Abstract
Purpose
The advent of immune checkpoint inhibitors (ICIs) is a revolutionary breakthrough. However, without the selection of a specific target population, the response rate of ICI therapy in lung adenocarcinoma (LUAD) is low, so a clinical challenge has arisen in effectively using biomarkers to determine which patients can benefit from ICI therapy.
Methods
In this study, patients were divided according to whether or not nonsynonymous mutations were present in the BCR signaling pathway, and univariate and multivariate Cox regression models were established based on a LUAD cohort treated with ICIs (Miao-LUAD). Then the relationship between the mutation status of the BCR signaling pathway and the prognosis of immunotherapy was examined. Finally, data from The Cancer Genome Atlas (TCGA) LUAD cohort, the Rizvi-LUAD, the Samstein-LUAD, and the Zhujiang Hospital of Southern Medical University LUAD (Local-LUAD) cohort were combined, and the mutation panorama, immunogenicity, tumor microenvironment (TME) and pathway enrichment analysis between the BCR signaling pathway mutant group (BCR signaling MUT) and the BCR signaling pathway wild group (BCR signaling WT) were comprehensively compared.
Results
It was found that, compared with the BCR signaling WT, the BCR signaling MUT had a significantly improved progression-free survival (PFS) rate and overall survival (OS) rate, higher immunogenicity (tumor mutational burden, neoantigen load, and DNA damage response signaling mutations), and anti-tumor immune microenvironment.
Conclusion
These results revealed that the mutation state of the BCR signaling pathway has potential as a biomarker to predict the efficacy of ICIs in LUAD.
Introduction
Among all cancer-related deaths, those caused by lung cancer rank first both globally and in China, posing a significant threat to human health.Citation1,Citation2 Lung adenocarcinoma (LUAD) is the main histological type in NSCLC, accounting for 40% of total lung cancer instances. Although surgery is the first choice of treatment for patients with early-stage LUAD, more than 70% of patients have reached advanced stages by the time they see a doctor. The 5-year survival rate of patients with advanced LUAD under traditional chemotherapy and radiotherapy is less than 5%.Citation3 The advent of immune checkpoint inhibition (ICI) therapy has brought about revolutionary changes in the treatment of solid tumors including lung cancer. The ICIs target the PD-1 or the PD-L1 axis, thus restoring the anti-tumor activity of T cells.Citation4 In the treatment of NSCLC, the use of ICIs alone or together with chemotherapy has demonstrated remarkable curative effects, and it has already become a standard treatment program for patients with advanced NSCLC. According to the results of a Phase III clinical trial,Citation5 the 5-year survival rate of NSCLC patients who have previously received platinum therapy is increased by more than 5 times (13.4% vs 2.6%) after PD-1 inhibitor treatment, compared with the docetaxel treatment group. However, due to factors including the exhaustion of T cells, the defect of antigen presentation, the influence of other immune checkpoint molecules and damage to the IFN-γ signaling pathway, the effective rate of ICI therapy rarely exceeds 40%.Citation6–8 Therefore, it is important to apply clinical biomarkers to screen potential beneficiaries of immunotherapy.
At present, many studies have revealed potential biomarkers for predicting the efficacy of ICI therapy such as PD-L1 expression and nonsynonymous tumor mutation burden (TMB).Citation9 However, some studies have found that NSCLC patients can benefit from nivolumab immune checkpoint therapy regardless of the expression level of PD-L1. Furthermore, the expression of PD-L1 is a dynamic process. It can be induced by IFN-γ secreted by tumor infiltrating lymphocytes (TILs), which can be affected by the process of treatment.Citation10 At present, TMB, which is documented as being positively correlated with the OS of ICI therapy, has uneven detection quality, and there is no unified cutoff standard.Citation11 Some studies have even shown that high TMB does not suggest a positive ICIs curative effect.Citation12 Neither PD-L1 nor TMB alone is sufficient as a predictor of the efficacy of ICIs. Therefore, more practical biomarkers are needed to screen the population of LUAD patients under ICI therapy.
The B cell receptor (BCR) is a membrane immunoglobulin that transmits signals downstream by binding extracellular antigens and ligands, thus regulating the proliferation, activation, differentiation, cell selection and apoptosis of B cells.Citation13 B cells infiltrated in a tumor microenvironment are thought to have a dual function in tumor promotion and anti-tumor immunity.Citation14 B cells activated by the BCR signaling pathway can act as antigen-presenting cells, presenting antigens to the cell surface, and then activating tumor-specific CD8+ T cells, thus enhancing anti-tumor immunity.Citation15 It is also reported that plasma cells differentiated from a B cell can infiltrate tumors and play an anti-tumor role by producing the tumor-specific antibody IgG1.Citation16 However, an overactivated BCR signaling pathway is also considered an important contributing factor in the occurrence and development of B-cell-derived malignant tumors such as chronic lymphocytic leukemia and diffuse large B-cell lymphoma.Citation17,Citation18 Clinically, there are many inhibitors of BCR and related pathways, such as Bruton’s tyrosine kinas inhibition.Citation19 However, at present, the relationship between the BCR signaling pathway and LUAD is not clear, and more research is needed on the relationship between the mutation of this pathway and the efficacy of ICIs. Therefore, we hope to explore the relationship between the mutation state of the BCR signaling pathway and the efficacy of ICIs through analysis of clinical, genome and transcriptome information.
