Abstract
Background
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by inflammation of multiple joints, resulting in irreversible cartilage and bone destruction. Chronic disease activity may be associated with metabolic disorders and premature atherosclerosis. Adipokines are involved not only in metabolism regulation, but also in inflammatory and immune response.
Aim
This study is designed to explore relationships between adipokines (adiponectin, leptin) and metabolic parameters, as well as disease activity, in patients with chronic RA.
Methods
This cross-sectional study enrolled 109 patients with RA. The clinical assessment was performed including tender and swollen joint counts, Disease Activity Score 28 (DAS28), body mass index (BMI). The following laboratory parameters were performed: erythrocyte sedimentation rate, C-reactive protein, glucose, lipid profile, creatinine. Serum levels of adiponectin and leptin were assessed by enzyme-linked immunosorbent assay (ELISA).
Results
The mean adiponectin and leptin serum concentrations remained within normal ranges. Both, adiponectin and leptin levels were not associated with current disease activity markers (clinical and laboratory), and type of treatment. Significant relationships were found between adipokines and metabolic parameters, as well as with coexistent conditions and RA characteristics. Higher leptin levels were noticed in patients with hypertension. In the multiple linear regression analysis, correlations were confirmed. Adiponectin was positively correlated with HDL-C (b = 0.37, p < 0.001), age (b = 0.39, p< 0.001), and negatively with glucose (b = −0.17, p = 0.03). Leptin was positively correlated with BMI (b = 0.58, p < 0.001), and negatively with estimated glomerular filtration rate (eGFR) (b = −0.30, p < 0.001).
Conclusion
The results of this study show the value of adipokines as indicators of metabolic disorders, rather than inflammatory markers in patients with chronic RA, treated with immunosuppressive or biological drugs. High leptin level may indicate poor prognostic factors, kidney and cardiovascular complications. Adiponectin seems to be protective against metabolic disorders in chronic RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by chronic inflammation of multiple joints, resulting in irreversible cartilage and bone destruction. RA is a systemic disease and may be associated with extra-articular manifestations and comorbidities, leading to increased morbidity and premature death. Extra-articular symptoms (eg, cardiovascular, kidney involvement) are classified as poor prognostic factors, alongside with high disease activity, high levels of acute phase reactants, positivity for antibodies (rheumatoid factor, RF and/or anti-citrullinated protein/peptide antibodies, ACPA), presence of early erosions, failure of therapy with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).Citation1–3
The exact etiology of RA remains uncertain, nevertheless it seems that RA pathogenesis is multifactorial, and results from the interaction between several genetic and environmental factors. It has been reported that obesity is the risk factor for RA, and not only for metabolic disorders such as metabolic syndrome or type 2 diabetes.Citation4,Citation5 On the contrary, high-grade inflammatory activity in the course of RA may be associated with loss of body cell mass and consequently body mass reduction, defined as rheumatoid cachexia.Citation6
Adipose tissue seems to be the largest endocrine organ that produces high amounts of several biologically active molecules, called adipokines. They are involved not only in appetite and metabolism regulation, and glucose homeostasis, but also in inflammatory and immune response.Citation5–8 Adipokines, such as adiponectin and leptin seem to be involved in the pathogenesis and activity of autoimmune diseases, including RA. However, their role remains unclear and conflicting reports are available in literature.Citation5–8
Leptin is regarded to be a proinflammatory adipokine since it stimulates production of proinflammatory cytokines (eg, tumor necrosis factor α, TNF-α; interleukin 6, IL-6), CC-chemokines, and favors the Th1 phenotype in Th1/Th2 balance.Citation8 It has been reported that leptin may stimulate fibroblast-like cells in RA to produce proinflammatory cytokines.Citation5 In most studies in literature, leptin levels were reported to be increased in patients with RA; some studies found no significant difference or reduced leptin levels when compared to healthy controls or patients with osteoarthritis.Citation6,Citation8 The positive correlation was found between leptin levels and disease activity score (DAS28) or with C-reactive protein (CRP) concentration.Citation6,Citation8 In another study, leptin levels were increased only in patients with RA and metabolic syndrome.Citation8 No correlation was also reported, between leptin and clinical activity, joint damage, laboratory parameters of RA activity.Citation8
Adiponectin is considered an anti-inflammatory adipokine in obesity, type 2 diabetes, metabolic syndrome, atherosclerosis. High adiponectin levels are reported to be a protective factor in those conditions.Citation8,Citation9 On the contrary, it was found that in RA adiponectin may exert proinflammatory effect especially within the joints, by stimulating the secretion of inflammatory mediators (prostaglandins, IL-6, IL-8, matrix metalloproteinases (MMPs)). A destructive role of adiponectin was also demonstrated and association with the radiographic progress in RA.Citation8 Although, no correlation with CRP, disease activity, disease duration or body mass index (BMI), was also reported, in spite of higher levels of adiponectin in RA patients than in controls.Citation6,Citation8
The purpose of this study is to determine relationships between adipokines (adiponectin, leptin) and metabolic parameters, as well as disease activity, in patients with chronic RA, treated with csDMARDs and biological DMARDs.
