Abstract
Purpose
Neurodegenerative diseases are associated with neuroinflammation along with activation of microglia and oxidative stress, but currently lack effective treatments. Punicalagin is a natural bio-sourced product that exhibits anti-inflammatory effects on several chronic diseases; however, the anti-inflammatory and anti-oxidative effects on microglia have not been well examined. This study aimed to investigate the effects of punicalagin on LPS-induced inflammatory responses, NLRP3 inflammasome activation, and the production of ROS using murine microglia BV2 cells.
Methods
BV2 cells were pre-treated with punicalagin following LPS treatment to induce inflammation. The secretion of NO and PGE2 was analyzed by Griess reagent and ELISA respectively, while the expressions of iNOS, COX-2, STAT3, ERK, JNK, and p38 were analyzed using Western blotting, the production of IL-6 was measured by ELISA, and the activity of NF-κB was detected using promoter reporter assay. To examine whether punicalagin affects NLRP3 inflammasome activation, BV2 cells were stimulated with LPS and then treated with ATP or nigericin. The secretion of IL-1β was measured by ELISA. The expressions of NLRP3 inflammasome-related proteins and phospho IκBα/IκBα were analyzed using Western blotting. The production of intracellular and mitochondrial ROS was analyzed by flow cytometry.
Results
Our results showed that punicalagin attenuated inflammation with reduction of pro-inflammatory mediators and cytokines including iNOS, COX-2, IL-1β, and reduction of IL-6 led to inhibition of STAT3 phosphorylation by LPS-induced BV2 cells. Punicalagin also suppressed the ERK, JNK, and p38 phosphorylation, attenuated NF-κB activity, inhibited the activation of the NLRP3 inflammasome, and reduced the production of intracellular and mitochondrial ROS by LPS-induced BV2 cells.
Conclusion
Our results demonstrated that punicalagin attenuated LPS-induced inflammation through suppressing the expression of iNOS and COX-2, inhibited the activation of MAPK/NF-κB signaling pathway and NLRP3 inflammasome, and reduced the production of ROS in microglia, suggesting that punicalagin might have the potential in treating neurodegenerative diseases.
Graphical Abstract
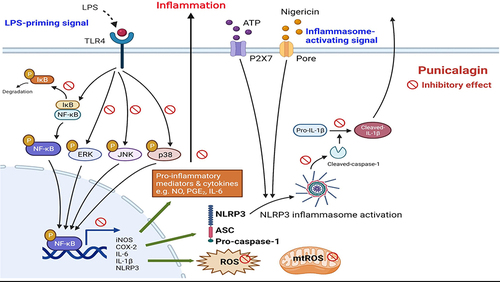
Introduction
Neurodegenerative disease, also known as the silent epidemic, is characterized by a progressive loss of neuronal cells in the brain. As a consequence of increase in the aging population, the range of medical resources used to treat and prevent senile-related diseases are also increasing year by year. Alzheimer’s disease is the most common type of neurodegenerative disease, and at present, there are ample studies to demonstrate that Alzheimer’s disease is characterized by the deposition of extracellular amyloid-beta and abnormal tau phosphorylation expression around the affected area of the brain;Citation1,Citation2 additionally, these cause dementia, progressive memory loss and the impairment of cognitive function.Citation3 Oxidative stress and neuroinflammation are important factors in the pathogenesis of neurodegenerative diseases.Citation4 Chronic oxidative stress is associated with neuroinflammation and neurodegeneration by activating signaling pathways of proinflammatory activities.Citation4 As the major resident macrophage-like immune cells in the central nervous system, microglia have a pivotal role in neuroinflammation and furnish multiple beneficial functions to neuron cells, including maintenance of cellular homeostasis and innate immunity.Citation5
Neuroinflammation is a crucial defense mechanism that competes with infection or trauma in the central nervous system. During neuroinflammation, microglia recognize pathogen-associated molecular patterns (PAMPs) (eg lipopolysaccharide (LPS) through Toll-like receptor-4 (TLR-4) and induce the robust activation of innate immune responses leading to an increase in the production of inflammatory mediators and cytokines such as nitric oxide (NO), prostaglandin E2 (PGE2) and cyclooxygenase (COX)-2, interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-1β as well as promoting the generation of reactive oxygen species (ROS) through nicotinamide-adenine dinucleotide phosphate oxidase.Citation6,Citation7 Additionally, microglial cells are stimulated by damage-associated molecular patterns (DAMPs) like adenosine triphosphate (ATP) and bind to P2X7 receptors then further induce the assembly of the nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3) inflammasome.Citation3 NLRP3 inflammasome is a cytosolic protein complex composed of NLRP3, an apoptotic speck-containing protein with a CARD (ASC) and caspase-1 that is formed in response to both PAMPs and DAMPs stimuli consequently leading to cleavage of pro-caspase-1 while promoting maturation of IL-1β and inducing pyroptosis.Citation8 Inhibition of NLRP3 inflammasome activation has been considered as a therapeutic strategy for ameliorating the progression of Alzheimer’s disease.Citation9–11
Polyphenols are found in various kinds of food sources such as tea, cocoa, fruits, berries, and vegetables, which have been demonstrated to exhibit antioxidative and anti-inflammatory propertiesCitation12,Citation13 and represent a protective role in many chronic diseases including cardiovascular disease, neurodegenerative diseases and diabetes.Citation14 Punicalagin is a large polyphenol compound with a molecular mass of 1084.7 and is the main compound of pomegranate (Punica granatum).Citation15 Although previous studies have demonstrated that punicalagin has benefits in inflammation-associated chronic diseases,Citation16–18 the anti-oxidative and anti-inflammatory effects of punicalagin on microglia have not been well examined; consequently, we aimed to investigate the anti-oxidative and anti-inflammatory effects of punicalagin on microglia while concurrently examining these effects on the activation of NLRP3 inflammasome in LPS-induced BV2 cells.
