Abstract
Purpose
Understanding the humoral immune response dynamics carried out by B cells in COVID-19 vaccination is little explored; therefore, we analyze the changes induced in the different cellular subpopulations of B cells after vaccination with BNT162b2 (Pfizer-BioNTech).
Methods
This prospective cohort study evaluated thirty-nine immunized health workers (22 with prior COVID-19 and 17 without prior COVID-19) and ten subjects not vaccinated against SARS-CoV-2 (control group). B cell subpopulations (transitional, mature, naïve, memory, plasmablasts, early plasmablast, and double-negative B cells) and neutralizing antibody levels were analyzed and quantified by flow cytometry and ELISA, respectively.
Results
The dynamics of the B cells subpopulations after vaccination showed the following pattern: the percentage of transitional B cells was higher in the prior COVID-19 group (p < 0.05), whereas virgin B cells were more prevalent in the group without prior COVID-19 (p < 0.05), mature B cells predominated in both vaccinated groups (p < 0.01), and memory B cells, plasmablasts, early plasmablasts, and double-negative B cells were higher in the not vaccinated group (p < 0.05).
Conclusion
BNT162b2 vaccine induces changes in B cell subpopulations, especially generating plasma cells and producing neutralizing antibodies against SARS-CoV-2. However, the previous infection with SARS-CoV-2 does not significantly alter the dynamics of these subpopulations but induces more rapid and optimal antibody production.
Introduction
COVID-19 is the great pandemic of the 21st century caused by the SARS-CoV-2 virus.Citation1 Immunization through vaccines is the most efficient measure to prevent emerging and re-emerging infectious diseases in the human population;Citation2 however, its effectiveness depends on the developed immune response.Citation3 The BNT162b2 (Pfizer-BioNTech) vaccine is based on mRNA technology, showing safety and high efficacy against SARS-CoV-2.Citation4
A successful immune response depends on the cooperation of different cells.Citation5 The immediate response is carried out by cells of the innate immune system, where they are quickly recruited at the site of damage (vaccine infiltration), initiating the immune response through inflammation; however, they also act as antigen-presenting cells.Citation6,Citation7 Subsequently, the adaptive immune system cells such as T and B lymphocytes are activated by contact with the antigen, resulting in the development of effector and memory cells.Citation3,Citation8
The impact of vaccination on cellular immunity has been extensively studied;Citation9,Citation10 however, the role of humoral immunity from the perspective of cell subpopulation dynamics remains to be explored further.
Activation of B cells is key to the effectiveness of the response to vaccination.Citation11 B cell subpopulations have different phenotypes and functions, such as the production of antibodies.Citation12 The transitional B cells (TrB cells; immunophenotype CD19+CD27−CD24+CD38+) are immature cells derived from bone marrow, considered precursors of mature B cells; these cells represent approximately 4% of all B CD19+ lymphocytes repertoire in healthy subjects.Citation13,Citation14 An increase in this population would indicate a stimulation to induce cell proliferation. When TrB cells encounter the antigen to which they are prone, mature B cells (immunophenotype, CD19+CD27−CD24−CD38−) can enter a germ center reaction and be able to differentiate into memory or plasma cells.Citation15
Memory B cells (immunophenotype, CD19+CD27+CD38−) are long-lived inactive cells prepared to respond rapidly to antigen reinfection,Citation16 whereas plasmablasts (immunophenotype CD19+CD27+CD38+) can be a transient population: plasma cell precursors (antibody-producing) or terminally differentiated effector cells.Citation17,Citation18 In peripheral blood, there are other less frequent subpopulations such as early plasmablasts (CD19+CD27−CD24−CD38+), a population with similar dynamics to plasmablasts, and the double-negative B cells (CD19+CD27−CD24−CD38-IgD−), which have been linked to age, autoimmune diseases or infections.Citation19,Citation20 In healthy adults, most B cells circulating in the blood are mature or with memory B-cell phenotypes, and there are low amounts of transitional B cells and plasma cells (plasmablasts).Citation12,Citation21,Citation22 These cells can be evaluated by flow cytometry immunophenotyping to determine the presence and ffoabsence of specific antigens by differentiation clusters (CD).Citation23
The immune system generates protection after natural infection and by vaccination against SARS-CoV-2, but the protection generated by the combination of infection before vaccination is little analyzed.Citation24 Studies have shown that the mentioned combination of the previous infection plus vaccination generates additional protection against SARS-CoV-2 reinfections.Citation24,Citation25
Regarding neutralizing antibody production, it has been shown that the role of a previous infection helps in the dynamics of the generation of these antibodies in a faster and more efficient way.Citation27,Citation28 There are no studies that analyze the role of previous infections in the dynamics of the generation of B cell subpopulations; however, it has been seen that the BNT162b2 vaccine generates memory and plasma B cells.Citation29,Citation30
Based on the above, it was interesting to study the differences in the effect of BNT162b2 on B-cell immunophenotypes and the neutralizing antibodies against SARS-CoV-2 production in individuals with and without prior COVID-19.
Materials and Methods
Subjects
We included 39 healthcare workers from Jalisco, Mexico, immunized with Pfizer-BioNTech (BNT162b2) vaccine. All individuals signed an informed consent report. They were divided into two groups: people with prior COVID-19 (n=22) and people without prior COVID-19 (n=17). Additionally, ten gender- and age-matched individuals without vaccination against SARS-CoV-2 were included as a reference group and were denominated control subjects (CS); five of these individuals had a history of prior asymptomatic COVID-19. This group was included because the immunophenotypes of B cell subpopulations were not determined before vaccination (baseline) in those vaccinated individuals. Therefore, these healthy unvaccinated individuals (CS) are a reference point to infer possible changes between B cell subpopulations before and after vaccination.
