Abstract
Objective
The present study was designed to evaluate the possible gastroprotective effects of different doses of azilsartan in ethanol-induced gastric ulcers in rats.
Methodology
Forty-eight male adult Wistar rats were used and allocated randomly into four groups: negative control treated with distilled water, positive control treated with ethanol, lansoprazole treated group, and azilsartan (1mg, 5mg, and 10mg/kg) treated group. The treatment protocol was for 15 days, and all the groups except for the negative control group received 1mL of ethanol on the last day 1hr before scarification. Gastric content was collected for measuring the volume, free acidity, and pH. The stomach was used for measuring the gastric lesion area and ulcer index. Blood samples were collected for measuring serum hydroxyproline, gastrin, CRP, TNF-α, MDA, and TAOC. Gastric tissues were sent for histopathological examinations.
Results
Ethanol administration significantly increased gastric lesion, gastric ulcer index, and gastric acidity. Ethanol also decreased serum levels of hydroxyproline and TAOC and increased serum gastrin, CRP, TNF-α, and MDA. Azilsartan 10mg/kg was able to decrease the lesion by 43.6% and increase gastric pH and significantly decreased MDA level. Both 5mg/kg and 10mg/kg azilsartan have successfully restored the level of hydroxyproline, gastrin, and TNF-α. The histopathological finding showed gastroprotection by azilsartan in a dose-dependent manner.
Conclusion
The study revealed that azilsartan possesses a gastroprotective effect. The proposed mechanisms could be increased blood flow to the stomach, antioxidant capacity, and anti-inflammatory activity along with restoring hydroxyproline and gastrin levels. These findings suggest azilsartan as a promising candidate to be tested in a clinical setting.
Introduction
Gastric ulceration is a lesion on the mucosal layer that covers the stomach. Many contributing factors may precipitate gastric ulcers such as exposure to excess acid, smoking, alcohol, H. pylori infection, ischemia, hypoxia, and NSAIDs.Citation1,Citation2 The stomach has been equipped with defense mechanisms that protect against these insults. Cytoprotection by prostaglandins causes stomach cells to release some products that function as a mucosal shield. Such a shield is so powerful that it protects against harm caused by strong chemicals as well as physical burns caused by boiling water.Citation3 Recently, the involvement of growth factors has been proved in accelerating ulcer healing through enhancing angiogenesis and mucosal regeneration.Citation4 Since the disease is multifactorial; the pathophysiology differs depending on the causative agent.Citation5 Generally, there is a balance between gastric irritants and defense mechanisms.Citation6 Acute or chronic exposure to irritants may lead to gastric lesions when the gut defense mechanisms fail to counteract the action of these insults. Alcohol is one of the agents that target the stomach affecting the digestion and absorption capacity of the gut.Citation7,Citation8 Although the exact mechanism through which the alcohol-induced gastric damage is still unknown; many proposed mechanisms may explain such toxicity.Citation9,Citation10 In the development of ethanol-induced gastric hemorrhagic erosions, vascular damage is an early pathogenetic component.Citation11 Gastric mucous membrane damage, generation of reactive species, and inflammatory reaction are among the most acceptable mechanisms of ethanol-induced gastric ulcers. The injury caused by ethanol enhances the recruitment of inflammatory cells such as neutrophils and these activated neutrophils can trigger the release of reactive species to the site of injury.Citation12,Citation13
The renin-angiotensin system (RAS) plays a vital role in regulating fluid and electrolyte homeostasis and systemic vascular resistance.Citation14 This system is found in many organs including the kidneys, heart, brain, and the GIT.Citation15 The presence of RAS and their local activity in the GIT have received pronounce attention lately; where many studies showed the involvement of local RAS in the gut in glycemic control and electrolyte regulation, besides having roles in carcinogenesis and inflammatory reactions.Citation16 Studies showed that blocking angiotensin II receptor AT1 protect against gastric ulcer,Citation17,Citation18 since angiotensin II can generate reactive oxygen species with inducing inflammatory reaction and cellular damage. These effects are mainly mediated through mucosal vasoconstriction.Citation19 When compared to other ARBs, azilsartan is closely linked to candesartan and has a higher potency and longer duration of effect. Azilsartan has an oxo-oxadiazole ring that is absent from other clinically authorized ARBs, making it less acidic and more lipophilic than other ARBs.Citation20 Studies showed the superiority of azilsartan as antihypertensive when compared with other ARBs.Citation21,Citation22 Some ARBs proven to have gastroprotective effects, however, the involvement of RAS in gastric homeostasis and protection still needed to be illuminated clearly. Accordingly, the present study was designed to evaluate the possible gastroprotective effects of different doses of azilsartan in ethanol-induced gastric ulcers in rats.