Materials and Methods
Study Population
This cross-sectional study enrolled 109 patients with RA, hospitalized in the Department of Rheumatology and Connective Tissue Diseases, Medical University of Lublin, Poland. The patients fulfilled the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA.Citation10 The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethical Committee of the Medical University of Lublin (approval number KE-0254/319/2016 dated 24 November 2016). Written informed consent was obtained from each patient, prior to the inclusion in this study.
Clinical and Laboratory Assessment
Clinical information was obtained through detailed interview, review of medical history, self-reported questionnaire, and physical examination. BMI was calculated as a ratio, body weight/height2 (kg/m2).
Disease activity was measured using disease activity score system (DAS28), calculated with tender joint count (TJC), swollen joint count (SJC), erythrocyte sedimentation rate (ESR), and patient global assessment (PGA) in visual analogue scale (VAS).Citation11 The cut point for low disease activity was DAS28 value ≤3.2 and high disease activity >5.1. The ability to perform daily activities was assessed using modified Health Assessment Questionnaire (M-HAQ), with range 0–3 (score 0 presenting no impairment of function).Citation12
All samples of blood were collected in the morning, after overnight fasting. Levels of ESR, CRP, creatinine, uric acid, glucose, lipid profile (total cholesterol, TC; high-density lipoproteins (HDL) cholesterol; triglycerides (TG); low-density lipoproteins (LDL) cholesterol) and complete blood cell count (CBC) were measured by the laboratory of the hospital. The estimated glomerular filtration rate (eGFR) was calculated by Modification of Diet in Renal Disease (MDRD) formula.Citation6 The aliquots of separated serum were stored at −80°C.
Adipokines Assessment
Serum concentrations of adipokines were measured by enzyme-linked immunosorbent assay (ELISA), using the commercial kits. Samples were prepared at appropriate dilutions and assayed following the manufacturer’s instructions. Adiponectin was measured by Human Total Adiponectin/Acrp30 Immunoassay, Quantikine, R&D System. The normal range of serum adiponectin according to manufacturer’s data is between 0.87 and 21.4 ug/mL (mean 6.64); the minimum detectable dose 0.246 ng/mL. Leptin was determined by Human Leptin Immunoassay, Quantikine, R&D System. The normal range of serum leptin according to manufacturer’s data is: in women between 3.88 and 77.27 ng/mL (mean 20.68), and in men between 2.21 and 11.15 ng/mL (mean 4.76); the minimum detectable dose is 7.8 pg/mL.
Statistical Analysis
Continuous variables were presented using the mean ± standard deviation (SD) or median and interquartile range (IQR) if the data were parametric or nonparametric, respectively. Categorical data were presented as absolute numbers and percentages. The results were tested for normality using the Kolmogorov–Smirnov’s test. The Student’s t-test or nonparametric Mann–Whitney U-test was used in order to compare continuous variables in subgroups of patients. Correlation between the quantitative variables was assessed by Spearman’s or Pearson’s correlation test. The multiple linear regression test was performed introducing variables that showed statistically significant association with certain parameters. For all tests, p values <0.05 were considered significant. All statistical analyses were performed using the StatSoft STATISTICA 12 application.
Results
The study group consisted mostly of women (almost 80%). The disease duration >10 years was observed in about 60% of the patients. The vast majority of patients were seropositive for IgM rheumatoid factor (RF-IgM) and/or anti-cyclic citrullinated peptide antibodies (anti-CCP). Extra-articular manifestations (rheumatoid nodules, sicca syndrome, interstitial lung disease, impaired kidney function, and vasculitis) in the course of the disease were observed in more than 50% of the patients ().