Individuals with a history of COVID-19 were diagnosed 3 to 5 months before vaccination. The verification of the group without a history of COVID-19 was performed by IgG/IgM Rapid Tests (Certum Diagnostics, Nuevo León, Mexico), confirming seronegativity to anti-SARS-CoV-2 antibodies. All groups answered a survey of clinical and demographic characteristics. For immunized people, blood samples were taken 21 days after each dose of the vaccine (two doses).
Sample Collection
Peripheral blood samples were obtained by venous puncture in Vacutainer tubes without anticoagulant for serum collection. Furthermore, peripheral blood mononuclear cells (PBMC) were collected from plastic BD Vacutainer EDTA tubes by a density gradient centrifugation protocol using Lymphoprep reagent (Axis-Shield Diagnostics, UK) and following the manufacturer’s instructions. Cells were suspended in 2 mL of phosphate-buffered saline (PBS) solution 1 X (Caisson Labs) and checked for viability in a Neubauer counting chamber using trypan blue (Sigma-Aldrich Co.).
Flow Cytometry
A 6-color multicolor flow cytometry panel was designed for the identification of B-cell immunophenotypes using the following antibodies: (Biolegend Inc., San Diego, CA, USA): CD19 PerCP/Cy5.5 (clone IA6-2), CD20 APC/Fire 750 (clone 2H7), CD24 PE (clone ML5), IgD FITC (clone IA6-2), CD27 PE/Cy7 (clone O323) and CD38 Alexa Fluor 700 (clone HIT2). As a negative control, the corresponding isotype control antibodies were included (Biolegend Inc., San Diego, CA, USA). After a cell viability verification, flow cytometry was performed immediately. For each sample, undyed cells were mixed with isotypes or antibodies, in each one, 300,000 cells were placed; after staining, these samples were incubated for 30 minutes at 4°C. Two washes with PBS 1X and centrifugation for 5 min at 1500 rpm in a volume of 500 μL were made. Finally, samples were resuspended in 500 μL PBS 1X with formaldehyde at 0.5% for subsequent reading immediately.
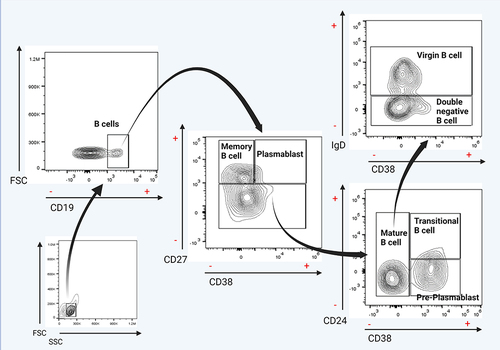
Samples were read in the acoustic focusing Attune® NxT flow cytometer (Life Technologies). The results were analyzed in the FlowJo software v10.0 program (Tree Star, Inc., Ashland, Oregon, USA). The analysis strategy started locating the lymphocytes based on their forward scatter (FC), and side scatters (SS) characteristics. After that, delimiting of subpopulations of interest (gating) was performed based on the CD19+ marker expression. The subsequent gating was to analyze the presence or absence of the CD27 and CD38 markers; subsequently, CD38± and CD27− population was taken for the next gating to analyze the CD24 marker. Finally, the presence or absence of the IgD marker was analyzed from the CD24−/+ and CD38− populations. illustrates those mentioned above. The following immunophenotype was used for the identification of cell subpopulations: memory B cell (CD19+CD27+CD38−), transitional B cell (CD19+CD27−CD24+CD38+), mature B cell (CD19+CD27−CD24−CD38−), virgin B cell (CD19+CD27−CD24−CD38−IgD+), plasmablasts (CD19+CD27+CD38+), early plasmablast (CD19+CD27−CD24−CD38+), and double-negative B cells (CD19+CD27−CD24−CD38−IgD−).
Figure 1 Flow cytometry analysis of B cell subsets and frequency of total B cells. Gating strategy for identifying the indicated B cell subsets in peripheral blood mononuclear cells (PBMCs) previously selected from singlets gate (FSC-A vs FSC-H).
Detection of Neutralizing Antibodies
Neutralizing antibodies were analyzed 21 days after applying each dose of BNT162b2. The neutralizing antibodies were quantified using the cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, Piscataway, NJ, USA) as described previously.Citation26 The inhibition rate was calculated with the following formula:
Statistical Analysis
According to the sample size calculation, the statistical power was assessed, performed with an estimated error margin of 2% with a confidence degree of 95%. The normal distribution of variables was assessed by the Shapiro–Wilk test. Categorical variables are expressed as frequencies, and the Fisher chiCitation2 test was used to compare proportions. Continuous variables with nonparametric variables are expressed as medians and interquartile ranges (IQR), whereas data with parametric distribution are represented as mean with standard deviation. For the analysis of variance, the Mann–Whitney U-test was applied for comparing two groups or Kruskal–Wallis for three or more in nonparametric variables, followed by Dunn’s multiple comparisons. The Wilcoxon signed-rank test was used to compare two related samples. Correlations were evaluated by Spearman’s rank correlation coefficient. Statistical analysis was performed using the GraphPad Prism v. 6.01 software. The significance level was set at p < 0.05.
Results
Clinical Characteristics of Study Groups
Age and gender were similar between the groups (p > 0.05). Individuals in the group with prior COVID-19 had more comorbidities than those without prior COVID-19 (p < 0.005). The percentage of neutralization of the antibodies against SARS-CoV-2 was 30.78% (IQR: 19.4–78.2) in the control group. Individuals with prior COVID-19 had a higher neutralization percentage than those without prior COVID-19 after 21 days post-application of the first BNT162b2 dose (median [IQR]: 98.08% [97.05–98.40] vs 88.79% [80.20–95.76], respectively; p = 0.0413). After the second BNT162b2 dose, no significant differences were observed in the percentage of neutralization ().