Materials and Methods
Forty-eight male adult Wistar rats, weighing 180–230 g, were purchased from the animal house of the University of Sulaimani and kept in well-ventilated plastic cages, at an ambient temperature of 25 ± 2°C and humidity of 55 ± 5% under 12 hrs dark-light cycle. The rats were fed standard laboratory chow with water and libitum. The animals were kept for one week before the experiment for acclimatization. The experimental protocols met the Guidelines for Animal Experimentation and approved by the Ethical Committee of the University of Sulaimani, College of Pharmacy (Certificate no. PH34-21 on 20th October 2021) following the institutional Animal Ethics Committee. The study was performed by the Canadian Council on Animal Care (CCAC) guidelines, 1998. All animals were randomly divided into six groups; the doses and the route of administrations of ethanol and each treated group have been chosen depending on the previous studies, respectively.Citation23,Citation24
The groups comprise eight rats each as follows:
Negative Control group: Treated with 1 mL of distilled water orally by gavage tube for 15 days.
Positive Control group: Treated with 1 mL of distilled water orally by gavage tube for 14 days then on day 15 two hours after administration of sodium chloride the animals received 1mL of 80% ethanol orally.
Lansoprazole treated group: Treated with lansoprazole 30 mg/kg given orally by gavage tube daily for 14 days with the ethanol treatment protocol.
Azilsartan treated group (24 animals 8 for each dose): Treated with either 1mg/kg, or 5mg/kg or 10mg/kg orally by gavage tube daily for 14 days with the ethanol treatment protocol.
On day 14 all the animals fasted for 24 hours with only free access to water. On day 15 two hours after administration of the treatment, they all received 1ml of 80% ethanol orally except for negative control group and the scarification was done 1 hour after the administration of ethanol.
Assessment of Gastric Mucosal Lesions
The stomachs were immediately removed, opened along the greater curvature and washed with saline solution (0.9% NaCl) and fixed on a cork plate, and areas of gastric hemorrhagic lesions were measured and calculated by planimetry using the Image Processing and Analysis in Java (ImageJ) by the National Institutes of Health (NIH, USA); ulcer index (UI) and percent of inhibition of UI in relation to the control group were estimated using the following equations:Citation25
UI = {ulcerated or hemorrhagic area (mm2) / total area of the stomach (mm2)}
The percentage of ulcer inhibition was calculated as below:
% I (Inhibition of Ulceration) = (Ulcer index Control - Ulcer index Test) × 100/Ulcer index Control
Measurement of Gastric pH and Volume
Samples of gastric contents were drained into a graduated tube and the volume of the juice was measured.Citation26 Then, the gastric juice was centrifuged (4000 rpm/10 min) and the volume of the supernatant was measured. Then, 1mL of supernatant was used for measuring the pH using a digital pH meter.Citation27
Determination of Total Acidity
The gastric juice was titrated with 0.1 N NaOH, then add two drops of Methyl orange reagent, which changes to a salmon color when the total amount of free hydrochloric acid was neutralized. For the determination of total acidity, phenolphthalein was used as an indicator. Methyl orange reagent (1–2 drops) was added to the gastric juice. A bright red color appears due to the presence of free HCl. Titration was with 0.1 NaOH from a burette, until the color changed to a canary yellow color. The numbers of milliliters of NaOH used were read from the burette. This represents the amount of free hydrochloric acid. 1–2 drops of phenolphthalein were added to the gastric juice. The titration was continued until the red color of phenolphthalein appeared (deep pink), titrated to the point at which the further addition of alkali did not deepen the color. Reading was taken (mL NaOH) for total acidity.Citation26
Y = 0.1 N NaOH (mL) X 10
Where, Y= Total acidity (m Eq/L)
Biochemical Parameters
Blood samples were collected by cardiac puncture at the end of the study on day 15; the blood centrifuged and the sera were separated and used for the assessment of serum gastrin, hydroxyproline, TNF-α, MDA, and total antioxidant capacity using ELISA kit (Bioassay technology laboratory, UK) according to the manufacturer’s instructions. C-reactive protein (CRP) was evaluated using an enzyme-linked immunosorbent assay kit (Elabscience, Houston, TX, USA) according to the manufacturer’s instructions.
Histotechnique Procedure
Initially, animals were fasted for at least 24 hours prior to sacrificing and then euthanized in a humane practice. Successively, after animal sacrificing necropsy started by collecting tissue samples first photographed then fixed in buffered formalin for histological preparation. Stomach sections were stained with Harris’s hematoxylin and eosin and then viewed under a bright field light microscope. In brief, gastric tissue samples were collected after scarification then fixed with 10% neutral buffered formaldehyde solution for about 48–72 hours. Thereafter, sections were aligned and immobilized in plastic tissue cassettes then dehydrated via passing through a series of ascending ethanol alcohol (50%, 60%, 70%, 90%, and 100%), followed by two to three steps of xylene clearance. Next, the processed gastric tissues were infiltrated and embedded in melted paraffin blocks using an automated wax embedder at (60 −70ᵒC). Paraffinized tissues were sectioned to 5 µm using a semi-automated rotary microtome. After that, tissue sections were fixed on glass slides and dried. Later on, the tissue sections were deparaffinized and cleaned with xylene solution for 30 minutes then dried with a hot oven at 50ᵒC for 5 minutes. Finally, tissue sections were stained with Harris’s hematoxylin and eosin solution, cleaned with xylene, and cover slipped.