Materials and Methods
Materials and Reagents
Punicalagin (purity ≥ 98%) was purchased from ChemFaces (catalog number: CFN99938, Wuhan, Hunan, China). Lipopolysaccharide (LPS from E. coli. O111:B4, catalog number: L3024), Griess reagent, and protease inhibitor cocktails were obtained from Sigma Aldrich (St. Louis, MO, USA). MTT reagent (3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was supported by MDBio, Inc. (catalog number: 101-298-93-1, Taipei, Taiwan, R.O.C). IL-1β (catalog number: 88-7013-86) and IL-6 (catalog number: 88-7064-86) enzyme-linked immunosorbent assay (ELISA) kits, reactive oxygen species (ROS) detection reagent (catalog number: D399), and mitosox red mitochondrial superoxide indicator (catalog number: M36008) were purchased from Invitrogen (Carlsbad, CA, USA). PGE2 ELISA kit (catalog number: 514010), nigericin (catalog number: 11437), and ATP (catalog number: 14498) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Phosphatase inhibitor cocktails and Radioimmunoprecipitation assay (RIPA) buffer (catalog number: RIPA-50) were obtained from FIVEphoton Biochemicals (San Diego, CA, USA). BCA protein assay kit (catalog number: 23225) was purchased from Thermo Scientific (Waltham, MA, USA). Zeocin (catalog number: ant-zn-1p) was purchased from Invivogen (San Diego, CA, USA). Antibodies for phospho-signal transducer and activator of transcription 3 (STAT3) (catalog number: 9145), STAT3 (catalog number: 9139), phospho-c-Jun N-terminal kinase (JNK) (catalog number: 9255), JNK (catalog number: 9258), phospho-p38 MAPK (catalog number: 4511), p38 MAPK (catalog number: 8690), phospho-extracellular signal-regulated kinase (ERK)1/2 (catalog number: 4370), ERK1/2 (catalog number: 4695), ASC (catalog number: 67824), cleaved-IL-1β (catalog number: 52718), IL-1β (catalog number: 12242), cleaved-caspase-1 (catalog number: 67314), caspase-1 (catalog number: 24232), phospho-IκBα (catalog number: 2859), and IκBα (catalog number: 4814) were purchased from Cell Signaling Technology (Danvers, MA, USA). COX-2 (catalog number: sc-514489) and iNOS (catalog number: sc-7271) antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). NLRP3 (catalog number: GTX00763) and β-actin (catalog number: TA328070) antibodies were purchased from GeneTex (Irvine, CA, USA) and OriGene Technologies (Rockville, MD, USA) respectively.
Cell Culture
Mouse microglial BV2 cell line was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan, R.O.C). Cells were cultured in a humidified atmosphere at 37°C under 5% CO2 in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 U/mL streptomycin (Gibco, Waltham, MA, USA).
MTT Assay for Cell Viability
Cell viability of BV2 cells was measured by MTT assay. Briefly, BV2 cells (1 × 105 cells/well) were cultured in 96-well culture plates and allowed to attach overnight. Afterward, cells were pre-treated with different concentrations of punicalagin for 30 min following 1 μg/mL LPS treatment for 24 h. Then, 10 μL of 5 mg/mL MTT solution was added to each well and further incubated at 37°C for 4 h. Subsequently, 100 μL acidic isopropanol/HCl (isopropanol with 0.04 N HCl) was added to dissolve the formazan crystals. The absorbance was determined by spectrophotometry at 570 nm, and the percentage of viable cells was normalized to the untreated control.
Nitric Oxide Assay
The concentration of nitric oxide in the medium was measured by Griess reagent. Briefly, BV2 cells (1 × 105 cells/well) were cultured in 96-well culture plates and allowed to attach overnight. Afterward, cells were pre-treated with various concentrations of punicalagin for 30 min and then treated with LPS (1 μg/mL) for 24 h. Cell culture supernatant was collected and the concentration of nitric oxide was assessed by Griess reagent. The absorbance was determined at 540 nm by spectrophotometrically and the standard curve of NaNO2 was used to calculate the nitric oxide concentration.
Western Blot Analysis
Cells were washed with PBS and lysed by RIPA buffer supplemented with protease inhibitor cocktails and phosphatase inhibitor cocktails. The protein concentration was determined by the BCA protein assay kit. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. After blocking with 5% non-fat milk or BSA in TBST for 1 h, the membranes were incubated overnight with primary antibodies at 4°C overnight. Afterward, blots were washed three times by TBST, blocked with anti-rabbit or anti-mouse horseradish peroxidase-conjugated immunoglobulin G secondary antibodies diluted in TBST (1:5000) for 1 h at room temperature, and then the blots were washed three times using TBST. The intensities of protein bands were determined by Molecular Imager® Gel Doc™ XR System (Bio-Rad Laboratories, Hercules, California, USA) with Image Lab™ Software using an ECL chemiluminescence substrate (Thermo Scientific, Waltham, MA, USA). The result of β-actin was used to normalize the quantity of the protein bands.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of IL-6, IL-1β, and PGE2 in the medium were measured by ELISA. Briefly, BV2 cells (1 × 105 cells/well) were cultured in 96-well culture plates and allowed to attach overnight. Cells were pre-treated with different concentrations of punicalagin for 30 min following 1 μg/mL LPS treatment for 24 h or 48 h. Cell culture supernatant was collected for analysis according to the manufacturer’s protocol.