Table 1 Clinical and Demographic Characteristics of Study Subjects
B-Cell Subpopulations After the First Dose of the BNT162b2 Vaccine
B-cell subpopulations were analyzed 21 days after the first dose of BNT162b2 immunization in individuals with and without prior COVID-19. The overall percentage of B cells (CD19+) did not show a significant difference between vaccinated or unvaccinated groups (p > 0.05, ).
Figure 2 Subpopulations of B cells after the first dose of the BNT162b2 vaccine. (A) Total B cells, (B) transitional B cells, (C) virgin B cells, (D) mature B cells, (E) memory B cells, (F) plasmablasts, (G) early plasmablasts, and (H) double-negative B cells.
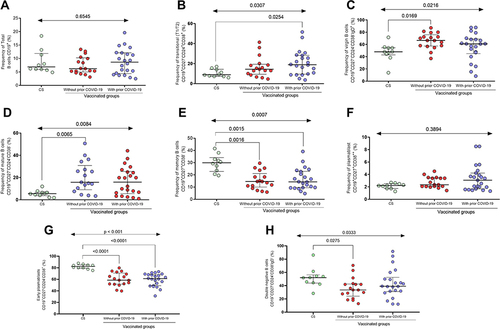
Regarding transitional B cells, we observed that vaccinated people with prior COVID-19 showed a higher percentage of this subpopulation than control subjects (CS) (median 18.9% vs 8.9%; p = 0.0254, ). In contrast, the group without prior COVID-19 shows a higher frequency of virgin B cells than the CS (66.4% vs 47.9%; p = 0.0169, ). In those two previous subpopulations, there were no statistical differences between the vaccinated groups with and without prior COVID-19 (p > 0.05).
People vaccinated with BNT162b2 with and without prior COVID-19 had a higher percentage of mature B cells than CS (median CS = 5.5%; vaccinated without prior COVID-19 = 15.7% and vaccinated with prior COVID-19 = 15.9%; p = 0.0084, ). The frequency of memory B cells was higher in the control group than in vaccinated groups with and without prior COVID-19 (p = 0.0007, ). There was no difference between the immunized groups with and without prior COVID-19 in mature B cell subpopulations and memory B cells (p > 0.05).
For plasmablasts, no statistical difference was observed between vaccinated people and CS (p > 0.05); however, a tendency of a higher percentage of this subpopulation in immunized individuals with prior COVID-19 was observed (median CS = 2.1%; vaccinated without prior COVID-19 = 2.37%, and vaccinated with prior COVID-19 = 3.07%, ).
In the case of early plasmablasts, both individuals vaccinated with and without prior COVID-19 present lower percentages unlike CS (median CS = 82.08%; vaccinated without prior COVID-19 = 59.88%, and vaccinated with prior COVID-19 = 59.11%; p < 0.0001, ). Finally, people vaccinated with BNT162b2 with and without prior COVID-19 had a lower percentage of double-negative B cells than CS (median CS = 52.11%; vaccinated without prior COVID-19 = 36.1% and vaccinated with prior COVID-19 = 43.12%; p = 0.0333, ). No differences were observed between the vaccinated groups (p > 0.05) for both subpopulations (early plasmablast and double-negative B cells).
B-Cell Subpopulations After the Second Dose of the BNT162b2 Vaccine
Only nine individuals with prior COVID-19 and twelve without prior COVID-19 were followed up for the second dose analysis. shows the percentage of B cell sub-populations after the second dose of BNT162b2 (21 days after), where the overall percentage of B cells (CD19+) did not vary significantly between groups (p > 0.05, ) as observed in the first dose. For transitional B cells, no statistical difference was observed between the three groups (p > 0.05, ); however, a trend of more frequent of these cells was observed in the vaccinated group with prior COVID-19 (median CS = 8.9%; vaccinated without prior COVID-19 = 9.4% and vaccinated with prior COVID-19 = 11. 7%).
Figure 3 Subpopulations of B cells after the second dose of the BNT162b2 vaccine. (A) Total B cells, (B) transitional B cells, (C) virgin B cells, (D) mature B cells, (E) memory B cells, (F) plasmablasts, (G) early plasmablasts, and (H) double-negative B cells.
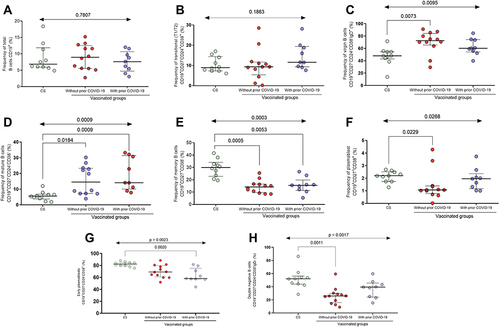
Regarding the subpopulation of virgin B cells, a higher frequency of this subpopulation was maintained in the vaccinated group without prior COVID-19 compared to the control group (median CS = 47.9% vs vaccinated without prior COVID-19 = 72.5%; p = 0.0073, ), as observed in the first dose. There was no difference between the vaccinated groups (p > 0.05).
Mature B cells predominated in the vaccinated group compared to CS (median CS = 5.5%; vaccinated without prior COVID-19 = 14.5% and vaccinated with prior COVID-19 = 14.1%; p < 0.01, ), whereas memory B cells predominated in the control group compared to the vaccinated groups (median CS = 29.9%; vaccinated without prior COVID-19 = 14.2% and vaccinated with prior COVID-19 = 15.5%; p = 0.0003, ). No differences were observed between the vaccinated groups (p > 0.05) for both subpopulations (mature and memory cells).