Table 1 Characteristics of Patients with RA
Pharmacological Treatment and the Disease Activity in Patients with RA
At the time of examination csDMARDs were used in 107 patients and included: methotrexate (MTX) in 98 (89.9%) patients (dose 10–25 mg/week, in monotherapy or combination), leflunomide (LEF) 14 (12.8%), hydroxychloroquine (HCQ) or chloroquine (CQ) 35 (32.1%), sulfasalazine (SS) 15 (13.8%) and cyclosporine 1 (0.9%) ().
Biological DMARDs (bDMARDs) were administered in 57 (52.3%) patients ().
Low-dose glucocorticoid (GC) therapy (prednisone ≤10 mg/day) was used in 71 (65.1%) patients ().
Remission or low disease activity (DAS28 ≤3.2) at the time of examination was noted in more than 1/3 of the patients. High disease activity (DAS28 >5.1) was found in about 1/3 of the patients ().
Table 2 Clinical and Laboratory Parameters in RA Patients
Adiponectin and Leptin Serum Concentrations in the Whole Group of Patients with RA
The median serum concentrations of adiponectin and leptin remained within the normal reference ranges in the study group ().
The serum adiponectin concentration above the normal range was noted in 9 patients (7 female and 2 male). The serum leptin concentration above the normal range was found in other 7 patients (1 female and 6 male). The demographic and disease-related characteristics of these patients were comparable to other patients.
Leptin level was significantly higher in female compared with male patients (respectively, 16.5 (10.0–29.1) vs 6.6 (2.4–9.6), p < 0.001). Adiponectin level was comparable in female and male patients.
Adiponectin and Leptin Levels in Certain Groups of Patients with RA
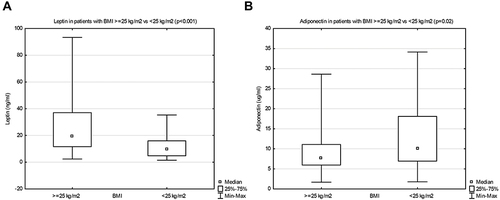
In patients with BMI ≥ 25 kg/m2 (overweight/obesity), when compared with those of normal BMI, we found significantly higher leptin levels (respectively, 19.4 (11.6–37.0) vs 9.7 (4.8–15.9), p < 0.001) () and significantly lower adiponectin levels (respectively, 7.7 (6.0–11.1) vs 10.1 (6.9–18.1), p=0.02) ().
Figure 1 Leptin (A) and adiponectin (B) serum concentrations in patients with body mass index (BMI) ≥ 25 kg/m 2 vs < 25 kg/m2.
The median concentration of adiponectin was significantly higher in patients with extra-articular manifestations during the course of RA, as compared to those with no extra-articular symptoms (respectively, 10.4 (7.1–16.7) vs 7.6 (5.5–12.1), p=0.02) (). The median leptin concentration was comparable in both groups.
Figure 2 Adiponectin serum concentrations in patients with vs without extra-articular manifestations (A), and with RA duration ≥10 years, vs <10 years (B).
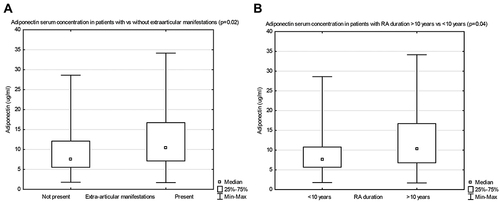
The median adiponectin level was significantly higher in patients with long-term RA (>10 years), compared with patients of shorter disease duration (respectively, 10.2 (6.7–16.7) vs 7.5 (5.7–10.8), p=0.04) (). The median leptin level was comparable in both groups.
In patients with arterial hypertension, as compared to those with normal blood pressure, the median concentration of leptin was significantly higher (respectively, 18.9 (12.2–37.0) vs 10.6 (5.9–17.9), p < 0.001) (). The median concentration of adiponectin was comparable in both groups.
Figure 3 Leptin serum concentrations in patients with vs without arterial hypertension (A), and with vs without cardiovascular disease (CVD) family history (B).