NF-κB Promoter Reporter Assay
BV2-Blue cells were derived from BV2 cells that were stably expressing a secreted embryonic alkaline phosphatase (SEAP) gene inducible by NF-κB as described in the previous studyCitation19 and maintained in Dulbecco’s modified Eagle’s medium supplemented with Zeocin (15 μg/mL) (InvivoGen, San Diego, CA, USA). Cells (1 × 105 cells/well) were seeded in a 96-well plate and allowed to attach overnight. Afterward, cells were pre-treated with different doses of punicalagin for 30 min and then treated with LPS (1 μg/mL) for 24 h. The medium then was harvested and mixed with QUANTI-Blue medium (100 μL cell culture supernatant to 100 μL QUANTI-Blue medium) (InvivoGen, San Diego, CA, USA) in 96-well plates and incubated at 37 °C for 45 min. SEAP activity was assessed by measuring the optical density at 655 nm using a microplate reader.
Flow Cytometry
The productions of intracellular ROS and mitochondrial ROS were measured by flow cytometry. BV2 cells (5 × 105 cells/well) were cultured in 6-well plates and allowed to attach overnight. Afterward, cells were pre-treated with different concentrations of punicalagin for 30 min following 1 μg/mL LPS treatment for 24 h. For the detection of intracellular ROS, cells were stained by the fluorescent probe dichloro-dihydro-fluorescein diacetate (DCFH-DA) reagent (Invitrogen, Carlsbad, California, USA) with 10 μM in HBSS buffer. For the detection of mitochondrial ROS, cells were stained by the MitoSOX™ Red reagent with 5 μM in HBSS buffer. After staining for 40 or 45 min, cells were washed with PBS and then analyzed using flow cytometry (Beckman Coulter Cytomics FC500 MCL Flow Cytometer System with CXP cytometer software, Elkin, NC, USA).
Immunofluorescence Staining
BV2 cells were seeded on the coverslips at a density of 2×105 cells/well, placed in 6-well plates and allowed to attach overnight. The cells were pre-treated with different doses of punicalagin for 30 min following 1 μg/mL LPS treatment for 24 h, then stained with DCFH-DA or MitoSOX™ Red following the above experimental conditions. Afterward, cells were washed with PBS and then stained with DAPI. Images were taken by fluorescence microscope (Nikon, Tokyo, Japan).
Statistical Analysis
The experimental results are presented as mean ± standard deviation (SD). All data are shown from three independent experiments. Statistical analysis was performed by one-way ANOVA followed by Tukey post-hoc test using GraphPad Prism 6 (San Diego, CA, USA). The significant difference between the groups was defined as *p < 0.05; **p <0.01; ***p <0.001.
Results
Punicalagin Reduces the Production of NO and PGE2 and Inhibits the Expression of COX-2 and NOS2 in LPS-Induced BV2 Cells
To determine the toxic effect of punicalagin, the effect of punicalagin on the cell viability of BV2 cells was examined. Cells were pre-treated with various doses (0, 25, 50, 75, 100 μM) of punicalagin for 30 min following 1 μg/mL LPS treatment for 24 h. The results showed that the LPS-alone group slightly reduced cell viability, whereas punicalagin had no cytotoxic effect on BV2 cells when cells were treated with 0 to 100 μM punicalagin (). In addition, at higher doses of punicalagin groups (75 and 100 μM), punicalagin rescued LPS-induced cell death compared with the LPS-alone group (). To examine whether punicalagin affected LPS-induced NO and PGE2 productions, BV2 cells were pre-treated with various doses (0 to 100 μM) of punicalagin for 30 min and then treated with LPS (1 μg/mL) for 24 h. The levels of NO and PGE2 in cell culture supernatants were examined by Griess reagent and ELISA respectively.
Figure 1 The effect of punicalagin on the production of pro-inflammatory mediators (NO and PGE2) by LPS-induced BV2 cells. BV2 cells were pre-treated with various concentrations (0, 25, 50, 75, 100 μM) of punicalagin for 30 min, and then treated with LPS (1 μg/mL) for 24 h. (A) The cell viability was determined by MTT assay. (B and C) The secretion of NO and PGE2 was measured by Griess reagent assay and ELISA respectively. Expressions of iNOS and COX-2 were analyzed using Western blot. The representative images are shown in (D) and the quantitative results of three independent experiments shown in (E and F). β-actin was used as a loading control. Statistical significance was indicated as *p < 0.05; **p <0.01; ***p <0.001.
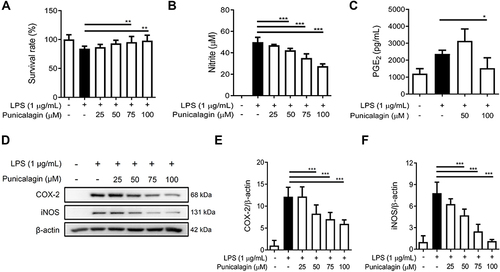
As shown in , punicalagin significantly attenuated NO production by LPS-induced BV2 cells in a concentration-dependent manner. In addition, a high dosage (100 μM) of punicalagin significantly decreased the secretion of PGE2 by LPS-induced BV2 cells (). Inducible nitric oxide synthase (iNOS) is a key enzyme generating nitric oxide (NO) from the amino acid L-arginine,Citation20 while cyclooxygenase-2 (COX-2) is a key enzyme converting arachidonic acid into PGE2.Citation21 We further examined whether punicalagin affected the expression of iNOS and COX-2. BV2 cells were pre-treated with various doses of punicalagin (0 to 100 μM) for 30 min following 1 μg/mL LPS treatment for 24 h. The expression of iNOS and COX-2 was detected by Western blot. As shown in , punicalagin significantly suppressed both iNOS and COX-2 expressions in a dose-dependent manner as compared with LPS alone.