Plasmablasts after the second dose of BNT162b2 had a higher frequency in the CS compared to the group without prior COVID-19 (median CS = 2.2 vs vaccinated without prior COVID-19 = 1.07; p = 0.0229, ). There was no difference between the vaccinated groups (p > 0.05).
Early plasmablasts had a higher frequency in the CS compared to the immunized group with prior COVID-19 (median CS = 82.08 vs vaccinated with prior COVID-19 = 63.14; p = 0.0020, ). On the other hand, a lower frequency of double-negative B cells was maintained in the vaccinated group without prior COVID-19 compared to the control group (median CS = 52.11% vs vaccinated without prior COVID-19 = 26.15%; p = 0.0011, ), as observed in the first dose. There was no difference in the frequency of these subpopulations between the vaccinated groups (p > 0.05).
Dynamics of Generation of B Cells Subpopulations After the First and Second BNT162b2 Dose in Individuals with and without Prior COVID-19
In and , we observed that the percentage of total B cells (CD19+) does not vary between doses in individuals with and without prior COVID-19 (p > 0.05).
Figure 4 Changes in B-cell subpopulations after the first and second BNT162b2 doses in the groups with and without prior COVID-19. (A) Percentages of total B cells in individuals without prior COVID-19 and (B) with prior COVID-19. (C) Percentages of transitional B cells in individuals without prior COVID-19 and (D) with prior COVID-19. (E) Percentages of virgin B cells in individuals without prior COVID-19 and (F) with prior COVID-19. (G) Percentages of early plasmablast in individuals without prior COVID-19 and (H) with prior COVID-19.
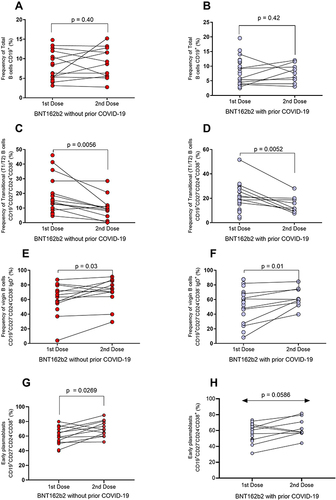
For the subpopulation of transitional B cells, a low percentage of this cell population after applying the second dose of BNT162b2 was observed in individuals without and with prior COVID-19 (p = 0.0056 and p = 0.0052, respectively; and ). Virgin B cells percentage increased after the second dose of BNT162b2 in the groups without and with prior COVID-19 (p = 0.03 and p = 0.01; and ). For early plasmablast, an increase after the second dose of BNT162b2 in the groups without and with prior COVID-19 was observed (p = 0.0269 and p = 0.0586, respectively; and ).
and show that mature B cells did not change in both groups after the first and second BNT162b2 dose (p > 0.05). Also, for memory B cells, there was no significant variation in both groups ( and ; p > 0.05). On the other hand, plasmablasts percentage decreased in both groups after the second dose of the BNT162b2 vaccine ( and ; p = 0.01, and p = 0.003).
Figure 5 Variation in B-cell subpopulations after the first and second doses of BNT162b2 in the groups with and without prior COVID-19. (A) Percentages of mature B cells in individuals without prior COVID-19 and (B) with prior COVID-19. (C) Percentages of memory B cells in individuals without prior COVID-19 and (D) with prior COVID-19. (E) Percentages of plasmablasts in individuals without prior COVID-19 and (F) with prior COVID-19. (G) Percentages of double-negative B cells in individuals without prior COVID-19 and (H) with prior COVID-19.
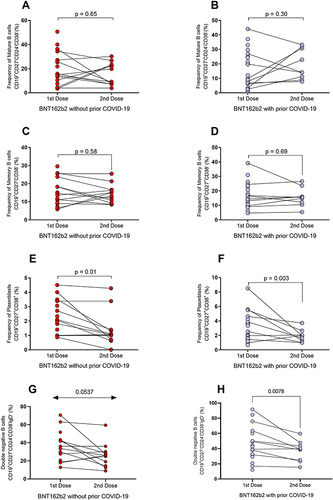
The double-negative B cells decreased after the second dose of BNT162b2 in the groups without and with prior COVID-19 (p = 0.0537 and p = 0.0078, respectively; and ).
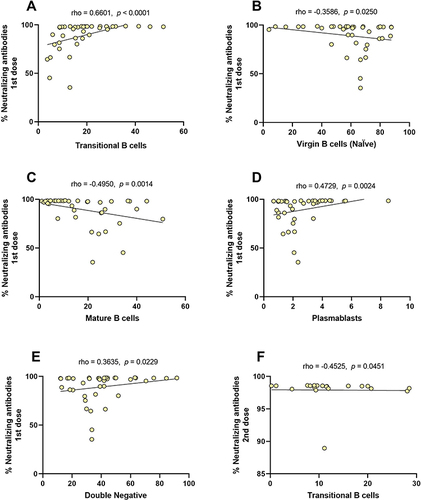
Correlation Between Neutralizing Antibodies and B Cell Subpopulations
Maturation and differentiation of B cells will result in cells capable of secreting antibodies, being the primary function of the humoral immune system, so we performed a general correlation analysis between the levels of neutralizing antibodies and the B cell subpopulations induced by the BNT162b2 vaccine at each dose. In , we observed that the levels of neutralizing antibodies after the first BNT162b2 dose correlate with subpopulations percentages. There was a negative correlation between the percentages of virgin and mature B cells ( and ), whereas a positive correlation was observed with subpopulations of transitional B cells, plasmablasts, and double-negative B cells ( and , and E; p < 0.05). After the second BNT162b2 dose, only a significant correlation was observed between the transitional B cells and the levels of neutralizing antibodies (; r = −0.4525, p = 0.0451).