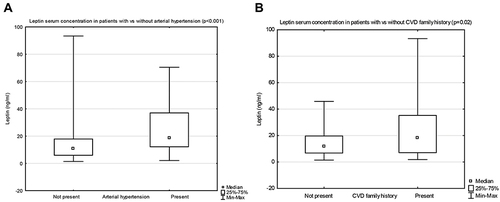
The median leptin level was significantly higher in patients with the family history of cardiovascular diseases (CVD) (arterial hypertension, ischemic heart disease, heart failure), when compared with those without CVD family history (respectively, 18.0 (7.1–35.3) vs 11.7 (6.8–19.6), p=0.02) (). The median adiponectin level was comparable in both groups.
No other significant differences of adipokines levels were observed in relation to disease activity, immunological status, method of treatment.
The Relationship Between Adipokines (Adiponectin, Leptin) and the Disease Activity Markers, the Disease Duration, and Metabolic Parameters
Positive correlations were found between adiponectin and patients’ age, disease duration, and HDL-C. Inverse correlations were found with other metabolic parameters (BMI, TG, TC/HDL-C, glucose) ().
Table 3 Significant Correlations Between Clinical and Laboratory Parameters and Adipokines in Patients with RA
Positive correlations were found between leptin and metabolic parameters (BMI, TC). There was an inverse correlation between leptin and eGFR value (). There were no other significant correlations between adipokines and disease activity parameters (joint counts, PGA, DAS28, morning stiffness, CRP, ESR), M-HAQ.
In the multiple linear regression analysis, significantly positive associations were confirmed for adiponectin with HDL-C (b = 0.37, p<0.001) and age (b = 0.39, p< 0.001), as well as an inverse association with glucose (b = −0.17, p=0.03). A positive association was found for leptin with BMI (b = 0.58, p< 0.001) and an inverse with eGFR (b = −0.30, p< 0.001).
Discussion
Our study showed that, in patients with RA, adipokines (adiponectin, leptin) were significantly associated with metabolic parameters (BMI, lipid profile components, glucose), as well as characteristics of RA (extra-articular manifestations of RA, disease duration), and coexistent conditions (arterial hypertension, CVD family history). However, we found no significant correlation between adipokines and the current disease activity markers, both clinical and laboratory. The median levels of adiponectin and leptin remained within the normal reference ranges. That could be related to long-term course of RA (≥10 years in almost 60% of patients), and chronic treatment with DMARDs including biological therapy (in more than 50% of patients).
In this study, we observed significant correlations between adipokine levels and BMI, the positive correlation between leptin and BMI, and the inverse correlation between adiponectin and BMI. In the literature, significant correlations have been reported, a negative between adiponectin and body fat mass,Citation13 and positive correlations between leptin concentration and the amount of body fatCitation13 and BMI.Citation6,Citation9 Both leptin and adiponectin are mainly produced in adipose tissue, that is why leptin concentration is associated with the amount of body fat. The negative association between adiponectin and body fat mass may result from TNF-α overproduction in obese subjects. Subsequently, TNF-α may induce suppression of adiponectin production in adipocytes.Citation13
In this study, adiponectin levels were higher in patients with extra-articular symptoms and in those with long-term disease (≥10 years). Adiponectin was positively correlated with the age of patients and duration of RA. We also found that adiponectin was related positively to HDL-C and inversely to TG and atherogenic index (TC/HDL-C). The inverse association was noticed between adiponectin and plasma glucose level.
Our results are consistent with data in the literature. The positive associations between adiponectin concentration and age, and the disease duration were established, as well as significantly higher adiponectin level in patients with long-standing RA.Citation6 It has been reported in the literature that adiponectin exerts protective effects against metabolic disorders (diabetes, atherosclerosis) and inflammation. Adiponectin regulates plasma glucose levels and increases fatty acid oxidation in skeletal muscles resulting in TG reduction.Citation9 The positive association with HDL-C has been also observed.Citation6
It has been reported that adiponectin concentration inversely correlates to weight, central obesity, insulin resistance and diabetes risk.Citation9 Physical activity stimulates production and release of adiponectin, which results in improved glucose and lipid metabolism. That is probably why lower adiponectin concentrations have been observed in obese subjects.Citation9 Additionally, in patients with long-term RA, the physical activity may be decreased due to chronic pain associated with irreversible joint damage and muscle mass reduction. In our study, adiponectin levels were significantly lower in overweight/obese patients as compared to RA patients with normal BMI.