Semi-Quantitative Lesion Scoring
Lesion scoring was estimated semi-quantitatively via image analyzer software (AmScope, 3.7) using a microscope eyepiece camera (MD500, 2019), and tissue samples were analyzed under the light microscope (NOVEL XSZ-N107T, China). In general, the gastric mucosal erosion-surface area was estimated and measured in percentage of calculated erosional depth and length from randomly selected different fields in µm, whereas areas of edema and inflammatory exudate were assessed in µm and statistically evaluated as a mean percentage. While inflammatory cells and necrotic debris were counted in randomly chosen ten fields under high power magnification (100X), then the mean average was calculated statistically in percentage. Moreover, the area of hemorrhage was evaluated using randomly selected four different tissue sections, then the mean average is statistically measured in percentage of µm. Finally, the mean percentage of all calculated values were expressed as the following lesion scoring and grading protocols (score 0–10% as no lesions; score 10–25% as mild; score 25–75% as moderate; score 75–100% as severe lesions).
Statistical Analysis
The statistical analysis was performed using GraphPad Prism 8. The values of the measured parameters were expressed as mean ± standard deviation (S.D.). For the comparisons between different groups, one-way analysis of variance (ANOVA), followed by Tukey multiple comparison tests were used. Unpaired t-tests were used to compare each group with the positive control group. The results were considered statistically significant when the p-value was less than 0.05.
Results
Effect of Different Doses of Azilsartan on Gastric Lesion Area, Gastric Lesion Index, and Percentage of Lesion Inhibition
shows a significant (P-value < 0.0001) decrease in gastric lesion area produced by lansoprazole. Azilsartan also showed a significant reduction in the gastric lesion and in a dose-dependent manner when compared to the positive control-treated group with the maximum effect produced by 10mg/kg (p-value < 0.0001) which was comparable with the protection achieved by lansoprazole. Gastric lesion index also revealed a significant decrease in both 5mg and 10mg/kg azilsartan with maximum effect achieved by the highest dose, which was comparable with that produced by lansoprazole. The use of 10mg/kg azilsartan was able to inhibit the ulcer by (43.62%) which was still less than that produced by lansoprazole (62.96%).
Table 1 Effect of Different Doses of Azilsartan on Gastric Lesion Area, Gastric Lesion Index and Percentage of Lesion Inhibition
Effect of Different Doses of Azilsartan on Gastric Juice Parameters
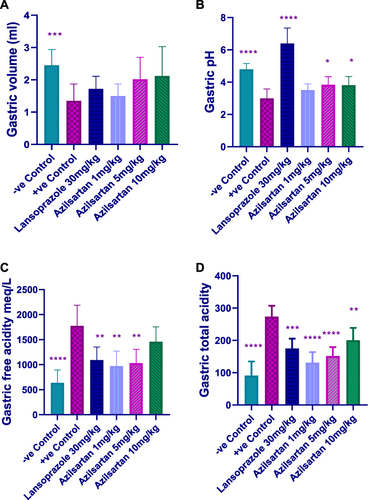
shows the effect of graded doses of azilsartan on the gastric juice in the rat model of ethanol-induced gastric ulcer. Azilsartan at doses (1 mg/kg, 5 mg/kg, and 10mg/kg) and lansoprazole showed no significant change in gastric volume when compared to ethanol-treated (positive control) group. The pH of the gastric juice revealed a significant increase in the negative control group, lansoprazole-treated group and azilsartan (5mg/kg) and (10mg/kg) compared to positive control group; (P- value < 0.0001), (P- value < 0.0001), (P- value = 0.013) and (P- value = 0.02) respectively (). A significant reduction also seen in the free and total acidity the negative control group (p-value < 0.0001), lansoprazole-treated group, (P- value = 0.007) and (P- value = 0.0003) and azilsartan (1mg/kg), (P- value = 0.0016) and (P- value < 0.0001), (5mg/kg), (P- value = 0.002) and (P- value < 0.0001), respectively, and (10mg/kg) in the total acidity only (p-value = 0.02) as compared to ethanol-treated group alone ( and ).
Figure 1 Effect of different doses of azilsartan on (A) the gastric volume, (B) gastric pH, (C) gastric free acidity and (D) gastric total acidity; *(p < 0.05), **(p < 0.01), ***(p < 0.001) and ****(p < 0.0001) significantly different compared to the positive control group using one-way ANOVA and unpaired t-test.
