Abstract
Low back pain (LBP) is a common problem worldwide, resulting in great patient suffering and great challenges for the social health system. Intervertebral disc (IVD) degeneration (IVDD) is widely acknowledged as one of the key causes of LBP. Accumulating evidence suggests that aberrant pyroptosis of IVD cells is involved in the pathogenesis of IVDD progression, however, the comprehensive roles of pyroptosis in IVDD have not been fully established, leaving attempts to treat IVDD with anti-pyroptosis approaches questionable. In this review, we summarize the characteristics of pyroptosis and emphasize the effects of IVD cell pyroptosis on the pathological progression of IVDD, including secretion of cytokines, nucleus pulposus cell apoptosis and autophagy, accelerated extracellular matrix degradation, annulus fibrosus rupture, cartilage endplate calcification, vascularization, sensory and sympathetic fiber neoinnervation, and infiltrating lymphatic vessels. Finally, we discuss several interventions used to treat IVDD by targeting pyroptosis. This review provides novel insights into the crucial role of IVD cell pyroptosis in IVDD pathogenesis, and could be informative for developing novel therapeutic approaches for IVDD and LBP.
Introduction
Low back pain (LBP) is a common symptom of the musculoskeletal system and a primary cause of disability.Citation1 Epidemiological studies have revealed that intervertebral disc (IVD) degeneration (IVDD) is clinically related to LBP and is one of the key causes of LBP.Citation2,Citation3 Despite the enormous efforts of researchers made to investigate the etiology and pathogenesis of IVDD, the exact mechanisms underpinning IVDD remain obscure, which accounts for the limited number of effective approaches to prevent and treat this pathological condition. Therefore, it is essential to further elucidate the cellular and molecular mechanism of IVDD to facilitate the development of novel therapeutic strategies.
IVD is fibrocartilage tissue that connects the bodies of adjacent vertebrae, accounting for 25–30% of the total length (height) of the spine and maintaining its stability.Citation4 IVD consists of a peripheral annulus fibrosus (AF) surrounding a central gelatinous nucleus pulposus (NP) and a thin layer of cartilage endplates (CEP) at the upper and lower ends. AF consists mainly of tough and elastic annular fibrous cartilage arranged in concentric circles. In normal adults, NP is a highly hydrated gelatinous structure consisting of NP cells derived from the embryonic notochord as well as NP cell-produced components of extracellular matrix (ECM), such as collagen type II (Col2) and proteoglycan, which are acknowledged as key contributors for resistance to axial stress and stress in the spine.Citation5,Citation6 Meanwhile, CEP is a layered composite consisting of semi-porous thickened cancellous bone and hyaline cartilage,Citation7 which can transmit the compressive loads containing and pressurizing NP, and transfer water, nutrients, and waste into and out of the IVD.Citation8
The etiology of IVDD is multifactorial, including aging, mechanical loading, trauma, genetic susceptibility and other factors leading to vertebral instability, spinal canal stenosis, and spinal segment deformity, resulting in LBP and mobility disability.Citation9 IVDD is characterized by abnormalities in local physiological structure, such as progressive NP dehydration, ECM degradation or degeneration, AF rupture, CEP calcification, and inflammation.Citation10 Interestingly, inflammatory factors such as interleukin (IL)-1β and tumor necrosis factor -α (TNF-α) have been identified as key cytokine mediators that participate in the pathological changes of IVDD and LBP, which can amplify inflammatory reaction, stimulate ECM degradation, accelerate cell senescence, impair IVD cell proliferation, aggravate oxidative stress, promote angiogenesis and neoinnervation in the process of IVDD.Citation9 It has been shown that AF rupture in IVD provides a microenvironment for sensory innervation and angiogenesis,Citation11 allowing ingrowth of sensory neurons and blood vessels, further accelerating the development of discogenic pain.Citation12 In addition, emerging evidence suggests that once the IVD tissue is compressed into the surrounding soft tissue, reparative fibrous tissue containing lymphatic vessels is induced to grow inwards, although it is widely acknowledged that normal adult IVDs do not contain lymphatic vessels.Citation13
Pyroptosis is a newly discovered inflammatory programmed cell death mediated by inflammasome, which differs from other types of cell death, such as apoptosis, necrosis, and autophagy. The remarkable features of pyroptosis include pore formation on the plasma membrane, cell swelling and rupture, and subsequent release of inflammatory cytokines (such as IL-1β and IL-18) and intracellular materials.Citation14 It has been established that aberrant activation of pyroptosis can result in a massive inflammatory response, leading to inappropriate repair of damaged tissue and organ, causing inflammatory diseases, including arthritis, sepsis, atherosclerosis, cancers, Parkinson and other diseases.Citation15–21 Growing evidence suggests that the inhibition of pyroptosis may be a potential therapeutic direction for these diseases.Citation22–25 Importantly, our latest findings also demonstrated a significant increase in the expression of pyroptosis-related proteins, such as nod-like receptor protein-3 (NLRP3), Caspase-1, and Gasdermin-D (GSDMD) in IVD cells of IVDD patients and murine IVDD model.Citation26–28 Meanwhile, other in vitro experiments corroborated that exogenous stimuli, including lipopolysaccharide (LPS) and hydrogen peroxide (H2O2), could induce inflammatory responses and degenerative phenotype of NP cells by activating NLRP3.Citation29,Citation30 The above findings suggest that pyroptosis is a prominent regulatory process in IVDD. However, the comprehensive roles of pyroptosis in IVDD have not been fully established, which accounts for the lack of consensus on attempts to treat IVDD via an anti-pyroptosis approach.