As opposed to metabolic disorders, in the pathogenesis of RA adiponectin seems to exert pro-inflammatory effect and may be involved in the development of RA. Higher levels of adiponectin in subjects with overweight/obesity were associated with an increased risk of developing RA, independently of other adipokines or risk factors. It has been suggested that a chronic pro-inflammatory state associated with obesity is necessary to demonstrate the relation between adiponectin level and RA incidence. It is hypothesized that overweight/obesity may uncover the link between adiponectin and the risk of RA development.Citation9
According to reports in the literature, adiponectin is able to activate pro-inflammatory response in different cell types, including fibroblast-like synoviocytes in the joints and stimulate production of pro-inflammatory factors (IL-6, IL-8, prostaglandin E2), enhance production of vascular endothelial growth factor (VEGF) and MMPs, which may result in joint destruction.Citation5,Citation8,Citation14 It seems that adiponectin is involved in the process of angiogenesis in the pannus of affected joints.Citation15 Several studies demonstrated higher adiponectin levels in patients with RA compared to controls and an association with radiographic progression, particularly at early disease stage.Citation5,Citation8,Citation15 A positive correlation between both serum and synovial adiponectin levels and disease activity was reported.Citation8 However, in other studies, no correlation was found between serum adiponectin and disease activity, DAS28, CRP concentration,Citation8,Citation16,Citation17 or a negative association with CRP.Citation18 In our study, no correlation was found between adiponectin and disease activity parameters. However, adiponectin was significantly higher in patients with extra-articular symptoms in the course of RA, associated with severe course of the disease and poor prognosis.
Leptin is the main adipokine secreted by adipocytes and related to white adipose tissue mass. The main function of leptin is appetite and energy balance regulation by the induction of anorexigenic factors and suppression of orexigenic neuropeptides.Citation19 However, leptin has other functions. It is regarded as a pro-inflammatory adipokine, related to “low-grade inflammatory status” which occurs in overweight/obese subjects. In the immune system, leptin stimulates monocytes, macrophages, neutrophils, natural killer (NK) cells and production of pro-inflammatory cytokines. Leptin seems to be involved in development and progression of RA.Citation19
In several studies, serum leptin concentration was reported to be higher in patients with RA, or not significantly different, or reduced when compared to controls.Citation8,Citation13,Citation15,Citation19–22 Leptin was positively correlated with disease activity, DAS28, CRP concentration,Citation8,Citation15,Citation18,Citation21 or no correlation was found between leptin and the disease activity.Citation8,Citation23 In another observation, an inverse correlation between leptin and inflammatory markers (CRP, IL-6) was found, suggesting that long-term in vitro stimulation of adipose tissue by pro-inflammatory cytokines (TNF-α, IL-1b) may inhibit leptin production.Citation22 The relationship between leptin and bone destruction in RA also remains controversial.Citation15 In our study, serum leptin concentration remained within the normal reference ranges.
According to data from the literature, leptin level was significantly higher in women than in men with RA and correlated positively with TC concentration and negatively with eGFR value.Citation6 Leptin could be considered an obesity marker since serum leptin concentrations correlated with body fat percentage in patients with RA.Citation19 In another study, higher leptin values were associated with disease activity, positive RF, obesity, tobacco, and a positive association was demonstrated between cardiovascular risk and serum leptin concentrations in patients with RA.Citation24
The results of animal studies suggest that higher leptin levels may cause hyperglycemia, chronic increase of blood pressure and renal dysfunction. Leptin may induce natriuresis which results in an increase of arterial pressure. Leptin may also promote glomerular hypertrophy and sclerosis by stimulating glomerular endothelial and mesangial cell proliferation and type IV collagen production.Citation25,Citation26 It has been demonstrated that plasma leptin levels are inversely related to eGFR and higher leptin values are associated with chronic kidney disease in adults.Citation25 Leptin is mainly cleared by kidneys and impaired kidney function is associated with leptin accumulation, which in turn induces inflammation, lipid disorders, reactive oxygen species production. Additionally, leptin induces endothelial dysfunction and vascular proliferation, which increases cardiovascular risk. Increased leptin levels may be considered a poor prognostic factor.Citation27
Our results are consistent with those in literature. In patients with RA we found the inverse correlation between leptin and eGFR value. In this study, we observed significantly higher leptin levels in patients with arterial hypertension and with positive CVD family history.