In this study, a TCGA-LUAD data set, Samstein-LUAD, Rizvi-LUAD and Miao-LUAD ICIs-treated data sets, as well as Local-LUAD data sets were analyzed; the prognosis of ICI treatment was compared after grouping according to the nonsynonymous mutation status of the BCR signaling pathway; the relationship between BCR signaling pathway mutation and ICI treatment efficacy from the gene level to the tumor microenvironment level was comprehensively analyzed using the bioinformatics method; and hypotheses were formed about the related mechanism.
Materials and Methods
LUAD Sample Collection
Three data sets of LUAD patients treated with ICIs were collected. The Rizvi-LUAD group included 186 advanced LUAD patients treated with anti–PD-(L)1 monotherapy alone or in combination with anti–cytotoxic T-cell lymphocyte-4.Citation20 The Miao-LUAD group included 47 advanced LUAD patients treated with anti-PD-1, anti-PD-L1, anti-CTLA-4, or a combination of these therapies.Citation21 The Samstein-LUAD included 266 LUAD patients who received at least one dose of immunotherapy (atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, or tremelimumab).Citation22 Both ICIs-treated cohorts included mutation data from before they had received ICI treatment and prognosis data. In addition, the LUAD dataset was downloaded from the TCGA database using the TCGA biolinks R package,Citation23 which included mutation data, expression data and clinical data of 511 patients.
70 LUAD samples were collected from Zhujiang Hospital of Southern Medical University, and targeted sequencing was used (HapOncoTM680 Panel) to obtain mutation data. (see Supplementary Table 1 for Panel on targeted sequencing). The written informed consent of all participants was obtained, and this study was approved by the Zhujiang Hospital Research Ethics Committee of Southern Medical University.
Status Analysis of BCR Receptor Signaling Pathway
The B cell receptor signaling pathway gene set (KEGG_B_CELL_RECEPTOR_SIGNALING_PATHWAY) comes from the MsigDB database (Supplementary Table 2).Citation24 The somatic mutation data of patients was processed (deleting synonymous mutation data and retaining nonsynonymous mutation data, and the nonsynonymous mutation type include “Frame_Shift_Del”, “Frame_Shift_Ins”, “Splice_Site”, “Translation_Start_Site”,“Nonsense_Mutation”, “Nonstop_Mutation”, “In_Frame_Del”,“In_Frame_Ins”, “Missense_Mutation”).Citation25 Then, the number of gene mutations in the B cell receptor signaling pathway was counted in every LUAD patient. If the number of gene mutations in the B cell receptor signaling pathway in LUAD patients is zero, then the patient is a B cell receptor signaling pathway wild-type (WT). Otherwise, the patient is a B cell receptor signaling pathway mutant-type (MUT). The baseline clinical information on Miao-LUAD, TCGA-LUAD is shown in the Supplementary Tables 3 and 4.
Immunogenicity, Immune Infiltration and Pathway Enrichment Analysis
Drawing on previously published research results, Immunogenicity data on tumor TMB and neoantigen loads (NAL) was included, NAL of the TCGA-LUAD cohort was directly obtained from a published literature.Citation26 TMB score of Samstein-LUAD cohort and Rizvi-LUAD cohort was directly obtained from the public datesets, and TMB score of Local-LUAD cohort and Miao-LUAD cohort was quantified by dividing the number of somatic mutations by 38 Mb. In addition, we collected the gene sets of DNA damage repair-related pathways from MSigDB,Citation27 and counted the number of nonsynonymous mutations in each patient’s DNA damage repair (DDR)-related pathways. The immune cell analysis and the immune related pathway score analysis were the main types of immune infiltration analyses used. In the analysis of immune cells, we applied a CIBERSORT algorithmCitation28 to estimate the relative abundance of 22 types of immune cells in each patient’s TME. Immune-related scores come from a previously published study.Citation26 The pathway enrichment analysis included a gene set enrichment analysis (GSEA), and a single sample gene set enrichment analysis (ssGSEA).Citation29,Citation30
Statistical Test
The Mann–Whitney U-test and Fisher’s exact test were used to evaluate the statistical differences between the two groups of categorical variables. The univariate and multivariate Cox risk proportional regression models and the Kaplan-Meier analysis were used to explore the prognosis of the B cell receptor signaling pathway for patients with LUAD who received ICI treatment. All data analysis and data visualization in this study are based on R software (Version 3.6). The p value is bilateral and p<0.05 has statistical difference.