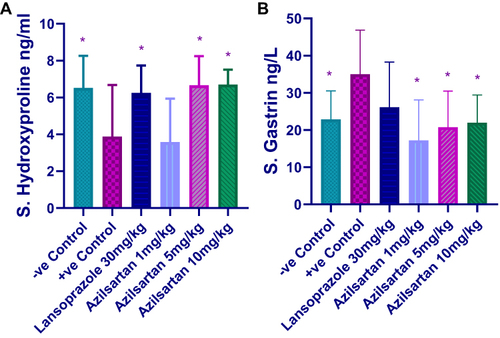
Effect of Different Doses of Azilsartan on Serum Level of Hydroxyproline and Gastrin
The results of the current study showed that both 5mg and 10mg/kg of azilsartan produced a significant reduction in the level of hydroxyproline (P-value = 0.032) compared to the ethanol-treated group. This result was comparable to that produced by lansoprazole and negative control groups (P-value = 0.046) and (P-value = 0.033), respectively, compared to the positive control group (). Regarding the effect on serum gastrin; significant reduction has been achieved by the three doses of azilsartan (1mg/kg, P-value = 0.014), (5mg/kg, P-value = 0.006) and (10mg/kg, P-value = 0.008). Lansoprazole also resulted in a significant decrease in gastrin level (P-value = 0.005) compared to the positive control group ().
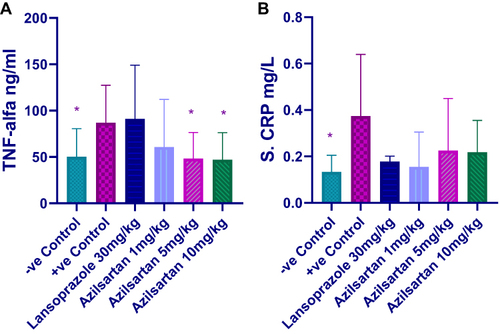
Effect of Different Doses of Azilsartan on Serum Level of Inflammatory Markers
Ethanol treated group revealed a significant increase in the serum level of TNF-α compared to the negative control group. Both 5mg and 10mg/kg azilsartan produced significant reduction in the level of TNF-α (P-value = 0.019), and (P-value = 0.04), respectively, in comparison to positive control group (). The level of CRP significantly increased in the positive control group; all the treatment protocols decreased its level but they were statistically non-significant ().
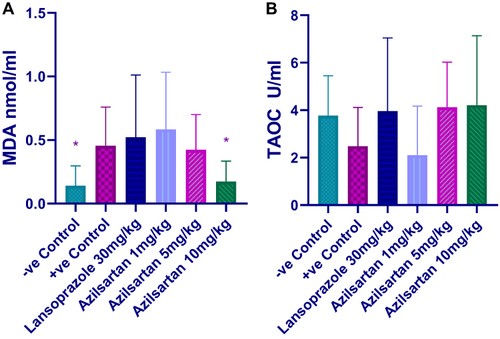
Effect of Different Doses of Azilsartan on Serum Lipid Peroxidation and Antioxidant Capacity
In the present study, MDA level significantly increased by ethanol compared to the negative control group and only 10mg/kg azilsartan was able to significantly decrease the level (P-value = 0.04) compared to the ethanol-treated group (). Total antioxidant capacity decreased by the use of ethanol and was restored by lansoprazole, and both 5mg and 10mg/kg azilsartan, however, the change in all groups did not reach a significant level ().
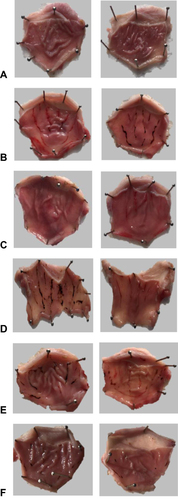
Gross Evaluation of Gastric Lesions
Macroscopic examination demonstrates a gastroprotective effect of azilsartan and in a dose-dependent manner. represents the images of the six-treated groups. The antiulcer activity of lansoprazole () and azilsartan 10mg/kg () in ethanol-induced gastric is clearly observed when compared with the positive control group ().
Histopathological Findings
As an overall concept, the semi-quantitative morphometric calculation and lesion scoring of gastric sections were illustrated in (). Remarkably, oral treatment with azilsartan 10mg/kg as a daily regimen dose for 15 days revealed significant P<0.05 mitigation in the number of inflammatory exudates together with a significant reduction in the percentage of necrotic cellular debris as well as inflammatory cells infiltration and overall lesion severity. On the other hand, depth, length, and the area of gastric erosion, as well as the area of hemorrhage, were significantly reduced in lansoprazole 30mg/kg along with azilsartan 10mg/kg treated groups in comparison to morphological changes in the positive control group G2 as demonstrated clearly in and (G2, G3, and G6). However, gastric sections from animals treated with azilsartan in medium dose 5 mg/kg and low dose 1 mg/kg as in G5 and G4 groups, show clear alleviation in the severity of mucosal lesions in a dose-dependent manner, which was more significant in G5 than in G4, even though still not significant as the treatment in G3 and G6. Additionally, tissue sections from all treatment groups showed significant improvement in lesion scoring in comparison with the non-treated control positive group (G2) as shown clearly in . Yet, this improvement is a dose-dependent manner, and it is more significant in G3 30 mg/kg of lansoprazole and G6 10 mg/kg of azilsartan, respectively.