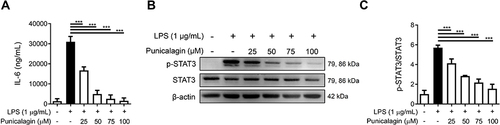
Punicalagin Reduces the Secretion of IL-6 and Inhibits the Phosphorylation of STAT3 by LPS-Induced BV2 Cells
The pro-inflammatory cytokine IL-6 is demonstrated as being involved in the etiopathology of Alzheimer’s disease.Citation22 To examine whether punicalagin affects the secretion of IL-6 by LPS-induced BV2 cells, cells were pre-treated with various doses of punicalagin (0 to 100 μM) for 30 min following 1 μg/mL LPS treatment for 24 h. The level of IL-6 in the cell culture medium was detected by ELISA. As shown in , punicalagin significantly decreased the secretion of IL-6 by LPS-induced BV2 cells in a dose-dependent manner. IL-6 is known to regulate the phosphorylation of the signal transducer and activator of transcription 3 (STAT3) during LPS/TLR4-driven inflammation.Citation23 To further examine whether punicalagin affects STAT3 activation by LPS-induced BV2 cells, cells were pre-treated with various doses of punicalagin (0 to 100 μM) for 30 min following 1 μg/mL LPS treatment for 2 h. The expression of phospho-STAT3 and STAT3 was determined by Western blot. As shown in and , the experimental results showed that punicalagin significantly suppressed the phosphorylation of STAT3 in a concentration-dependent manner.
Figure 2 The effect of punicalagin on the production of IL-6 and the activation of STAT3 in LPS-induced BV2 cells. Cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 mins and then treated with LPS (1 μg/mL) for 24 h. (A) The level of IL-6 was measured by ELISA. The data are presented as the means ± SD of three independent experiments. Statistical significance was assessed by one-way ANOVA represented as follows: ***p < 0.001 vs LPS alone. Cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min and then treated with LPS (1 μg/mL) for 2 h. The expression of phospho-STAT3 and STAT3 was determined by Western blot. The representative images are shown in (B) and the quantitative results of three independent experiments shown in (C). β-actin was used as a loading control. Statistical significance was indicated as ***p <0.001.
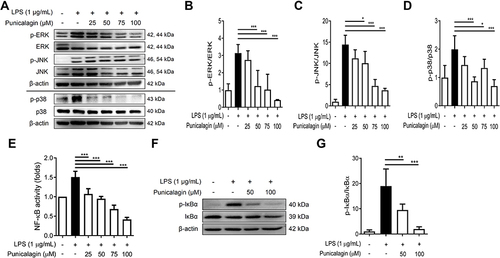
Punicalagin Attenuates the Activation of Both MAPK and NF-κB Signaling Pathways in LPS-Induced BV2 Cells
Both MAPK and NF-κB signaling pathways are known to drive inflammation-associated gene expressions during LPS-induced inflammation.Citation24,Citation25 To assess whether punicalagin regulated the activation of MAPK and NF-κB signaling pathways by LPS-induced BV2 cells, cells were pre-treated with various doses of punicalagin (0 to 100 μM) for 30 min following 1 μg/mL LPS treatment for 6 h. The expression of phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, and p38 was examined by Western blot. As shown in , punicalagin significantly decreased the phosphorylation of ERK, JNK, and p38 in LPS-induced BV2 cells in a dose-dependent manner. In addition, we also determined the effect of punicalagin on the activation of NF-κB by LPS-induced BV2 cells using promoter reporter assay. The NF-κB reporter BV2 cells, BV2-Blue cells, were pre-treated with various doses of punicalagin (0 to 100 μM) for 30 min following 1 μg/mL LPS treatment for 24 h. The SEAP activity in the cell culture medium was examined. As shown in , the experimental results demonstrated that punicalagin significantly attenuated the activation of NF-κB by LPS-induced BV2-Blue cells. Since IκBα phosphorylation is an essential event driving NF-κB activation,Citation25 we further assessed whether punicalagin affected IκBα phosphorylation by LPS-induced BV2 cells using Western blot. As shown in and , punicalagin significantly inhibited the phosphorylation of IκBα by LPS-induced BV2 cells.
Figure 3 The effects of punicalagin on the activation of both MAPK and NF-κB signaling pathways by LPS-activated BV2 cells. BV2 cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min following 1 μg/mL LPS treatment for 6 h. The expressions of phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, p38 were determined by Western blot. The representative images are shown in (A) and the quantitative results of three independent experiments shown in (B–D). β-actin was used as a loading control. (E) BV2-Blue cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min following 1 μg/mL LPS treatment for 24 h. The activation of NF-κB was measured by detected SEAP activity. BV2 cells were pre-treated with punicalagin (0, 50, 100 μM) for 30 min following with 1 μg/mL LPS treatment for 50 min. The expressions of phospho-IκBα and IκBα by LPS-activated BV2 cells were determined by Western blot. The representative images are shown in (F) and the quantitative results of three independent experiments shown in (G). β-actin was used as a loading control. Statistical significance was indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 vs LPS alone.
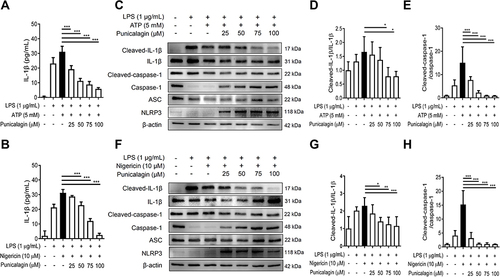
Punicalagin Attenuates the Secretion of IL-1β and Inhibits the Cleavage of IL-1β and Caspase-1 in LPS/ATP-Induced and LPS/Nigericin-Induced BV2 Cells
The activation of NLRP3 inflammasome has been demonstrated to associate with neuroinflammatory diseases including Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis.Citation26,Citation27 To examine whether punicalagin affected NLRP3 inflammasome activation, BV2 cells were pre-treated with various doses of punicalagin for 30 min, primed with LPS for 47.5 h, and then stimulated with ATP or nigericin for 30 min. The secretion of IL-1β was analyzed by ELISA and the expression of inflammasome-associated proteins was examined using Western blot. As shown in and , the experimental results revealed that punicalagin significantly reduced the secretion of IL-1β by both LPS/ATP-induced and LPS/ nigericin-induced BV2 cells in a dose-dependent manner. Furthermore, our results also showed that punicalagin significantly decreased the expressions of cleave-IL-1β and cleave-caspase-1 by both LPS/ATP-induced and LPS/ nigericin-induced BV2 cells ().