Results
LUAD Patients with BCR Signaling Pathway Mutation Have Better PFS and OS After ICI Treatment
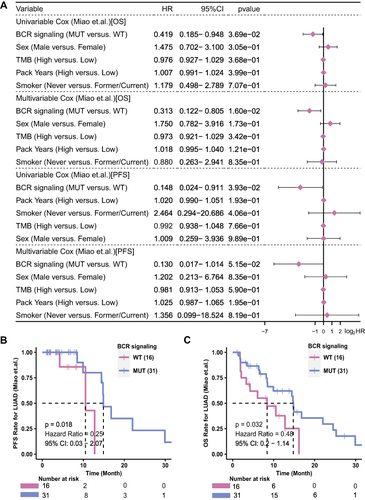
is a brief flowchart of this study. The Miao-LUAD cohort was used as our discovery cohort, and the Samstein-LUAD and Riziv-LUAD, TCGA-LUAD cohort and Local-LUAD cohort were used as verification cohorts. The detailed analysis process is showed in the flow chart. According to whether or not the BCR signaling pathway gene showed nonsynonymous mutation, the 47 patients in the Miao-LUAD cohort were divided into a BCR signaling MUT group (31) and a BCR signaling WT group (16). The results of the univariate Cox regression model showed that the BCR signaling pathway was a protective factor in the mutant group, which could predict better PFS and OS. After adjusting for related clinical confounding factors such as gender, pack years, TMB, and smoking, the results of the multivariate Cox regression model further confirmed that the mutation of the BCR signaling pathway can be used as an independent protective factor for LUAD patients receiving ICI treatment (). Next, a Kaplan-Meier survival analysis of the Miao-LUAD cohort between BCR signaling MUT and WT groups was carried out to establish whether the mutant state of this pathway can effectively predict the prognosis of ICI treatment. The results showed that the BCR signaling MUT group had significantly longer PFS (Logrank test, HR = 0.25, 95% CI [0.03–2.07], p = 0.018) () and longer OS (Logrank test, HR = 0.48, 95% CI [0.20–1.14], p = 0.032) ().Citation21
Figure 1 Work flowchart of clinical cohort establishment and subsequent analysis in this study.
Figure 2 The predictive value of clinical characteristics and the mutation status of the BCR signaling pathway for ICI efficacy. (A) Forest plot of the results of the univariate and multivariate Cox regression analyses in the Miao-LUAD cohort (ICI-treated cohort). The main portion of the forest plot presents the hazard ratios (HR) and 95% confidence intervals (95% CI). The p value represents the statistical significance of the variable. The HR indicates whether the factors are predictors of favorable (HR < 1) or poor (HR > 1) outcomes. KM survival curves for (B) PFS and (C) OS in 47 LUAD patients from the Miao-LUAD cohort.
Overview of Gene Mutation Between BCR Signaling MUT and WT Groups
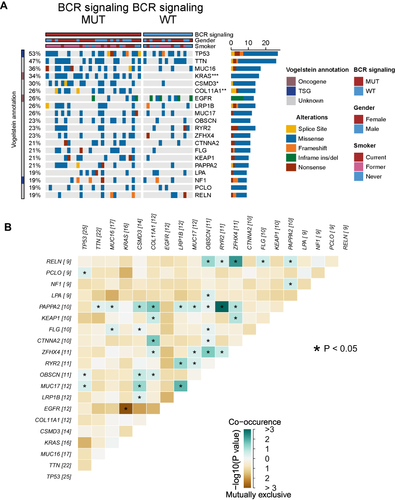
In the Miao-LUAD cohort, the somatic mutant genes were compared with the top 20 mutation frequencies across BCR signaling MUT patients and BCR signaling WT patients (). It was found that in the BCR signaling MUT group, KRAS (52% vs 0%; p<0.05), CSMD3 (42% vs 0.06%; p<0.05), and COL11A1 (39% vs 0; p<0.05), the mutation frequency of the three genes, of which only the KRAS gene is tumorigenic, increased significantly. It is also worth noting that there was no statistically significant difference in gender or in the proportion of smokers and non- smokers between the MUT and WT groups, suggesting that these two clinical factors may not be related to the mutation of the BCR signaling pathway. Subsequently, mutually exclusive co-occurrence analyses were conducted on these 20 genes (). We then used the lollipop diagram to show the mutation sites of the KRAS gene and COL11A1 gene which mutated only in the BCR signaling MUT group (Supplementary Figure 1).
Figure 3 Genomic profiles of 47 LUAD patients in the Miao-LUAD cohort. (A) The 20 genes with the highest mutation frequencies and corresponding clinical information. (B) Mutual exclusion co-occurrence analysis of the top 20 mutated genes. BCR signaling MUT, b cell receptor signaling mutation group, BCR signaling WT, b cell receptor signaling wild group (*p<0.05; **p<0.01; and ***p<0.001).
The BCR Signaling MUT Group Indicates Higher Immunogenicity Compared with the BCR Signaling WT Group
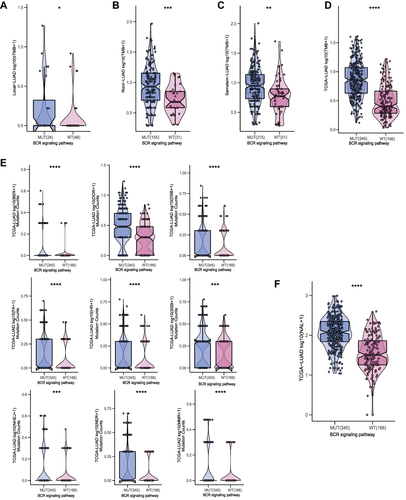
Sufficient immunogenicity is the basis of the immune response. In order to explore the relationship between the mutation status of the BCR signaling pathway and immunogenicity, the TMB, NAL and mutation numbers of DNA damage repair (DDR) related pathways were compared. The results of TMB analysis showed that among the Local-LUAD, Rizvi-LUAD, Samstein-LUAD and TCGA-LUAD, the TMB of the BCR signaling MUT group was significantly higher than that of the BCR signaling WT group (; all p<0.05). Furthermore, in the TCGA-LUAD, the analysis results on the number of DDR related pathways mutations also support the hypothesis that the BCR signaling MUT group had higher immunogenicity; that is, the BCR signaling MUT group had higher numbers of BER, DDR, DSB, FA, HR, SSB, NHEJ, NER and MMR pathway gene mutations than the BCR signaling WT group (; all p<0.05). In addition, the TCGA-LUAD cohort BCR signaling MUT group had higher NAL than the BCR signaling WT group (; p<0.05).