Table 2 Correlation Between Neutralizing Antibodies After the First and Second BNT162b2 Dose with B Cell Subpopulations
Figure 6 Correlation between neutralizing antibodies after the first and second BNT162b2 dose with B cell subpopulations. (A) Transitional B cells, (B) virgin B cells, (C) mature B cells, (D) plasmablast, (E) double-negative B cells in the first dose, and (F) transitional B cells in the second dose.
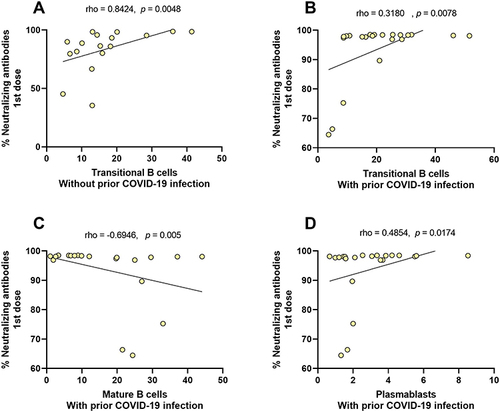
To assess the effect of the previous infection, we analyze stratified groups with and without prior COVID-19. shows that after the first BNT162b2 dose, there was a positive correlation between neutralizing antibodies percentages and Transitional B cells in the group without prior COVID-19 (; r = 0.8424, p = 0.0048). In patients with prior COVID-19, there were also positives correlation between neutralizing antibodies percentages with Transitional B cells (; r = 0.3180, p = 0.0078) and plasmablasts (; r = 0.4854, p = 0.0174), whereas a negative correlation was observed with mature B cells (; r = −0.6946, p = 0.005). Regarding the second BNT162b2 dose, no significant correlations were observed in any study group.
Table 3 Correlation Between Neutralizing Antibodies and Subpopulations of B Cells According to the Antecedent of COVID-19
Figure 7 Correlation between neutralizing antibodies and subpopulations of B cells according to antecedent with and without prior COVID-19. (A) Transitional B cells in the group without prior COVID-19, (B) transitional B cells, (C) mature B cells, and (D) plasmablasts in the group with prior COVID-19, all of the first BNT162b2 dose.
Discussion
SARS-CoV-2 vaccines have reduced death and severe cases of COVID-19.Citation31 However, the immunological mechanisms of immunization of novel platforms as mRNA-based vaccines still have specific questions, one of which is related to humoral immunity. The study’s objective was to analyze the dynamics of the subpopulations of B lymphocytes in response to the BNT162b2 mRNA vaccine in people with and without a history of COVID-19, comparing the results with a group of individuals not vaccinated against SARS-CoV-2.
Analyzing the absolute numbers (frequency) of total B cells (CD19+), we did not detect significant differences between unvaccinated and vaccinated individuals (first and second doses) with BNT162b2 with and without previous COVID-19. There is no similar previous study to compare; however, Sosa et al reported a similar pattern in healthy subjects and people who had fallen ill with COVID-19. Although no change was reported in the total number of B cells, the frequency of some B cell subpopulations analyzed was modified in response to COVID-19 infection; therefore, they suggested that changes in B cell subpopulations could serve as predictors of the antiviral response by the adaptive system against SARS-CoV-2 and even as a potential marker.Citation32 Based on these findings, it is possible that COVID-19 vaccination could have similar effects on these cells.
In the present study, transitional B-cells predominated in vaccinated individuals with prior COVID-19 in the first and second doses; especially, there was a significant difference compared to unvaccinated individuals in the first dose. This cellular population has been involved in response to infectious processes;Citation14,Citation33 Giltiay et al suggested a direct role of this cell subpopulation in protecting against infections since they can be differentiated into plasma cells, mediated by pattern recognition receptors.Citation34 In COVID-19, these cells have been involved in the innate immune response;Citation33 therefore, it is necessary to characterize their role in this disease and other infectious processes. Based on the above, we suggest that this increase in the frequency of transitional B cells in people vaccinated with BNT162b2 would be involved in developing and maturation towards antibody-secreting cells.Citation35 People vaccinated with prior COVID-19 reach slightly higher values of neutralizing antibodies, unlike those without prior COVID-19,Citation36 which could be due to the increase of this cellular subpopulation maintained in both doses of the BNT162b2 vaccine.
Instead, the frequency of mature B cells after the first and second doses in both vaccinated groups was higher than in those unvaccinated individuals. It has been reported that this cell subpopulation correlates with the transition cells. This can be explained because the increase in both subpopulations can indicate a stimulation process,Citation37 which the BNT162b2 vaccine could induce.
Mature B cells possess a B-cell receptor (BCR) with sufficient avidity of antigens that can interact with T follicular helper cells to be activated and go into clonal expansion to generate germinal centers where somatic hypermutation takes place for generating optimal and specific antibodies.Citation38 Brewer et al demonstrated that SARS-CoV-2-specific memory B cells derive from virgin B cells (naïve) in response to the BNT162b2 vaccine.Citation39 Our study does not have this analytical capability; however, we infer that the increase of this subpopulation in the group without prior COVID-19 compared to the control group may indicate a cell maturation process for differentiation to memory B cells or plasma cells. Schulz et al analyzed the role of virgin B cells in a particular group of immunocompromised people (where this subpopulation is diminished) immunized with the mRNA-1273 vaccine (another mRNA-based vaccine) and concluded that virgin B cells are associated with antibody levels and are a strong predictor for generating antibody levels (titers) comparable to the vaccinated control group,Citation40 which is consistent with the above.