Table 2 Histological Quantitative Evaluation of Gastric Tissue Sections
Figure 6 Photomicrograph of gastric tissue from groups; (G1) Shows no obvious lesions, represented by intact and typically arranged gastric layers started with the mucosa (M) with normally appearing gastric glands (G), then a loose connective tissue of submucosa (SM), two layers of smooth muscle representing the muscularis externa (ME), and finally the serosa (S) which consist of adipose tissue. (G2) Reveal significant area of mucosal erosion (ER) together with the presence of hemorrhage (HR) mixed with necrotic tissue, and diffusely distributed inflammatory exudates (yellow arrows) throughout the mucosal (M) erosion and the submucosa which contains also a pinkish edematous fluid (ED). (G3) Elucidate the presence of light pinkish edema (ED) within the submucosa mixed with inflammatory exudates (yellow arrows). The inflammatory exudates can also be seen among the gastric glands (G) which show a regenerative capacity in the area of gastric mucosal erosion (ER). (G4) Show pinkish-proteinaceous inflammatory exudates (yellow arrows) mixed with mucosal necrotic debris (ND) in the area of mucosal erosion (ER). The inflammatory exudates are also distributed among the gastric glands, together with the presence of submucosal (S) inflammatory edema (ED). (G5) Display slight mucosal necrotic debris (yellow arrows) in the area of gastric erosion (ER) mixed with some inflammatory cells (IF). Regeneration of some damaged gastric glands (G) and some edema with a scant number of inflammatory cells in the submucosa (S). (G6) show clear regeneration of mucosal gastric glands (G), reduction in the area of mucosal erosion (ER) together with few inflammatory cell infiltration (yellow arrows), and pinkish inflammatory edema (ED) can be noted clearly within the submucosal layer. H&E. Scale bar: 4 mm.
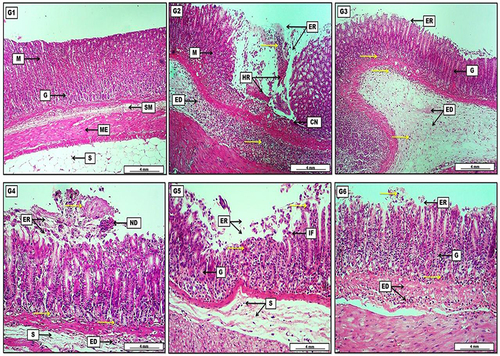
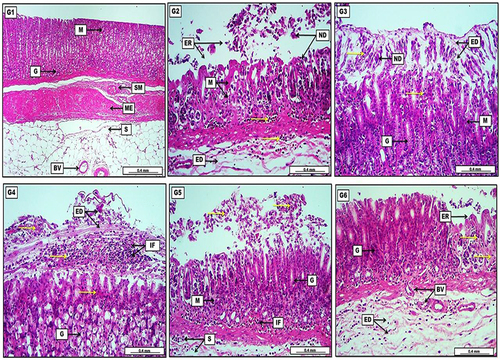
Figure 7 Photomicrograph of gastric tissue from groups; (G1) Reveal no prominent lesions with intact mucosal layers, evident by typical gastric glands (G) and normal submucosa (SM). Muscularis Externa (ME) and the serosa (S) display no significant lesions with the presence of slightly congested blood vessels (BV). (G2) Show significant and severe mucosal destruction, evident by the presence of much cellular necrotic debris (ND) within the mucosal (M) damage area or erosion (ER), together with inflammatory cells (yellow arrows), in addition to edema in the submucosa (ED) with inflammatory exudates. (G3) Display the presence of light eosinophilic edematous fluid (ED) mixed with necrotic debris (ND) and many inflammatory cells (yellow arrows) which cover the mucosal layer (M). The section also reveals moderate glandular regeneration (G) evident by increasing their depth within the lamina propria. (G4) Reveal significant infiltration of inflammatory cells (IF) mixed with rose-tinged edema (ED) and many areas of inflammatory exudates (yellow arrows). The section shows many affected gastric glandular acinar cells (G) with eosinophilic cytoplasm and condensed nuclei. (G5) Display moderate and obvious sloughed necrotic cellular debris (yellow arrows) in the lining epithelium of the mucosa (M), together with typical regenerative gastric gland (G). The section also reveals the presence of infiltrated inflammatory cells within the mucosal (M) and submucosal (S) layers. (G6) Show mild to moderate acidophilic inflammatory exudates (yellow arrows) in the areas of mucosal erosion (ER). Gastric glands (G) show a significant regenerative appearance. The section also displays marked angiogenesis as newly formed blood vessels (BV) in the submucosal connective tissue with still existence of light eosinophilic edema (ED) mixed with scattered inflammatory cells. H&E. Scale bar: 0.4 mm.