Figure 4 Comparison of TMB between the MUT and WT groups in the (A) Local-LUAD cohort, (B) Rizvi-LUAD cohort, (C) Samstein-LUAD cohort, and (D) TCGA-LUAD cohort. (E) Comparison of DDR related signaling pathways alterations between the MUT and WT groups in the TCGA-LUAD cohort. (F) Comparison of NAL between the MUT and WT groups in the TCGA-LUAD cohort. MUT, B cell receptor signaling pathway mutant type; WT, B cell receptor signaling pathway wild type; (*p<0.05; **p<0.01; ***p<0.001; and ****p<0.0001).
Differences in the Tumor Microenvironments Between BCR Signaling MUT and BCR Signaling WT
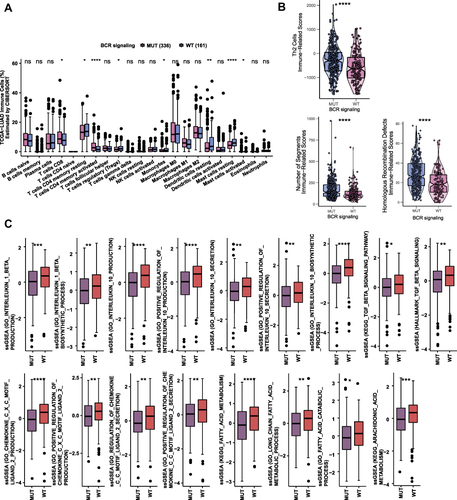
Based on the mutation data and transcriptome data of the TCGA-LUAD cohort, a comparative evaluation was conducted to determine the difference between the mutation status of the BCR signaling pathway and the 22 kinds of immune cells infiltrated in tumors, using a CIBERSORT algorithm (). The results showed that the numbers of CD8+ T cells, activated CD4 memory T cells and activated mast cells in the BCR signaling MUT group were higher (all p<0.05) than in the BCR signaling WT group. However, the numbers of regulatory T cells (Tregs), monocytes, resting CD4 T memory cells, resting mast cells, and resting dendritic cells increased significantly in BCR signaling WT group (all p<0.05). Then the immune-related scores (TH2 score, number of segment score and Homologous recombination score) were calculated in the TCGA-LUAD cohort (), and all of them were significantly higher in the BCR signaling MUT group (all p<0.05).
Figure 5 (A) Comparison of the proportions of immune cells estimated by the CIBERSORT method between MUT and WT groups in the TCGA-LUAD cohort. (B) Comparison of immune related scores between MUT and WT groups in the TCGA-LUAD cohort. The immune related scores are Th2 Cell, number of segment score and Homologous recombination score. (C) Results of ssGSEA analysis between MUT and WT groups in the TCGA-LUAD cohort (*p<0.05; **p<0.01; ***p<0.001; and ****p<0.0001; Wilcoxon rank-sum test).
Pathway Enrichment Analysis Between BCR Signaling MUT and BCR Signaling WT
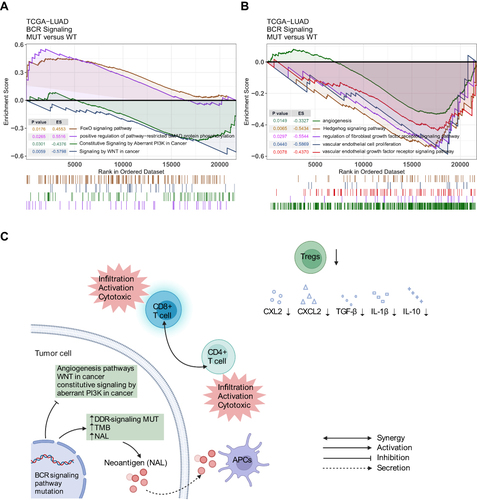
Based on transcriptome data of the TCGA-LUAD cohort, ssGSEA and GSEA were used to enrich and analyze the functional gene sets. The results of ssGSEA () showed that the ssGSEA scores of several cytokines and chemokines pathways related to IL-1β, IL-10, CXCL2, CXC2 and TGF-β, were significantly lower in the BCR signaling MUT group (all p<0.05). The ssGSEA score of arachidonic acid and fatty acid metabolism pathways in the BCR signaling MUT group was also significantly lower than that in the WT group (all p<0.05). In addition, GSEA results showed that the SMAD signaling pathway and the FoxO signaling pathway were significantly activated in the BCR signaling MUT group (p<0.05, ES>0), whereas pathways of the signaling by WNT in cancer and constitutive signaling by aberrant PI3K in cancer were down-regulated in the BCR signaling MUT group (p<0.05, ES<0) (). Similarly, within the BCR signaling MUT group the signal pathways relating to angiogenesis such as vascular endothelial cell proliferation, vascular endothelial growth factor signaling pathway, Hedgehog signaling pathway and fibroblast growth factor receptor signaling pathway were significantly down-regulated (p<0.05, ES<0 (). Finally, based on the results of the analysis, we hypothesized the possible underlying mechanism for the better prognosis in the BCR signaling MUT group ().