In recent years, increasing numbers of patients with RA have received treatment with biological DMARDs. However, limited data exist on relationships between adipokines and biological therapy. Long-term (2 years) anti-TNFα therapy in RA and ankylosing spondylitis (AS) patients was associated with no changes of serum leptin, total adiponectin, but a significant decrease of high molecular weight (HMW) adiponectin, as well as increase in body weight, BMI, total fat mass. The results raised the issue of cardiovascular (CV) risk in these patients.Citation28 In meta-analysis of studies, it has been reported that in patients with RA treated with anti-TNFα therapy, a significant increase in fat mass, adiposity and a significant decrease in lean mass were observed. The authors suggested, that the use of anti-TNFα drugs associated with a further increase in fat mass, could potentially have CV consequences.Citation29 In another study, patients with RA treated with biological DMARDs (various anti-TNFα, tocilizumab, abatacept) had lower leptin concentration than those not receiving them, however no significant difference of adiponectin concentration.Citation13 In the group of patients with RA treated with tocilizumab both total and HMW adiponectin levels significantly increased at month 3, and persisted until month 6 of therapy, with in parallel, a significant gain in BMI, waist circumference and lean mass without changes in fat mass.Citation30 In our study, more than 50% of patients were treated with biological DMARDs, and almost all the patients received csDMARDs. The leptin and adiponectin serum concentrations maintained within the normal ranges, which may be related to chronic treatment with several DMARDs.
Our study has some potential limitations. First, the relatively small number of patients included in the study; a higher number of patients could enable better statistical evaluation. Second, this was a cross-sectional study, without a control group. Third, we could not exclude the impact of concomitant treatment (DMARDs or GC), because the study was performed in real-life RA patients. Fourth, heterogenicity of DMARDs treatment, both conventional synthetic and biological.
Our study also has several strengths. First, the detailed characteristics of the patients, which were considered in all aspects of RA pathology. Second, this was a study performed in a clinical setting, not experimental or animal one; most of current data available in the literature, related to adipokines come from experimental studies. Third, patients were not selected for the study—they are real-life patients. Fourth, more than 50% of patients were treated with biological DMARDs.
Conclusion
In this study, we found significant relationships between adipokines (adiponectin, leptin) and metabolic parameters, coexistent conditions, and characteristics of RA. The results of our study indicated that in patients with chronic RA, and treated with DMARDs, adiponectin and leptin levels were not associated with the current disease activity markers (clinical, laboratory), and type of treatment.
These results point to the value of adipokines as biomarkers of metabolic disorders, rather than inflammatory markers in patients with chronic RA. High leptin level may indicate the risk of kidney and cardiovascular complications. Adiponectin seems to be protective against metabolic disorders in chronic RA.
Data Sharing Statement
All data reported in this study are available upon request by contact with the corresponding author.
Ethics Approval and Informed Consent
The study was approved by the Ethical Committee of the Medical University of Lublin and all procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient, following a detailed description of the aim of this study, prior to the inclusion in the study.
Author Contributions
The authors contributed significantly to the work reported, conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, critically reviewing the article; gave final approval of the version to be published, have agreed on the journal to which the article has been submitted.
Disclosure
Prof. Dr. Bożena Targońska-Stępniak reports personal fees from Medac GmbH, Accord Healthcare Poland, Sandoz, MSD, and Gedeon Richter, outside the submitted work. The authors declare no other conflicts of interest in this work.
Acknowledgments
We are indebted to Dr Magdalena Dryglewska, Research Laboratory of Autoimmune Diseases, Department of Rheumatology and Connective Tissue Diseases, Medical University of Lublin, Poland, for performing laboratory assessment of adipokines.
Additional information
Funding
References
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
- Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20(4):102776. doi:10.1016/j.autrev.2021.102776
- Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther. 2017;19(1):68. doi:10.1186/s13075-017-1266-4
- Feng X, Xu X, Shi Y, et al. Body mass index and the risk of rheumatoid arthritis: an updated dose-response meta-analysis. Biomed Res Int. 2019;2019:3579081. doi:10.1155/2019/3579081
- Zhang Y, Johansson L, Andersson-Assarsson J, et al. Adiponectin associates with rheumatoid arthritis risk in overweight and obesity independently of other adipokines. J Clin Med. 2021;10(13):2791. doi:10.3390/jcm10132791
- Targońska-Stepniak B, Dryglewska M, Majdan M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol Int. 2010;30(6):731–737. doi:10.1007/s00296-009-1053-x
- Del Prete A, Salvi V, Sozzani S. Adipokines as potential biomarkers in rheumatoid arthritis. Mediators Inflamm. 2014;2014:425068. doi:10.1155/2014/425068
- Fatel ECS, Rosa FT, Anc S, Dichi I. Adipokines in rheumatoid arthritis. Adv Rheumatol. 2018;58:25. doi:10.1186/s42358-018-0026-8
- Khoramipour K, Chamari K, Hekmatikar AA, et al. Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients. 2021;13(4):1180. doi:10.3390/nu13041180
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
- Prevoo ML, Van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi:10.1002/art.1780380107
- Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol. 2005;32(8):1432–1439.