Figure 4 The effects of punicalagin on the activation of NLRP3 inflammasome by LPS/ATP-activated and LPS/nigericin-activated BV2 cells. BV2 cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min, and then treated with LPS (1 μg/mL) for 47.5 h followed by ATP (5 mM) or Nigericin (10 μM) treatments for 30 min. (A and B) The secretion of IL-1βwas determined by ELISA. The expressions of cleaved-IL-1β, IL-1β, cleaved-caspase-1, caspase-1, ASC and NLRP3 were analyzed by Western blot. The representative images are shown in (C and F) and the quantitative results of three independent experiments shown in (D, E, G and H). β-actin was used as a loading control. Statistical significance was indicated as *p < 0.05, **p < 0.01 and ***p < 0.001.
Punicalagin Attenuates the Production of Both Intracellular and Mitochondrial ROS in LPS-Induced BV2 Cells
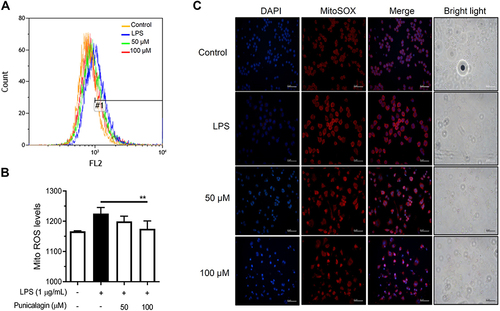
ROS plays a vital role in immunity by enhancing immunological defense and causing oxidative damage.Citation28 Next, we further investigated the anti-oxidation potential of punicalagin by determining the levels of reactive oxygen species (ROS) in intracellular and mitochondrial using DCFH‐DA and MitoSOX red staining respectively. The results were examined by flow cytometry and fluorescent microscopy. As shown in , punicalagin significantly inhibited intracellular ROS production in LPS-induced BV2. Moreover, punicalagin also attenuated the mitochondrial ROS levels by LPS-induced BV2 cells ().
Figure 5 The effects of punicalagin on the production of intracellular ROS in LPS-activated BV2 cells. BV2 cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min and then treated with LPS (1 μg/mL) for 24 h. The production of (A) intracellular ROS was analyzed by flow cytometry. The quantitative value (mean fluorescence intensity) compared to the peak of LPS group (#1) was shown in (B). The data are presented as the means ± SD of three independent experiments. Statistical significance was indicated as ***p < 0.001 vs LPS alone. The immunofluorescence staining of ROS in (C) cytoplasm was examined using DCFH-DA.
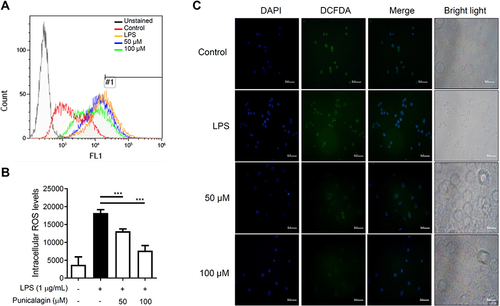
Figure 6 The effects of punicalagin on the production of mitochondrial ROS in LPS-activated BV2 cells. BV2 cells were pre-treated with punicalagin (0, 25, 50, 75, 100 μM) for 30 min and then treated with LPS (1 μg/mL) for 24 h. The production of (A) mitochondrial ROS was analyzed by flow cytometry. The quantitative value (mean fluorescence intensity) compared to the peak of LPS group (#1) was shown in (B). The data are presented as the means ± SD of three independent experiments. Statistical significance was indicated as **p < 0.01 vs LPS alone. The immunofluorescence staining of ROS in (C) mitochondria was examined using MitoSOX red staining.
Discussion
Neurodegenerative diseases are common in aging adults globally and cause serious health problems by progressive morbidity, memory and cognitive impairment.Citation29 By 2020, about 50 million people worldwide were suffering from dementia, and as the most common type of neurodegenerative disease, Alzheimer’s disease accounts for 60% to 80% of causes of dementia.Citation30 Neuroinflammation and oxidative damage are the key features of neurodegenerative diseases, and the hallmark of neuroinflammation is the activation of microglia in the central nervous system. Activated microglia in neurodegenerative processes induce the release of proinflammatory cytokines and mediators including IL-1β, IL-6, TNF-α and ROS, thereby causing neuronal cell degeneration.Citation31,Citation32 Currently, there is no effective treatment to slow or stop the progression of neurodegenerative diseases other than relying on supportive and symptomatic care;Citation29,Citation33 therefore, suppressing activated microglia-induced inflammatory responses have been considered as a therapeutic strategy for treating these diseases.Citation31 In this study, our experimental results revealed that punicalagin effectively inhibited LPS-activated murine microglial BV2 cells by attenuating the secretion of inflammatory mediators and cytokines, inhibiting the activation of the NLRP3 inflammasome, and suppressing the production of intracellular and mitochondrial ROS.