Regarding memory B cells, in both doses of the BNT162B2, vaccinated people had less percentage of these cells than unvaccinated individuals. So far, no studies report this pattern in vaccination; however, this result is consistent with those reported by Biasi et al and Sosa et al, studies performed in patients with COVID-19 of different severity and control groups. They reported lower memory B cells percentages than control subjects, proposing that this decrease is due to the activation of pre-existing memory cells (specific for a different coronavirus of SARS-CoV-2, eg, endemic coronavirus [endemic HCoVs] or SARS-CoV-1), to differentiate into plasma cells by cross-reaction.Citation32,Citation41,Citation42 However, a more detailed study is necessary to clear this finding since we find plasmablasts and not plasma cells in peripheral blood because these cells are mainly found in the bone marrow.
Ciabattini et al reported the persistence of specific memory B cells against SARS-CoV-2 by vaccination with the BNT162b2 vaccine for six months after the complete schema, with a peak seven days after the second dose and a decrease after 180 days.Citation29 We did not perform a long-term study, but with the above, we can speculate that the BNT162b2 vaccine helps in the generation of memory B cells and that added to this would be necessary a long-term comparative study between groups of people vaccinated with and without prior COVID-19 to determine the role of pre-existing natural immunity.
Concerning plasmablasts, we observed an interesting behavior. During the first dose of the BNT162b2 vaccine, plasmablasts were more frequent in vaccinated individuals than in unvaccinated; however, that result did not show a statistical difference. Surprisingly, at the second dose, vaccinated individuals showed a lower frequency of plasmablasts than those unvaccinated. This could be due to differentiation towards plasma cells in vaccinated individuals, as this differentiation is necessary for antibody production.Citation30 This hypothesis is supported by the study of Amanat et al, where plasmablasts are related to the production of non-neutralizing antibodies; therefore, these cells must be differentiated to produce neutralizing antibodies.Citation43,Citation44 On the other hand, He et al relate this subpopulation with a polyclonal response; therefore, differentiation is necessary for a more specific response.Citation43,Citation44 Additionally, the values of neutralizing antibodies against SARS-CoV-2 shown in are indicators that mRNA-type vaccines induce plasma cell differentiation in vaccinated individuals, especially with a faster synthesis in those individuals with prior SARS-CoV-2.
Early plasmablasts are proliferative cells characterized by the central markers CD38+CD24− expressing low levels of CD2719; they have been identified in response to the tetanus vaccine, showing a similar kinetics response to conventional plasmablasts.Citation45 In our study, we found a lower frequency of early plasmablasts in the immunized groups (without and with prior COVID-19) at both doses of the BNT162b2 vaccine compared to CS; however, the intergroup dose analysis shows an increase in this subpopulation after the second dose being more marked in the group without prior COVID-19. Kardava et al studied the response of B cells in the Moderna COVID-19 vaccine (mRNA-1273), showing a brief burst of this subpopulation on day seven post-vaccination followed by a slower phase studied until day 60 post-vaccination.Citation46 This elevation is consistent with the intergroup increase in our analysis; on the other hand, the decrease of this subpopulation in immunized individuals compared to CS could be due to the probable differentiation toward plasmablast and plasma cells, as this subset increases the expression of BLIMP-1 while maintaining the expression of Pax5, two transcriptional factors involved in differentiation to plasma cells.Citation47 Complementary to the above, early plasmablasts have also been related to an extrafollicular response, being producers of IgM-type antibodies before a rapid response to antigen.Citation48
Double-negative B cells (CD27−IgD−) are a subpopulation discovered in Systemic Lupus Erythematosus, an autoimmune disease;Citation49 however, recently, they have been involved in infectious processes, cancer, and vaccination.Citation20 The present study shows that this cell subpopulation is lower mainly in vaccinated individuals without prior COVID-19 than CS. We did not find studies with similar findings to us; however, Ruschil et al reported an increase in circulating double-negative B cells on days 4, 7, and 14 after vaccination against the influenza virus, being the peak on day 7; a similar finding was observed for the vaccine against the encephalitis virus.Citation50 We detected a low frequency of this subpopulation approximately 21 days after vaccination with BNT162b2, which could be an apparently opposite result to that previously described; however, we suggest that the vaccination response to these cells could be fast (4–14 days) and then these cells could be differencing after having presented an increase. This hypothesis is based on the fact that double-negative B cells have characteristics of B cells experimented with antigen in terms of surface phenotype, proliferation response, and patterns of somatic hypermutation to a lesser degree;Citation50,Citation51 moreover, other studies have described that this subset comes from activated virgin B cells and that they can further differentiate into plasmablasts through an extrafollicular maturation pathway.Citation19
We are aware that the study has some limitations; the main one is that we do not have the baseline immunophenotypes of B cell subpopulations of people vaccinated with BNT162b2 to observe the main changes. However, the comparison with healthy unvaccinated individuals as a control group helps us to make an inference. The other limitation is that we do not have the complete follow-up of all the individuals vaccinated in the first dose for issues beyond our control.