Discussion
The presence of Angiotensin II receptors in various organs indicates the involvement of the RAS in many physiological activities and drives the researchers to target this system. Additionally, the pleiotropic effects of ARBs render them a good candidate to be tested for various disease models.Citation28 These include antioxidant, anti-inflammatory, peroxisome proliferator-activated receptor gamma (PPAR-γ) activity, and many other poorly known mechanisms.Citation29 Gastric mucosa was formerly known to be protected by prostaglandin only; however, later on, many other mediators were shown to contribute in protecting the GIT such as endothelial growth factor,Citation30 as well as nitric oxide, carbon monoxide, and hydrogen sulfide.Citation31,Citation32 Recently, angiotensin II has been implicated in the pathogenesis of gastric lesions, where, injury to the gastric mucosa is shown to activate the local RAS in the microcirculation of the gastric epithelial cells.Citation29 Blocking the action of angiotensin II through occupying angiotensin I and II receptor proven to play a pivotal role in protecting against ulcerogens.Citation33,Citation34
Azilsartan, is one of the ARBs that possess better antihypertensive activity when compared with the older ARBs which could be mainly attributed to the more potent binding with angiotensin receptor in comparison with the other ARBs.Citation35 Azilsartan also showed a superior effect in lowering blood pressure and protein urea compared to candesartan,Citation36 Furthermore, it has been proven for possessing a reno-protective effect.Citation37,Citation38 All the aforementioned advantages of azilsartan encouraged us to test it in the ulcer model.
To the best of our knowledge, this is the first study of azilsartan in gastric ulcers. In the present study, azilsartan produced a remarkable protection against ethanol in a dose-dependent manner. Administration of ethanol directly contributed to gastric mucosal damage via inducing intragastric bleeding and tissue ischemia.Citation10 The gastric ulcer area and gastric ulcer index have been improved by the use of azilsartan. Additionally, the pH of the stomach significantly increased by the use of 5mg and 10mg/kg azilsartan. The exact mechanism through which azilsartan produces these effects is unclear, however, increasing gastric blood flow along with upregulation of PPAR-γ activity could contribute to the observed outcome.Citation39 Other ARBs like telmisartan also has been proven to have gastroprotective effect when used in stress-induced gastric ulcer.Citation33 Gastric ulcer is thought to occur when there is an imbalance between the causative agent and gastric protective mechanisms. Hydroxyproline level serves as one of the new serum biomarkers of gastric ulcers. During gastric lesion by ethanol; a noticeable decline in serum hydroxyproline occurs as a result of lesion-induced attenuation of the amount of collagen in the GIT.Citation40 Hydroxyproline levels were completely restored by azilsartan (5mg and 10mg/kg) and lansoprazole, which was comparable to the negative control group. Another finding of this study is the attenuation in the level of serum gastrin; where, pretreatment with azilsartan resulted in a significant decrease in gastrin level, while lansoprazole produced no significant change when compared to ethanol-treated group. Serum gastrin usually increases during gastric ulcer or secondary to infection like H. pylori.Citation41 Gastrin is a hormone produced by gastrin cells and has a critical role in regulating cell growth and acid secretion through binding to gastrin receptors.Citation42 Gastrin shown to exert proinflammatory effect in rats,Citation43 via stimulating the release histamine, which mediates acute inflammatory reactions.Citation42 Gastrin may also act on endothelial cells where it seems to modify the expression of adhesion molecules and increase chemokine secretion.Citation44
Another mechanism of ethanol-induced gastric ulcer is mediated through initiating lipid peroxidation and weakening the antioxidant system;Citation45 and this finding is clearly observed in the current study. The highest dose of azilsartan used in this study was successful in inhibiting lipid peroxidation manifested by the normal level of MDA, which was comparable to that of the negative control. Furthermore, total antioxidant capacity is also maintained by azilsartan.Citation46 Additionally, it was able to attenuate the inflammatory markers like CRP and TNF-α and this finding is in tune with other studies.Citation24,Citation47,Citation48 These outcomes could be explained depending on the fact that blocking the effects of angiotensin II is directly related to decreasing the oxidative damage and inflammatory process.Citation49–51 The histopathological findings of the current study were compatible with the biochemical results, where azilsartan showed pronounced protection against ethanol-induced gastric ulcer and in a dose-dependent manner and this was clearly demonstrated as an attenuation in the infiltration of inflammatory cells and reduction in the percentage of necrotic cellular debris. This protective effect could be attributed to accelerating the healing process via enhancing the formation of granulation tissue and anti-inflammatory activity.Citation24
Conclusion
Azilsartan was able to protect the gastric mucosa challenged with ethanol and the highest protection was achieved by 10mg/kg azilsartan. The proposed mechanisms could be increased blood flow to the stomach, antioxidant and anti-inflammatory activity, along with restoring hydroxyproline and gastrin levels. These findings render azilsartan a promising candidate to be tested in a clinical setting.
Disclosure
The authors report no conflicts of interest in this work.