- Chihara K, Hattori N, Ichikawa N, Matsuda T, Saito T. Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis. Sci Rep. 2020;10(1):15932. doi:10.1038/s41598-020-73068-2
- Kamareddine L, Ghantous CM, Allouch S, et al. Between inflammation and autophagy: the role of leptin-adiponectin axis in cardiac remodeling. J Inflamm Res. 2021;14:5349–5365. doi:10.2147/JIR.S322231
- Cici D, Corrado A, Rotondo C, et al. Adipokines and chronic rheumatic diseases: from inflammation to bone involvement. Clin Rev Bone Miner Metab. 2020;18:58–71. doi:10.1007/s12018-021-09275-w
- Cao H, Lin J, Chen W, Xu G, Sun C. Baseline adiponectin and leptin levels in predicting an increased risk of disease activity in rheumatoid arthritis: a meta-analysis and systematic review. Autoimmunity. 2016;49(8):547–553. doi:10.1080/08916934.2016.1230847
- Lee YH, Bae SC. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Int J Rheum Dis. 2018;21(3):664–672. doi:10.1111/1756-185X
- Yoshino T, Kusunoki N, Tanaka N, et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern Med. 2011;50(4):269–275. doi:10.2169/internalmedicine.50.4306
- Carrión M, Frommer KW, Pérez-García S, Müller-Ladner U, Gomariz RP, Neumann E. The adipokine network in rheumatic joint diseases. Int J Mol Sci. 2019;20(17):4091. doi:10.3390/ijms20174091
- Tian G, Liang JN, Wang ZY, Zhou D. Emerging role of leptin in rheumatoid arthritis. Clin Exp Immunol. 2014;177(3):557–570. doi:10.1111/cei.12372
- Lee YH, Bae SC. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: a meta-analysis. Z Rheumatol. 2016;75(10):1021–1027. doi:10.1007/s00393-016-0050-1
- Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis. 2005;64(8):1195–1198. doi:10.1136/ard.2004.032243
- Oner SY, Volkan O, Oner C, Mengi A, Direskeneli H, Tasan DA. Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol Port. 2015;40(1):50–54.
- Batún-Garrido JAJ, Salas-Magaña M, Juárez-Rojop IE. Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis. Clin Rheumatol. 2018;37(3):631–637. doi:10.1007/s10067-017-3897-x
- Shankar A, Syamala S, Xiao J, Muntner P. Relationship between plasma leptin level and chronic kidney disease. Int J Nephrol. 2012;2012:269532. doi:10.1155/2012/269532
- Lim CC, Teo BW, Tai ES, et al. Elevated serum leptin, adiponectin and leptin to adiponectin ratio is associated with chronic kidney disease in Asian adults. PLoS One. 2015;10(3):e0122009. doi:10.1371/journal.pone.0122009
- Mao S, Fang L, Liu F, Jiang S, Wu L, Zhang J. Leptin and chronic kidney diseases. J Recept Signal Transduct Res. 2018;38(2):89–94. doi:10.1080/10799893.2018.1431278
- É T, Mourot L, Dehecq B, Wendling D, É G, Dumoulin G. TNFα blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: a 2-year prospective study. Eur J Nutr. 2014;53(3):951–961. doi:10.1007/s00394-013-0599-2
- Marouen S, Barnetche T, Combe B, Morel J, Daïen CI. TNF inhibitors increase fat mass in inflammatory rheumatic disease: a systematic review with meta-analysis. Clin Exp Rheumatol. 2017;35(2):337–343.
- Toussirot E, Marotte H, Mulleman D, et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: a 12-month multicentre study. Arthritis Res Ther. 2020;22(1):224. doi:10.1186/s13075-020-02297-7