Discussion
In this study, it was found that nonsynonymous BCR signaling pathway mutation status before treatment could predict better PFS and OS after ICI treatment in the Miao-LUAD cohort. This was then combined with other published data sets and the Local-LUAD group from our own hospital to calculate the degree of immune cell infiltration, the number of TMB, NAL and DDR related pathway mutations from the perspective of immunogenicity and immune microenvironment, and were then further combined with pathway enrichment analysis. With these results the ability of nonsynonymous mutation status in the BCR signaling pathway as a predictive marker of the efficacy of ICIs was further validated.
Immunogenicity of tumors is considered to be a prerequisite for exerting anti-tumor immunity,Citation31 and it can be assessed via NAL, TMB, or DDR pathway mutation.Citation26 The number of NAL measures the quantity of neoantigen in tumor cells, so as to evaluate the ability of the tumor to induce a tumor-specific immune response. TMB is now a clinically used ICI treatment predictor in LUAD, and it is documented that a damaged DNA repair mechanism can result in enhanced immunogenicity and a high TMB status.Citation32,Citation33 Wang et al showed that DDR pathway mutations can act as potential biomarkers of ICI therapy in multiple tumor types.Citation32 Furthermore, Teo et al. Showed that DDR pathway mutations are related to a better clinical response and prolonged PFS and OS in urothelial carcinoma patients who had received ICI therapy.Citation33 In our study, we found that the BCR signaling MUT group in TCGA cohort had higher TMB, NAL and DDR-related pathways mutations, and the TMB was higher in all five cohorts in the BCR signaling MUT group.
In addition, TME is a complex system mainly composed of tumor cells, surrounding immune and inflammatory cells, tumor-related fibroblasts, nearby interstitial tissues, capillaries, and various cytokines and chemokines.Citation34 Before ICI baseline treatment, TILs infiltrated in the TME were reported to be positively correlated with ICI treatment efficacy.Citation35–37 Therefore, we analyzed the infiltration degree of 22 types of immune cells in the TCGA-LUAD cohort via a CIBERSORT method. There were shown to be more CD8+ T cells and activated memory CD4+ T cells infiltrating in BCR signaling MUT group. CD8+ T cells were shown to be specific anti-tumor T cells. They can kill tumor cells by producing IFN-γ, TNF and granzyme B.Citation38 CD4+ T cells play an important role in helping CD8+ T cells to enhance anti-tumor immunity.Citation39,Citation40 Once activated by tumor-associated antigens, CD4+ T cells can promote the maturation of dendritic cells, present more antigens, and promote the differentiation of naive CD8+ cells into effector CD8+ T cells, thus increasing the number of CD8+ T cells. CD4+ T cells can also simultaneously act as cytotoxic cells by secreting IFN-γ and TNF or express receptors of TNF family factors such as FASL and TRAIL, mediating a direct cytotoxic immune response. MHC class II restricted antigen recognition of CD4+ T cells, as opposed to MHC class I restricted antigen recognition of CD8+ T cells, are an important supplementary part of antigen recognition in anti-tumor immunity.
On the contrary, the degree of Tregs, can directly inhibit CD8+ T cells infiltration in LUAD.Citation41 Infiltration in the BCR signaling MUT group was significantly lower than that in the WT group. In addition, our results showed that there were significant differences in the degree of mast cell infiltration between the BCR signaling MUT group and the WT group. The role of mast cells in tumor immunity has been controversial. According to epidemiological surveys, the existence of mast cells in TME is negatively correlated with the progress of lung cancer,Citation42,Citation43 and a humanized-mouse melanoma model proves that tumor-infiltrated mast cells are related to the tolerance of anti-PD-1 antibody treatment, which is a negative factor in immunotherapy efficacy.Citation44 However, mast cell-derived TNF can kill tumor cells.Citation45 Further research is required to determine the role that it plays. Furthermore, the anti-tumor immune microenvironment clearly activated in BCR signaling MUT group could be a reason why this group has significantly better ICIs treatment efficacy.