Natural products are important sources for new drug development.Citation34 Punicalagin is a major active component mainly found in pomegranate, a plant widely distributed in the tropics and subtropical regions.Citation35 Previous studies have reported that punicalagin alleviates macrophage-mediated acute inflammation,Citation36,Citation37 acute lung injury and acute respiratory distress syndrome,Citation38,Citation39 acute kidney injury,Citation40 and chronic diseases including arthritis, diabetes, obesity, cardiovascular and neurodegenerative diseases.Citation16,Citation17,Citation41 Neuroinflammation is mediated by several proinflammatory molecules. As an inflammatory mediator, iNOS is one of the three different isoforms of NOS that expresses in glial cells, macrophages and neutrophils, and is only generated after induction by inflammatory mediators like cytokines or endotoxins.Citation42 It has a neurotoxic effect on neurodegenerative diseases when generating higher concentrations of NO.Citation42 Furthermore, inhibiting COX-2 and subsequent synthesis of PGE2 leads to a decrease in neuronal degeneration.Citation43 Proinflammatory cytokine IL-6 is reported to be associated with the pathogenesis of Alzheimer’s disease,Citation22 and it may cause neuroinflammation by recruiting leukocytes across the blood-brain barrier and promoting the LPS-driven inflammatory responses by regulating the phosphorylation of STAT3.Citation23 In addition, the MAPK cascade and NK-κB signaling pathway both regulate the expression and production of LPS-induced proinflammatory cytokines.Citation24 NF-κB has been demonstrated to play an integral role in the progression of Alzheimer’s diseaseCitation44 and ischemic stroke.Citation45 A previous murine model in vivo and in vitro study revealed the anti-neuroinflammatory effect of punicalagin on microglia and astrocytes by inhibiting the production of proinflammatory cytokines including IL-1β, IL-6 and TNF-α and interfering with NF-κB signaling via binding to its subunit p50 directly.Citation46 Similarly, our results demonstrated that punicalagin suppressed LPS-induced inflammatory responses by reducing the expression of iNOS, COX-2, inhibiting the secretion of NO, PGE2 and IL-6 while suppressing the phosphorylation of STAT3, IκBα, and MAPKs in BV2 cells, indicating that punicalagin has potential in attenuating microglia-induced inflammation.
Previous studies have indicated that activation of either NF-κB and MAPK signaling pathways was partly responsible for inducing the expression and activation of NLRP3 inflammasome proteins in neurons and brain tissue.Citation34,Citation47,Citation48 Activation of NLRP3 inflammasome is associated with neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis.Citation26,Citation27 Neuroinflammation and activation of microglia around the degenerating neurons with subsequent secretion of proinflammatory molecule IL-1β lead to neuronal damage.Citation31 The canonical activation of the NLRP3 inflammasome, a multiprotein complex composed of NLRP3, ASC and caspase-1, is responsible for the production of IL-1β from microglia upon cellular stress as well as induction of pyroptosis, a type of programmed cell death that causes rupture of the cell membrane resulting in the release of more pro-inflammatory cytokines thereby promoting the inflammatory response.Citation8,Citation49
Previous studies have indicated that the levels of NLRP3, ASC, and caspase-1 proteins were upregulated in the brain and plasma of patients with neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease,Citation27,Citation50 as well as in an Alzheimer’s disease mice model.Citation9,Citation10 Inactivation of NLRP3 inflammasome has been suggested as a therapeutic target for neurodegenerative diseases.Citation9–11,Citation48,Citation50,Citation51 Punicalagin has been reported to possess anti-inflammatory effects on a diabetic nephropathy mice model by inhibiting pyroptosis based on the NLRP3 pathwayCitation52 and a neuroinflammation mice model via inhibition of NF-κB.Citation46 Our results demonstrated that punicalagin suppressed the secretion of IL-1β and inhibited the cleavage of caspase-1 and IL-1β by LPS/ATP- and LPS/nigericin-activated BV2 cells, indicating that punicalagin can inhibit NLRP3 inflammasome activation in activated microglia.
Oxidative stress is an imbalance state between prooxidant and antioxidant species and is characterized by an increasing level of reactive species including ROS and nitrogen reactive species (RNS).Citation4,Citation28 Previous evidence shows that oxidative stress and neuroinflammation play important roles in the development and progression of neurodegenerative diseases because reactive species can become injurious under chronic oxidative stress by oxidizing intracellular proteins and lipids, causing DNA damage, and mediating activation of microglia and astrocytes that promote inflammatory responses.Citation4 Alzheimer’s disease is the most common type of neurodegenerative disease and its pathogenesis is associated with oxidative stress and amyloid-β, a major component of the senile plaques found in the pathology of patients with Alzheimer’s disease.Citation1,Citation2,Citation53 Altogether, the increase of ROS production and intracellular Ca2+ cause excessive Ca2+ influx into mitochondria resulting in mitochondrial impairment and subsequently releasing pro-apoptotic molecules leading to neuronal degeneration and damage.Citation53 Mitochondria are important cellular organelles and as being considered the powerhouse in cells, mitochondrial impairment leads to defective energy metabolism and excessive production of ROS.Citation53
Furthermore, damaged mitochondria might promote the activation of caspase-1, release of proinflammatory cytokines and activation of inflammasome formation.Citation8 Recent studies have suggested antioxidants counteract the oxidative damage conferred by ROS as a therapeutic target in treating neurodegenerative diseases including Alzheimer’s disease.Citation12,Citation53 Punicalagin has been reported to have the potential of being an effective antioxidant in attenuating cardiac mitochondrial impairment in an obesity rat modelCitation15 while reducing the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α as well as reactive species hydrogen peroxide (H2O2) in brain tissue of an LPS-stimulated mouse model.Citation46 In line with our study, our results indicate that punicalagin presented an anti-oxidative effect by attenuating the production of intracellular ROS and mitochondrial ROS in LPS-activated microglia.