References
- Khushtar M, Siddiqui HH, Dixit RK, Khan MS, Iqbal D, Rahman MA. Amelioration of gastric ulcers using a hydro-alcoholic extract of Triphala in indomethacin-induced Wistar rats. Eur J Integr Med. 2016;8(4):546–551. doi:10.1016/J.EUJIM.2016.01.004
- Scida S, Russo M, Miraglia C, et al. Relationship between helicobacter pylori infection and GERD. Acta Biomed. 2018;89(4):40–43. doi:10.23750/abm.v89i8-S.7918
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats prevention of gastric necrosis produced by alcohol, HCl, NaOH, Hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77(3):433–443. doi:10.1016/0016-5085(79)90002-7
- Tarnawski AS, Ahluwalia A. The critical role of growth factors in gastric ulcer healing: the cellular and molecular mechanisms and potential clinical implications. Cells. 2021;10(8):1964. doi:10.3390/CELLS10081964
- Karampour NS, Arzi A, Rezaie A, Pashmforoosh M, Kordi F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina. 2019;55(3):1–9. doi:10.3390/medicina55030064
- Dimaline R, Varro A. Attack and defence in the gastric epithelium - A delicate balance. Exp Physiol. 2007;92(4):591–601. doi:10.1113/expphysiol.2006.036483
- Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95(12):3374–3382. doi:10.1016/S0002-9270(00)02140-7
- Alimi H, Hfaiedh N, Bouoni Z, Sakly M, Ben Rhouma K. Evaluation of antioxidant and antiulcerogenic activities of Opuntia ficus indica f. inermis flowers extract in rats. Environ Toxicol Pharmacol. 2011;32(3):406–416. doi:10.1016/J.ETAP.2011.08.007
- Cadirci E, Suleyman H, Aksoy H, et al. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem Biol Interact. 2007;170(1):40–48. doi:10.1016/J.CBI.2007.06.040
- Rocco A, Compare D, Angrisani D, Sanduzzi Zamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20(40):14652–14659. doi:10.3748/wjg.v20.i40.14652
- Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88(1 Pt 2):228–236. doi:10.1016/S0016-5085(85)80176-1
- Pan J-S, He S-Z, Xu H-Z, et al. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14(38):5857–5867. doi:10.3748/wjg.14.5857
- Arda-Pirincci P, Bolkent S, Yanardag R. The role of zinc sulfate and metallothionein in protection against ethanol-induced gastric damage in rats. Dig Dis Sci. 2006;51(12):2353–2360. doi:10.1007/S10620-006-9301-3
- Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin-Angiotensin-Aldosterone Syst. 2010;11(1):33–36. doi:10.1177/1470320309347786
- Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747–803. doi:10.1152/physrev.00036.2005
- Jaworska K, Koper M, Ufnal M. Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol. 2021;321(4):G355–G366. doi:10.1152/ajpgi.00099.2021
- Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents gastric ulcers during cold-restraint stress. Ann N Y Acad Sci. 2004;1018:351–355. doi:10.1196/ANNALS.1296.044
- Yamane S, Amano H, Ito Y, et al. The role of Angiotensin II type 1A receptor signaling in gastric ulcer healing. Kitasato Med J. 2019;49:52–63.
- Bregonzio C, Armando I, Ando H, et al. Anti-inflammatory effects of angiotensin II AT 1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol. 2003;285(2):G414–23. doi:10.1152/ajpgi.00058.2003
- Pradhan A, Tiwari A, Sethi R. Azilsartan: current evidence and perspectives in management of hypertension. Int J Hypertens. 2019;2019:1824621. doi:10.1155/2019/1824621
- Sica D, White WB, Weber MA, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens. 2011;13(7):467–472. doi:10.1111/J.1751-7176.2011.00482.X
- Bakris GL, Sica D, Weber M, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens. 2011;13(2):81–88. doi:10.1111/J.1751-7176.2010.00425.X
- Mshelia HS, Karumi Y, Dibal NI. Therapeutic effect of Momordica balsamina leaf extract on ethanol-induced gastric ulcer in Wistar rats. Ann Res Hosp. 2017;1:1. doi:10.21037/ARH.2017.04.03
- De Araujo AA, Varela H, De Medeiros CACX, et al. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS One. 2015;10(2):1–16. doi:10.1371/journal.pone.0116799
- Kim YS, Nam YS, Song J, Kim H. Gastroprotective and healing effects of Polygonum cuspidatum root on experimentally induced gastric ulcers in rats. Nutrients. 2020;12(8):1–15. doi:10.3390/nu12082241
- Rao G, Sujatha D, Rupa V, Priya E, Rao K, Venkateswarlu M. Study on anti-ulcer activity of Daucus carota juice. Res J Pharm Tech. 2010;3(2):547–550.
- Ketuly KA, Abdulla MA, Hadi HA, Mariod AA, Ibrahim S, Wahab A. Anti-ulcer activity of the 9alpha-bromo analogue of Beclomethasone dipropionate against ethanol-induced gastric mucosal injury in rats. J Med Plants Res. 2011;5(4):514–520. doi:10.5897/JMPR.9000114
- Chrysant SG, Chrysant GS. The pleiotropic effects of angiotensin receptor blockers. J Clin Hypertens. 2006;8(4):261–268. doi:10.1111/J.1524-6175.2005.05264.X
- Brzozowski T, Ptak-Belowska A, Kwiecien S, et al. Novel concept in the mechanism of injury and protection of gastric mucosa: role of renin-angiotensin system and active metabolites of angiotensin. Curr Med Chem. 2012;19(1):55–62. doi:10.2174/092986712803413953
- Wozniak-Holecka J, Josko J, Tyrpien M, Kasperczyk J, Steplewska K, Holecki T. Influence of vascular endothelial growth factor (VEGF) on gastroprotection in stress-induced gastric mucosal ulcers in rats. Methods Find Exp Clin Pharmacol. 2009;31(8):523–531. doi:10.1358/MF.2009.31.8.1419716
- Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20(5):9099–9123. doi:10.3390/MOLECULES20059099
- Takeuchi K, Aihara E, Kimura M, Dogishi K, Hara T, Hayashi S. Gas mediators involved in modulating duodenal HCO3- secretion. Curr Med Chem. 2012;19(1):43–54. doi:10.2174/092986712803413962
- Tawfik M. Protective role of telmisartan, candesartan and olmesartan on cold restraint stress -induced gastric ulcer in rats: influence of Ppar Γ Mrna. Al-Azhar J Pharm Sci. 2012;45(1):365–382. doi:10.21608/ajps.2012.7248
- Morsy M, Ashour O, Amin E, Rofaeil R. Gastroprotective effects of telmisartan on experimentally-induced gastric ulcers in rats. Pharmazie. 2009;64(9):590–594.