Chemokines and cytokines secreted by immune cells in TME are important components of LUAD anti-tumor immunity.Citation46 The ssGSEA results showed that cytokines and chemokine produced pathways related to immunosuppression, such as IL-10, IL-1β, CCL2 and CXCL2, were significantly down-regulated in the BCR signaling MUT group.Citation47 These factors recruit tumor-associated macrophages (TAMs), Tregs and Myeloid-derived suppressor cells (MDSCs) into the TME, helping tumor cells escape detection by the immune system and promoting the development and metastasis of tumors.Citation48,Citation49 Evidence has been shown that IL-10 secreted by cancer cells activated type II macrophages, the latter promoting tumor growth by inhibiting T cells. Tumor cells can also recruit a large number of Tregs and MDSCs for themselves by secreting IL-10 and TGF-β and up-regulating the expression of CXCR2 ligand.Citation49,Citation50 Both Tregs and MDSCs are notorious immunosuppressive cells, which jointly inhibit the infiltration and function of CD8+ T cells and mediate the adaptive immune tolerance of tumors. Anti-tumor immunity can be further weakened through the up-regulation of IL-10, which can promote the differentiation of native CD4 T cells into Tregs.Citation51 IL-1β is a pro-inflammatory factor, and in the NSCLC mouse model, IL-1β derived from mast cells and tumor-associated neutrophils has been proven to be beneficial to tumor growth.Citation52,Citation53 The effects of IL-1β are significantly related to a poor prognosis for NSCLC.Citation54 At the same time, a drug clinical trial using IL-1β inhibitor showed that, after the secretion of IL-1β is inhibited, the mortality rate related to NSCLC decreased significantly.Citation55,Citation56 In addition, in the study of the mouse model of pleural cancer with lung cancer metastasis, researchers found that the vicious circle of IL-1β and CCL2 working together explained the occurrence and development of malignant pleural effusion in NSCLC.Citation57–59
At last, in our GSEA results, some classic signaling pathways that promote tumor occurrence and development were significantly down-regulated in the BCR signaling MUT group,Citation60–63 such as signaling by WNT in cancer, and constitutive signaling by aberrant PI3K in cancer. It is worth noting that the pathways related to tumor angiogenesis, which are basic to the survival and metastasis of tumor cells, were also significantly down-regulated in the BCR signaling MUT group.Citation64–66 Tumor angiogenesis in solid tumors can also prevent tumor-specific T cells from infiltrating TME.Citation67 Finally, the analysis revealed that the signal pathways related to tumor inhibition were significantly up-regulated in MUT group, such as positive regulation of pathway restricted SMAD protein phosphorylation;Citation68 activated SMAD protein inhibited the activation of TGF-β signal pathway, which was reported to be related to the enhancement of tumor invasiveness and metastasis in advanced NSCLC. Correspondingly, in the ssGSEA results, the BCR signaling MUT group had lower scores of TGF-β signaling pathway. FoxO signaling pathway not only inhibits the abnormal expression of Fox family proteins, but also, consequently, inhibits the role of these proteins in promoting tumor growth.Citation69 In addition, we also found that the MUT group had lower scores in ssGSEA, a signaling pathway related to fatty acid metabolism, which was related to immune failure and tumor metastasis.Citation70
This study explored the impact of BCR signaling pathway nonsynonymous mutation status on the progress of LUAD patients treated with ICIs from the perspective of immune microenvironment (ie immune cells, immunogenicity and cytokines). However, this study still had several limitations. First, because the survival data of the LUAD cohort treated with ICIs is very limited, it was only possible to sufficiently explore the relationship between the BCR signaling pathway and the ICI treatment survival rate in the Miao-LUAD cohort. Secondly, due to the lack of information on TNM classification, Gene-altering events and the ICI treatment program, including factors such as whether ICI therapy was conducted alone or in combination with chemotherapy/radiotherapy, we cannot exclude the influence of these clinical factors on the relationship between BCR pathway mutations and ICIs prognosis. In future studies, we will try to collect more clinical data to explore the potential bias of these clinical factors. Thirdly, because of the quantity and complexity of mutation sites in the BCR signaling pathway, we did not explore the specific molecular mechanism between mutation and ICI efficacy, but we compared the TME differences between the wild group and the mutant group, and formed a hypothesis for the mechanism, using immune infiltration analysis and pathway enrichment analysis combined with previous literature reports. Finally, we recommend that readers interpret our findings with caution, since we did not carry out related cell and animal experiments to further verify our results, and we hope to supplement with further studies in the future.
Conclusion
In this study, we found that there is a significant relationship between the mutation state of BCR signaling pathway and the therapeutic effect of ICIs. As a result, the BCR signaling MUT group had better PFS and OS. With further analysis of RNA-seq data, mutation data and clinical data in multiple cohorts, it was found that the MUT group had higher immunogenicity and a more anti-tumor activated microenvironment, and these results indicate that the BCR signaling MUT group has longer PFS and OS. To sum up, these results support the mutation status of the BCR signaling pathway as a potential biomarker to predict the efficacy of ICI therapy in LUAD patients.
Abbreviations
ICIs, immune checkpoint inhibitors; LUAD, lung adenocarcinoma; TCGA, The Cancer Genome Atlas; MUT, mutant-type; WT, wild-type; TME, tumor microenvironment; TILs, tumor infiltrating lymphocytes; PD-L1, programmed cell death ligand 1; PD-1, programmed cell death 1; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; TMB, tumor mutation burden; BCR, B cell receptor; NAL, neoantigen loads; GSEA, gene set enrichment analysis; ssGSEA, single sample gene set enrichment analysis; PFS, Progression-free survival; OS, overall survival; TAMs, tumor-associated macrophages; Tregs, regulatory T cells; MDSCs, Myeloid-derived suppressor cells.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors Peng Luo at [email protected] and Jian Zhang at [email protected].