Conclusion
Our study demonstrated the anti-inflammatory effects of punicalagin through alleviating LPS-induced inflammation through MAPK and NF-κB signaling pathways and suppressing the activation of NLRP3 inflammasome in microglia. Furthermore, punicalagin also presented anti-oxidative effects in LPS-activated microglia by attenuating the production of both intracellular and mitochondrial ROS. Our results shed light on the molecular mechanism of anti-inflammatory and anti-oxidative effects of punicalagin as an agent possessing potential for treating neurodegenerative diseases.
Abbreviations
ASC, apoptotic speck containing protein with a CARD; ATP, adenosine triphosphate; COX-2, cyclooxygenase-2; DAMPs, damage-associated molecules patterns; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal–regulated kinase; IL, interleukin; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharides; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; NLRP3, NOD-like receptor family pyrin domain containing 3; NO, nitric oxide; NOD, nucleotide binding oligomerization domain; PAMPs, pathogen-associated molecular patterns; PGE2, prostaglandin E2; ROS, reactive oxygen species; SEAP, secreted embryonic alkaline phosphatase; TLR-4, Toll-like receptor-4; TNF-α, tumor necrosis factor-α.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by Kaohsiung Medical University [Grant No. NHRIKMU-111-I002 and KMU-DK(A)111003], Ministry of Science and Technology, Taiwan, R.O.C [Grant No. MOST 109-2320-B-037-007-MY3], and Chang Gung Memorial Hospital [Grant No. CMRPG8J1221].
References
- Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis. 2017;57(4):975–999. doi:10.3233/JAD-160612
- DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi:10.1186/s13024-019-0333-5
- Tejera D, Mercan D, Sanchez-Caro JM, et al. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 2019;38(17):e101064. doi:10.15252/embj.2018101064
- Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4(+) T cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114. doi:10.3389/fncel.2018.00114
- Park J, Min JS, Kim B, et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett. 2015;584:191–196. doi:10.1016/j.neulet.2014.10.016
- Avallone R, Lucchi C, Puja G, et al. BV-2 microglial cells respond to rotenone toxic insult by modifying pregnenolone, 5alpha-dihydroprogesterone and pregnanolone levels. Cells. 2020;9(9):2091. doi:10.3390/cells9092091
- Alam MB, Ju MK, Kwon YG, Lee SH. Protopine attenuates inflammation stimulated by carrageenan and LPS via the MAPK/NF-kappaB pathway. Food Chem Toxicol. 2019;131:110583. doi:10.1016/j.fct.2019.110583
- Wang S, Yuan YH, Chen NH, Wang HB. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol. 2019;67:458–464. doi:10.1016/j.intimp.2018.12.019
- Cai Y, Chai Y, Fu Y, et al. Salidroside ameliorates Alzheimer’s disease by targeting NLRP3 inflammasome-mediated pyroptosis. Front Aging Neurosci. 2021;13:809433. doi:10.3389/fnagi.2021.809433
- Shao L, Dong C, Geng D, He Q, Shi Y. Ginkgolide B inactivates the NLRP3 inflammasome by promoting autophagic degradation to improve learning and memory impairment in Alzheimer’s disease. Metab Brain Dis. 2022;37(2):329–341. doi:10.1007/s11011-021-00886-2
- Abu-Elfotuh K, Al-Najjar AH, Mohammed AA, Aboutaleb AS, Badawi GA. Fluoxetine ameliorates Alzheimer’s disease progression and prevents the exacerbation of cardiovascular dysfunction of socially isolated depressed rats through activation of Nrf2/HO-1 and hindering TLR4/NLRP3 inflammasome signaling pathway. Int Immunopharmacol. 2022;104:108488. doi:10.1016/j.intimp.2021.108488
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278. doi:10.4161/oxim.2.5.9498
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi:10.1155/2016/7432797
- Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr. 2018;5:87. doi:10.3389/fnut.2018.00087
- Cao K, Xu J, Pu W, et al. Punicalagin, an active component in pomegranate, ameliorates cardiac mitochondrial impairment in obese rats via AMPK activation. Sci Rep. 2015;5:14014. doi:10.1038/srep14014
- Xu J, Cao K, Liu X, Zhao L, Feng Z, Liu J. Punicalagin regulates signaling pathways in inflammation-associated chronic diseases. Antioxidants. 2021;11(1):29. doi:10.3390/antiox11010029
- Venusova E, Kolesarova A, Horky P, Slama P. Physiological and immune functions of punicalagin. Nutrients. 2021;13(7):2150. doi:10.3390/nu13072150
- Huang M, Wu K, Zeng S, et al. Punicalagin inhibited inflammation and migration of fibroblast-like synoviocytes through NF-kappaB pathway in the experimental study of rheumatoid arthritis. J Inflamm Res. 2021;14:1901–1913. doi:10.2147/JIR.S302929
- Hung YL, Wang SC, Suzuki K, et al. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine. 2019;59:152785. doi:10.1016/j.phymed.2018.12.008
- Lirk P, Hoffmann G, Rieder J. Inducible nitric oxide synthase–time for reappraisal. Curr Drug Targets Inflamm Allergy. 2002;1(1):89–108. doi:10.2174/1568010023344913
- Norregaard R, Kwon TH, Frokiaer J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract. 2015;34(4):194–200. doi:10.1016/j.krcp.2015.10.004
- Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004;295(1):245–257. doi:10.1016/j.yexcr.2004.01.002
- Greenhill CJ, Rose-John S, Lissilaa R, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186(2):1199–1208. doi:10.4049/jimmunol.1002971