- Ojima M, Igata H, Tanaka M, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336(3):801–808. doi:10.1124/JPET.110.176636
- Suehiro T, Tsuruya K, Yoshida H, et al. Stronger effect of azilsartan on reduction of proteinuria compared to candesartan in patients with CKD: a randomized crossover trial keywords azilsartan · candesartan · CKD · crossover trial · proteinuria. Res Artic Kidney Blood Press Res. 2021;46:173–184. doi:10.1159/000512365
- Shiga Y, Miura SI, Motozato K, et al. Comparison of efficacy and safety of azilsartan and olmesartan in patients with essential hypertension. Int Heart J. 2017;58(3):416–421. doi:10.1536/IHJ.16-285
- Takami T, Okada S, Saito Y, Nishijima Y, Kobori H, Nishiyama A. Effects of olmesartan and azilsartan on albuminuria and the intrarenal renin-angiotensin system. World J Res Rev. 2018;6(1):7. doi:10.31871/wjrr.6.1.18
- ViJardi R. Effects of azilsartan on PPAR gamma gene expression and oxidative stress in 3T3 L1 cells; 2012.
- Takeuchi K, Ohishi M, Endo K, et al. Hydroxyproline, a serum biomarker candidate for gastric ulcer in rats: a comparison study of metabolic analysis of gastric ulcer models induced by ethanol, stress, and aspirin. Biomark Insights. 2014;9:61–66. doi:10.4137/BMI.S15918
- Eissele R, Brunner G, Simon B, Solcia E, Arnold R. Gastric mucosa during treatment with lansoprazole: helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology. 1997;112(3):707–717. doi:10.1053/GAST.1997.V112.PM9041231
- Álvarez Á, Ibiza MS, Andrade MM, Blas-García A, Calatayud S. Gastric antisecretory drugs induce leukocyte-endothelial cell interactions through gastrin release and activation of CCK-2 receptors. J Pharmacol Exp Ther. 2007;323(1):406–413. doi:10.1124/JPET.107.122754
- Álvarez Á, Ibiza S, Hernández C, et al. Gastrin induces leukocyte‐endothelial cell interactions in vivo and contributes to the inflammation caused by Helicobacter pylori. FASEB J. 2006;20(13):2396–2398. doi:10.1096/FJ.05-5696FJE
- Clarke PA, Dickson JH, Harris JC, Grabowska A, Watson SA. Gastrin enhances the angiogenic potential of endothelial cells via modulation of heparin-binding epidermal-like growth factor. Cancer Res. 2006;66(7):3504–3512. doi:10.1158/0008-5472.CAN-05-0280
- Antonisamy P, Duraipandiyan V, Aravinthan A, et al. Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur J Pharmacol. 2015;750:167–175. doi:10.1016/J.EJPHAR.2015.01.015
- Hou N, Li L-R, Shi Y-Y, et al. Azilsartan ameliorates ventricular hypertrophy in rats suffering from pressure overload-induced cardiac hypertrophy by activating the Keap1–Nrf2 signalling pathway. J Pharm Pharmacol. 2021;73(12):1715–1725. doi:10.1093/JPP/RGAB097
- Aziz TA, Kareem AA, Othman HH, Ahmed ZA. The anti-inflammatory effect of different doses of aliskiren in rat models of inflammation. Drug Des Devel Ther. 2020;14:2841–2851. doi:10.2147/DDDT.S255607
- Dong Q, Li Y, Chen J, Wang N. Azilsartan suppressed LPS-induced inflammation in U937 macrophages through suppressing oxidative stress and inhibiting the TLR2/MyD88 signal pathway. ACS omega. 2020;6(1):113–118. doi:10.1021/acsomega.0c03655
- Pacurari M, Kafoury R, Tchounwou PB, Ndebele K. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam. 2014;2014:689360. doi:10.1155/2014/689360
- Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–374. doi:10.1016/J.TIPS.2008.05.003
- Zablocki D, Sadoshima J. Angiotensin II and oxidative stress in the failing heart. Antioxidants Redox Signal. 2013;19(10):1095–1109. doi:10.1089/ars.2012.4588