Ethics Approval and Informed Consent
The patients/participants provided their written informed consent to participate in this study and the research presented here has been performed in accordance with the Declaration of Helsinki and has been approved by the ethics committee of the Zhujiang Hospital of Southern Medical University.
Disclosure
The authors declare no conflicts of interest in relation to this work and that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks to the funding providers of the Natural Science Foundation of Guangdong Province, the Science and Technology Planning Project of Guangdong Province and the National Natural Science Foundation of China.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
- Gao S, Li N, Wang S, et al. Lung cancer in People’s Republic of China. J Thorac Oncol. 2020;15(10):1567–1576. doi:10.1016/j.jtho.2020.04.028
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
- Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15(6):452–462. doi:10.2174/156800961506150805145120
- Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials checkMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi:10.1200/JCO.20.01605
- Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. doi:10.1038/s41577-019-0218-4
- Yuan Y, Adam A, Zhao C, Chen H. Recent advancements in the mechanisms underlying resistance to PD-1/PD-L1 blockade immunotherapy. Cancers. 2021;13(4):663. doi:10.3390/cancers13040663
- Juarez-Garcia A, Sharma R, Hunger M, Kayaniyil S, Penrod JR, Chouaïd C. Real-world effectiveness of immunotherapies in pre-treated, advanced non-small cell lung cancer Patients: a systematic literature review. Lung Cancer. 2022;166:205–220. doi:10.1016/j.lungcan.2022.03.008
- Niu M, Yi M, Li N, Luo S, Wu K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp Hematol Oncol. 2021;10(1). doi:10.1186/s40164-021-00211-8
- Wojas-Krawczyk K, Kubiatowski T. Imperfect predictors for lung cancer immunotherapy-a field for further research. Front Oncol. 2020;10:568174. doi:10.3389/fonc.2020.568174
- Büttner R, Longshore JW, López-Ríos F, et al. Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open. 2019;4(1):e000442. doi:10.1136/esmoopen-2018-000442
- McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–672. doi:10.1016/j.annonc.2021.02.006
- Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41(6–7):599–613. doi:10.1016/j.molimm.2004.04.008
- Kim S, Summer W, Miyauchi S, Cohen EE, Califano JA, Sharabi AB. Role of B cells in responses to checkpoint blockade immunotherapy and overall survival of cancer patients. Clin Cancer Res. 2021;27(22):6075–6082. doi:10.1158/1078-0432.CCR-21-0697
- Ghosh D, Jiang W, Mukhopadhyay D, Mellins ED. New insights into B cells as antigen presenting cells. Curr Opin Immunol. 2021;70:129–137. doi:10.1016/j.coi.2021.06.003
- Ye J, Lee PP. B cell receptor signaling strength modulates cancer immunity. J Clin Invest. 2022;132(6):e157665. doi:10.1172/JCI157665
- Profitós-Pelejà N, Santos JC, Marín-Niebla A, Roué G, Ribeiro ML. Regulation of B-cell receptor signaling and its therapeutic relevance in aggressive B-cell lymphomas. Cancers. 2022;14(4):860. doi:10.3390/cancers14040860
- Taylor J, Wilmore S, Marriot S, et al. B-cell receptor signaling induces proteasomal degradation of PDCD4 via MEK1/2 and mTORC1 in malignant B cells. Cell Signal. 2022;94:110311. doi:10.1016/j.cellsig.2022.110311
- Jebaraj BMC, Müller A, Dheenadayalan RP, et al. Evaluation of vecabrutinib as a model for noncovalent BTK/ITK inhibition for treatment of chronic lymphocytic leukemia. Blood. 2022;139(6):859–875. doi:10.1182/blood.2021011516
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi:10.1200/JCO.2017.75.3384
- Miao D, Margolis CA, Vokes NI, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–1281. doi:10.1038/s41588-018-0200-2
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi:10.1038/s41588-018-0312-8
- Colaprico A, Silva TC, Olsen C, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71. doi:10.1093/nar/gkv1507
- Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi:10.1093/bioinformatics/btr260
- Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–1756. doi:10.1101/gr.239244.118
- Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2019;51(2):411–412. doi:10.1016/j.immuni.2019.08.004
- Luo P, Lin A, Li K, Wei T, Zhang J. DDR pathway alteration, tumor mutation burden, and cisplatin sensitivity in small cell lung cancer: difference detected by whole exome and targeted gene sequencing. J Thorac Oncol. 2019;14(12):e276–e279. doi:10.1016/j.jtho.2019.08.2509
- Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi:10.1007/978-1-4939-7493-1_12
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
- Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14(1):7. doi:10.1186/1471-2105-14-7
- Wang S, He Z, Wang X, Li H, Liu XS. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife. 2019;8:e49020. doi:10.7554/eLife.49020
- Wang Z, Zhao J, Wang G, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78(22):6486–6496. doi:10.1158/0008-5472.CAN-18-1814
- Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–1694. doi:10.1200/JCO.2017.75.7740
- Dougan M, Dougan SK. Targeting immunotherapy to the tumor microenvironment. J Cell Biochem. 2017;118(10):3049–3054. doi:10.1002/jcb.26005
- Zhang J, Zhou N, Lin A, et al. ZFHX3 mutation as a protective biomarker for immune checkpoint blockade in non-small cell lung cancer. Cancer Immunol Immunother. 2021;70(1):137–151. doi:10.1007/s00262-020-02668-8
- Huang W, Lin A, Luo P, et al. EPHA5 mutation predicts the durable clinical benefit of immune checkpoint inhibitors in patients with lung adenocarcinoma. Cancer Gene Ther. 2021;28(7–8):864–874. doi:10.1038/s41417-020-0207-6
- Jacquelot N, Pitt JM, Enot DP, et al. Immune biomarkers for prognosis and prediction of responses to immune checkpoint blockade in cutaneous melanoma. Oncoimmunology. 2017;6(8):e1299303. doi:10.1080/2162402X.2017.1299303
- Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi:10.1155/2016/8941260
- Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi:10.1126/science.1251102
- Cui C, Wang J, Fagerberg E, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. 2021;184(25):6101–6118.e13. doi:10.1016/j.cell.2021.11.007
- Ganesan AP, Johansson M, Ruffell B, et al. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol. 2013;191(4). doi:10.4049/jimmunol.1301317
- Sinnamon MJ, Carter KJ, Sims LP, Lafleur B, Fingleton B, Matrisian LM. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis. 2008;29(4):880–886. doi:10.1093/carcin/bgn040
- Hodges K, Kennedy L, Meng F, Alpini G, Francis H. Mast cells, disease and gastrointestinal cancer: a comprehensive review of recent findings. Transl Gastrointest Cancer. 2012;1(2):138–150.
