Baran W. Increased serum level and high tissue immunoexpression of interleukin 17 in cutaneous Lichen planus: a novel therapeutic target for recalcitrant cases? Dis Markers. 2020;2020:6521274. doi:10.1155/2020/6521274
- Weber B, Schlapbach C, Stuck M, et al. Distinct interferon-gamma and interleukin-9 expression in cutaneous and oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31(5):880–886. doi:10.1111/jdv.13989
- Pashirzad M, Shafiee M, Rahmani F, et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. J Cell Physiol. 2017;232(7):1611–1616. doi:10.1002/jcp.25687
- Santoro A, Majorana A, Bardellini E, et al. NF-kappaB expression in oral and cutaneous lichen planus. J Pathol. 2003;201(3):466–472. doi:10.1002/path.1423
- Gerdes S, Laudes M, Neumann K, et al. Wnt5a: a potential factor linking psoriasis to metabolic complications. Exp Dermatol. 2014;23(6):438–440. doi:10.1111/exd.12413
- Aghamajidi A, Raoufi E, Parsamanesh G, et al. The attentive focus on T cell-mediated autoimmune pathogenesis of psoriasis, lichen planus and vitiligo. Scand J Immunol. 2021;93(4):e13000. doi:10.1111/sji.13000
- Al-Bachir M, Bakir MA. Predictive value of body mass index to metabolic syndrome risk factors in Syrian adolescents. J Med Case Rep. 2017;11(1):170. doi:10.1186/s13256-017-1315-2
- Iyer A, Fairlie DP, Prins JB, et al. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6(2):71–82. doi:10.1038/nrendo.2009.264
- Koutaki D, Michos A, Bacopoulou F, et al. The emerging role of Sfrp5 and Wnt5a in the pathogenesis of obesity: implications for a healthy diet and lifestyle. Nutrients. 2021;13(7):2459. doi:10.3390/nu13072459
- Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454–457. doi:10.1126/science.1188280