Regarding the role of prior SARS-CoV-2 infection in the dynamics of B cell subpopulations after vaccinations with the BNT162b2, we observed that the groups with and without prior COVID-19 had the same behavior in all subpopulations between the first and second dose of the vaccine. However, five subpopulations showed significant changes: transitional B cells decrease after the second dose of the vaccine, virgin B cells (naïve) increase with the second dose, early plasmablast increase with the second dose, double-negative B cells decrease after the second dose of the vaccine and plasmablasts are decreased by the second dose. These findings may suggest that transitional B cells increase after vaccine-induced antigenic stimulation and that, similar to virgin B cells, they increase after the full vaccination schedule. Therefore, these transitional B cells are maturing and differentiating to produce mature B cells capable of entering a germinal center and generating antibody-producing cells.Citation38 The latter could be supported because there was a reduction of plasmablasts after the second dose since these cells need to differentiate into plasma cells in the bone marrow. Other less frequent subpopulations, such as the early plasmablasts and double-negative B cells, could also differentiate into plasma cells; however, they could be related to an extrafollicular response or thymus-independent.
This study has been unable to demonstrate that previous SARS-CoV-2 infection can significantly alter the dynamics of B cells after immunization with the BNT162b2 vaccine. However, the production of neutralizing antibodies correlates in a significant way with the subpopulations of B cells (transitional, mature, virgin, plasmablasts, and double-negative B cells), specifically in the first dose of the BNT162b2 vaccine, where the neutralizing antibodies from individuals with prior COVID-19 strongly correlate with transitional B cells (r=0.8424).
Therefore, it seems that after a second exposure to the antigen, there are no noticeable changes in the production or proliferation of new B cells in peripheral blood; however, there is a faster and optimal production of neutralizing antibodies with a single dose of the BNT162b2 vaccine in individuals with a history of COVID-19. This finding supports the proposal of some authors that a single dose of the BNT162b2 vaccine in individuals with prior COVID-19 had similar results in neutralizing antibody production than those without prior COVID-19 that receive a complete scheme (2 doses).Citation52–55
Conclusion
Our results showed differences in B lymphocyte subpopulations in response to the BNT162b2 vaccine among vaccinated (with and without prior SARS-CoV-2) and unvaccinated individuals. The plasmablasts were key cells for determining the effect of the BNT162b2 vaccine. We observed a decrease of these cells after the second dose in vaccinated individuals, which its differentiation could explain to plasmatic cells. On the other hand, there were slight variations among vaccinated individuals with and without prior COVID-19 without significant differences. The findings support that this mRNA vaccine can induce B cells’ cell differentiation and generates plasma cells that produce specific antibodies against SARS-CoV-2.
Ethics approval and informed consent
The Ethics Committee of the University Center of Health Sciences of the University of Guadalajara (protocol code 21–10) approved this study conducted according to the guidelines of the Declaration of Helsinki. Written informed was obtained from all the patients to publish this paper.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author thank all whose comments improved the paper.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Li J, Huang DQ, Zou B et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458. doi:10.1002/jmv.26424
- Guiso N. Impact de la vaccination sur l’épidémiologie des maladies infectieuses : exemple de la coqueluche. [Impact of vaccination on the infectious diseases epidemiology: example of pertussis]. Med Sci. 2007;23(4):399–403. French. doi:10.1051/medsci/2007234399
- Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. 2011;3(1):73–78. doi:10.4103/0974-777X.77299
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi:10.1038/s41577-020-00479-7
- Lee S, Ryu JH. Influenza viruses: innate immunity and mRNA vaccines. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.710647
- Platt A, Wetzler L. Innate immunity and vaccines. Curr Top Med Chem. 2013;13(20):2597–2608. doi:10.2174/15680266113136660185
- Jiskoot W, Kersten GFA, Mastrobattista E, Slütter B. Vaccines. In: Crommelin DJA, Sindelar RD, Meibohm B, editors. Pharmaceutical Biotechnology: Fundamentals and Applications. Springer International Publishing; 2019:281–304.
- Casado JL, Haemmerle J, Vizcarra P, et al. T-cell response after first dose of BNT162b2 SARS-CoV-2 vaccine among healthcare workers with previous infection or cross-reactive immunity. Clin Transl Immunol. 2021;10(9):e1341. doi:10.1002/cti2.1341
- Almendro-Vázquez P, Laguna-Goya R, Ruiz-Ruigomez M, et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021;17(12):e1010211. doi:10.1371/journal.ppat.1010211
- Dunn-Walters DK, Stewart AT, Sinclair EL, Serangeli I. Age-related changes in B cells relevant to vaccine responses. Interdiscip Top Gerontol Geriatr. 2020;43:56–72.