- Somasundaram R, Connelly T, Choi R, et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat Commun. 2021;12(1). doi:10.1038/s41467-020-20600-7
- Liu J, Zhang Y, Zhao J, et al. Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011;30(2):177–184. doi:10.1007/s10555-011-9276-1
- Lin A, Zhang H, Meng H, et al. TNF-alpha pathway alternation predicts survival of immune checkpoint inhibitors in non-small cell lung cancer. Front Immunol. 2021;12:667875. doi:10.3389/fimmu.2021.667875
- Paval DR, Patton R, McDonald J, et al. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. 2022;13(2):824–838. doi:10.1002/jcsm.12912
- Srivastava MK, Andersson Å, Zhu L, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy. 2012;4(3):291–304. doi:10.2217/imt.11.178
- Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5(5):585–590. doi:10.1097/JTO.0b013e3181d60fd7
- Zaynagetdinov R, Stathopoulos GT, Sherrill TP, et al. Epithelial nuclear factor-κB signaling promotes lung carcinogenesis via recruitment of regulatory T lymphocytes. Oncogene. 2012;31(26):3164–3176. doi:10.1038/onc.2011.480
- Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. 2016;4(4):354–365. doi:10.1158/2326-6066.CIR-15-0241
- Lilis I, Ntaliarda G, Papaleonidopoulos V, et al. Interleukin-1β provided by KIT-competent mast cells is required for KRAS-mutant lung adenocarcinoma. Oncoimmunology. 2019;8(7):1593802. doi:10.1080/2162402X.2019.1593802
- McLoed AG, Sherrill TP, Cheng DS, et al. Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Rep. 2016;16(1):120–132. doi:10.1016/j.celrep.2016.05.085
- Kim JW, Koh Y, Kim DW, et al. Clinical implications of VEGF, TGF-β1, and IL-1β in patients with advanced non-small cell lung cancer. Cancer Res Treat. 2013;45(4):325–333. doi:10.4143/crt.2013.45.4.325
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
- Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi:10.1016/S0140-6736(17)32247-X
- Agalioti T, Giannou AD, Krontira AC, et al. Mutant KRAS promotes malignant pleural effusion formation. Nat Commun. 2017;8(1):15205. doi:10.1038/ncomms15205
- Marazioti A, Lilis I, Vreka M, et al. Myeloid-derived interleukin-1β drives oncogenic KRAS-NF-κΒ addiction in malignant pleural effusion. Nat Commun. 2018;9(1):672. doi:10.1038/s41467-018-03051-z
- Giannou AD, Marazioti A, Spella M, et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125(6):2317–2334. doi:10.1172/JCI79840
- Xi Y, Chen Y. Wnt signaling pathway: implications for therapy in lung cancer and bone metastasis. Cancer Lett. 2014;353(1):8–16. doi:10.1016/j.canlet.2014.07.010
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28(21):2163–2172. doi:10.1038/onc.2009.82
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi:10.1038/nrm2882
- Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6(10):569–579. doi:10.1038/nrclinonc.2009.130
- Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9(3):570–579. doi:10.4161/cc.9.3.10591
- Desai A, Adjei AA. FGFR signaling as a target for lung cancer therapy. J Thorac Oncol. 2016;11(1):9–20. doi:10.1016/j.jtho.2015.08.003
- Hu H, Chen Y, Tan S, et al. The research progress of antiangiogenic therapy, immune therapy and tumor microenvironment. Front Immunol. 2022;13:802846. doi:10.3389/fimmu.2022.802846
- Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi:10.3389/fonc.2013.00231
- Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev. 1996;7(1):93–102. doi:10.1016/1359-6101(96)00001-9
- Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328(2):198–206. doi:10.1016/j.canlet.2012.09.017
- Zhao G, Cardenas H, Matei D. Ovarian cancer-why lipids matter. Cancers. 2019;11(12):E1870. doi:10.3390/cancers11121870