- Huang CH, Wang SC, Chen IC, et al. Protective effect of piplartine against LPS-induced sepsis through attenuating the MAPKs/NF-kappaB signaling pathway and NLRP3 inflammasome activation. Pharmaceuticals. 2021;14(6):588. doi:10.3390/ph14060588
- Huang MY, Tu CE, Wang SC, et al. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complement Altern Med. 2018;18(1):221. doi:10.1186/s12906-018-2287-5
- Ravichandran KA, Heneka MT. Inflammasome activation in neurodegenerative diseases. Essays Biochem. 2021;65(7):885–904. doi:10.1042/EBC20210021
- Rui W, Xiao H, Fan Y, et al. Systemic inflammasome activation and pyroptosis associate with the progression of amnestic mild cognitive impairment and Alzheimer’s disease. J Neuroinflammation. 2021;18(1):280. doi:10.1186/s12974-021-02329-2
- Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467(1–2):1–12. doi:10.1007/s11010-019-03667-9
- Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4):a033118. doi:10.1101/cshperspect.a033118
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi:10.1016/S0140-6736(20)30367-6
- Muzio L, Viotti A, Martino G. Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Front Neurosci. 2021;15:742065. doi:10.3389/fnins.2021.742065
- Naeem K, Tariq Al Kury L, Nasar F, et al. Natural dietary supplement, carvacrol, alleviates LPS-induced oxidative stress, neurodegeneration, and depressive-like behaviors via the Nrf2/HO-1 pathway. J Inflamm Res. 2021;14:1313–1329. doi:10.2147/JIR.S294413
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. doi:10.1002/alz.12328
- Riaz M, Al Kury LT, Atzaz N, et al. Carvacrol alleviates hyperuricemia-induced oxidative stress and inflammation by modulating the NLRP3/NF-kappaB pathwayt. Drug Des Devel Ther. 2022;16:1159–1170. doi:10.2147/DDDT.S343978
- Wu S, Tian L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules. 2017;22(10):1606. doi:10.3390/molecules22101606
- Xu X, Yin P, Wan C, et al. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-kappaB activation. Inflammation. 2014;37(3):956–965. doi:10.1007/s10753-014-9816-2
- Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients. 2019;11(11):2794. doi:10.3390/nu11112794
- Zeng Y, Zhao H, Zhang T, et al. Lung-protective effect of punicalagin on LPS-induced acute lung injury in mice. Biosci Rep. 2022;42(1):BSR20212196. doi:10.1042/BSR20212196
- Peng J, Wei D, Fu Z, et al. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38(2):493–499. doi:10.1007/s10753-014-9955-5
- Fouad AA, Qutub HO, Al-Melhim WN. Nephroprotection of punicalagin in rat model of endotoxemic acute kidney injury. Toxicol Mech Methods. 2016;26(7):538–543. doi:10.1080/15376516.2016.1211207
- Atrahimovich D, Samson AO, Khattib A, Vaya J, Khatib S. Punicalagin decreases serum glucose levels and increases PON1 activity and HDL anti-inflammatory values in balb/c mice fed a high-fat diet. Oxid Med Cell Longev. 2018;2018:2673076. doi:10.1155/2018/2673076
- Panthi S, Manandhar S, Gautam K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl Neurodegener. 2018;7:3. doi:10.1186/s40035-018-0108-x
- Ryan JC, Cross CA, Van Dolah FM. Effects of COX inhibitors on neurodegeneration and survival in mice exposed to the marine neurotoxin domoic acid. Neurosci Lett. 2011;487(1):83–87. doi:10.1016/j.neulet.2010.10.001
- Imran M, Shah FA, Nadeem H, et al. Synthesis and biological evaluation of benzimidazole derivatives as potential neuroprotective agents in an ethanol-induced rodent model. ACS Chem Neurosci. 2021;12(3):489–505. doi:10.1021/acschemneuro.0c00659
- Ali A, Shah FA, Zeb A, et al. NF-kappaB inhibitors attenuate MCAO induced neurodegeneration and oxidative stress-a reprofiling approach. Front Mol Neurosci. 2020;13:33. doi:10.3389/fnmol.2020.00033
- Kim YE, Hwang CJ, Lee HP, et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi:10.1016/j.neuropharm.2017.01.025
- Fann DY, Lim YA, Cheng YL, et al. Evidence that NF-kappaB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55(2):1082–1096. doi:10.1007/s12035-017-0394-9
- Usman H, Tan Z, Gul M, et al. Identification of novel and potential PPARgamma stimulators as repurposed drugs for MCAO associated brain degeneration. Toxicol Appl Pharmacol. 2022;446:116055. doi:10.1016/j.taap.2022.116055
- Pellegrini C, Antonioli L, Lopez-Castejon G, Blandizzi C, Fornai M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front Immunol. 2017;8:36. doi:10.3389/fimmu.2017.00036
- Gordon R, Albornoz EA, Christie DC, et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10(465):eaah4066. doi:10.1126/scitranslmed.aah4066
- Xue J, Jia P, Zhang D, Yao Z. TTP488 ameliorates NLRP3-associated inflammation, viability, apoptosis, and ROS production in an Alzheimer’s disease cell model by mediating the JAK1/STAT3/NFkappaB/IRF3 pathway. Cell Biochem Funct. 2021;39(4):555–561. doi:10.1002/cbf.3623
- An X, Zhang Y, Cao Y, Chen J, Qin H, Yang L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi:10.3390/nu12051516
- Walia V, Kaushik D, Mittal V, et al. Delineation of neuroprotective effects and possible benefits of antioxidants therapy for the treatment of Alzheimer’s diseases by targeting mitochondrial-derived reactive oxygen species: bench to bedside. Mol Neurobiol. 2022;59(1):657–680. doi:10.1007/s12035-021-02617-1