- Diks AM, Overduin LA, Van Leenen LD, et al. B-cell immunophenotyping to predict vaccination outcome in the immunocompromised - A systematic review. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.690328
- Martin VG, Wu YCB, Townsend CL, et al. Transitional B cells in early human B cell development – time to revisit the paradigm? Front Immunol. 2016;7. doi:10.3389/fimmu.2016.00546
- Zhou Y, Zhang Y, Han J, Yang M, Zhu J, Jin T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J Transl Med. 2020;18(1):131. doi:10.1186/s12967-020-02289-w
- Sagaert X, Sprangers B, De Wolf-Peeters C. The dynamics of the B follicle: understanding the normal counterpart of B-cell-derived malignancies. Leukemia. 2007;21(7):1378–1386. doi:10.1038/sj.leu.2404737
- Palm AKE, Henry C. Remembrance of things past: long-term B cell memory after infection and vaccination. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.01787
- Mitchell R, Kelly DF, Pollard AJ, Trück J. Polysaccharide-specific B cell responses to vaccination in humans. Hum Vaccin Immunother. 2014;10(6):1661–1668. doi:10.4161/hv.28350
- Ellebedy AH, Jackson KJL, Kissick HT, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17(10):1226–1234. doi:10.1038/ni.3533
- Sanz I, Wei C, Jenks SA, et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.02458
- Li Y, Li Z, Hu F. Double-negative (DN) B cells: an under-recognized effector memory B cell subset in autoimmunity. Clin Exp Immunol. 2021;205(2):119–127. doi:10.1111/cei.13615
- van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJM. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. 2007;204(3):645–655. doi:10.1084/jem.20060964
- Blanco E, Pérez-Andrés M, Arriba-Méndez S, et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141(6):2208–2219.e16. doi:10.1016/j.jaci.2018.02.017
- Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111(8):3941–3967. doi:10.1182/blood-2007-11-120535
- Kent SJ, Juno JA. Vaccination after prior COVID-19 infection: implications for dose sparing and booster shots. eBioMedicine. 2021;72:103586. doi:10.1016/j.ebiom.2021.103586
- Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi:10.1056/NEJMoa2118691
- Hammerman A, Sergienko R, Friger M, et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med. 2022;386(13):1221–1229. doi:10.1056/NEJMoa2119497
- Dalle Carbonare L, Valenti MT, Bisoffi Z, et al. Serology study after BTN162b2 vaccination in participants previously infected with SARS-CoV-2 in two different waves versus naïve. Commun Med. 2021;1(1):1–11. doi:10.1038/s43856-021-00039-7
- Morales-Núñez JJ, Muñoz-Valle JF, Meza-López C, et al. Neutralizing antibodies titers and side effects in response to BNT162b2 vaccine in healthcare workers with and without prior SARS-CoV-2 infection. Vaccines. 2021;9(7):742. doi:10.3390/vaccines9070742
- Ciabattini A, Pastore G, Fiorino F, et al. Evidence of SARS-CoV-2-specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12:3751. doi:10.3389/fimmu.2021.740708
- Giannotta G, Giannotta N. mRNA COVID-19 vaccines and long-lived plasma cells: a complicated relationship. Vaccines. 2021;9(12):1503. doi:10.3390/vaccines9121503
- Wang H, Paulson KR, Pease SA, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. doi:10.1016/S0140-6736(21)02796-3
- Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, et al. B cell subsets as severity-associated signatures in COVID-19 patients. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.611004
- Li S, Ma F, Hao H, et al. Marked elevation of circulating CD19+CD38hiCD24hi transitional B cells give protection against neonatal sepsis. Pediatr Neonatol. 2018;59(3):296–304. doi:10.1016/j.pedneo.2017.10.005
- Giltiay NV, Giordano D, Clark EA. The plasticity of newly formed B cells. J Immunol. 2019;203(12):3095–3104. doi:10.4049/jimmunol.1900928
- Oliviero B, Varchetta S, Mele D, et al. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol Immunol. 2020;17(10):1101–1103. doi:10.1038/s41423-020-00542-2
- Gobbi F, Buonfrate D, Moro L, et al. Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13(3):422. doi:10.3390/v13030422
- Shahaf G, Zisman-Rozen S, Benhamou D, Melamed D, Mehr R. B cell development in the bone marrow is regulated by homeostatic feedback exerted by mature B cells. Front Immunol. 2016;7:77. doi:10.3389/fimmu.2016.00077
- Chan TD, Brink R. Affinity-based selection and the germinal center response. Immunol Rev. 2012;247(1):11–23. doi:10.1111/j.1600-065X.2012.01118.x
- Brewer RC, Ramadoss NS, Lahey LJ, Jahanbani S, Robinson WH, Lanz TV. BNT162b2 vaccine induces divergent B cell responses to SARS-CoV-2 S1 and S2. Nat Immunol. 2022;23(1):33–39. doi:10.1038/s41590-021-01088-9
- Schulz E, Hodl I, Forstner P, et al. CD19+IgD+CD27- Naïve B cells as predictors of humoral response to COVID 19 mRNA vaccination in immunocompromised patients. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.803742
- De Biasi S, Lo Tartaro D, Meschiari M, et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur J Immunol. 2020;50(9):1283–1294. doi:10.1002/eji.202048838
- Song G. H Wting, Callaghan S et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat Commun. 2021;12(1):2938. doi:10.1038/s41467-021-23074-3
- Amanat F, Thapa M, Lei T, et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies and targets both the NTD and the RBD. medRxiv. 2021. doi:10.1101/2021.03.07.21253098
- He X-S, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365(1–2):67–75. doi:10.1016/j.jim.2010.12.008
- Qian Y, Wei C, Eun-Hyung Lee F, et al. Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identification of cell populations in multidimensional flow cytometry data. Cytometry B Clin Cytom. 2010;78B(S1):S69–S82. doi:10.1002/cyto.b.20554
- Kardava L, Rachmaninoff N, Lau WW, et al. Pre-vaccination and early B cell signatures predict antibody response to SARS-CoV-2 mRNA vaccine. medRxiv. 2021. doi:10.1101/2021.07.06.21259528
- Garimalla S, Nguyen DC, Halliley JL, et al. Differential transcriptome and development of human peripheral plasma cell subsets. JCI Insight. 2019;4(9). doi:10.1172/jci.insight.126732.
- Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183(5):3139–3149. doi:10.4049/jimmunol.0901690
- Jenks SA, Cashman KS, Zumaquero E, et al. Distinct effector B Cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49(4):725–739.e6. doi:10.1016/j.immuni.2018.08.015
- Ruschil C, Gabernet G, Lepennetier G, et al. Specific induction of double negative B cells during protective and pathogenic immune responses. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.606338
- Wei C, Anolik J, Cappione A, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624–6633. doi:10.4049/jimmunol.178.10.6624
- Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93(7):4612–4615. doi:10.1002/jmv.26982
- Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;3:1–4.
- Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58). doi:10.1126/sciimmunol.abi6950.
- Krammer F. ARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi:10.1038/s41586-020-2798-3