In this review, we briefly introduce the characteristics of pyroptosis and provide a brief overview of the latest findings on aberrant pyroptosis activation of IVD cells during IVDD progression and emphasize the comprehensive roles of pyroptosis on various pathological phenotypes of IVDD, including NP cell apoptosis and autophagy, ECM degradation, AF rupture, CEP calcification, sensory innervation, lymphoid ingrowth, and cytokine secretion. Finally, currently available anti-pyroptotic strategies for IVDD are listed and analyzed.
Characterization of Pyroptosis
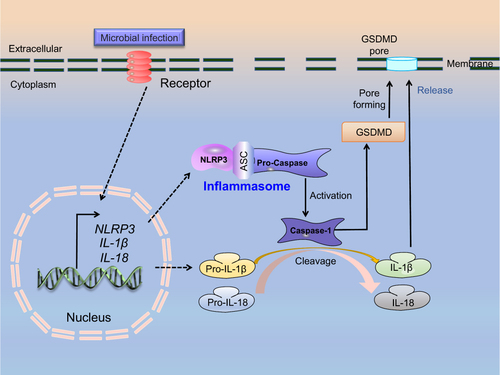
Pyroptosis is a newly discovered caspase-dependent cell death, the characteristics of which are different from the well-known apoptosis, autophagy, and ferroptosis (), mainly manifested as cell swelling until the cell membrane rupture, resulting in the inability to regulate the ingress and egress of cell contents and the activation of a strong inflammatory response.Citation14,Citation19 Pyroptosis is mediated by the formation of inflammasome complexes, which are cytosolic heptameric oligomers composed of the nucleotide-binding domain and leucine-rich repeat (NLR) pattern recognition receptors. The best-characterized NLR, NLRP3, is a redox-sensitive cytosolic sensor that triggers the recruitment and activation of pro-Caspase-1, thus cleaving pro-IL-1β and pro-IL-18 into the mature active form of pro-inflammatory factors, IL-1β and IL-18. In addition, Caspase-1 activation results in the oligomerization of the N-terminal of GSDMD and pore formation in the plasma membrane, which allows mature IL-1β/IL-18 of a diameter of 4.5 nm and Caspase-1 of a diameter of 7.5 nm to pass through.Citation31 Meanwhile, water entering through the pores causes cell swelling and osmotic lysis, resulting in the rupture of the plasma membrane and release of IL-1β and IL-18 ().Citation14,Citation32–35 A more in-depth discussion of the mechanisms underlying pyroptosis is available in previous reviews.Citation36–38
Table 1 Characterizations and Key Molecules Involving Four Forms of Cell Death
Aberrant Pyroptosis of IVD Cells During IVDD Progression
Accumulating evidence suggests that pyroptosis of IVD cells is induced during IVDD progression.Citation44–46 A previous study based on IVD tissues from IVDD patients showed that the expression of inflammasome-related proteins, including NLRP3 and its downstream targets CASPASE-1 and IL-1β, were upregulated in degenerative IVDs, and their expressions were positively correlated with the degree of disc degeneration.Citation47 It has been shown that NP cells from grade V disc degeneration patients express a higher cleaved-Caspase-1, IL-1β, and IL-18 than grade IV patients, depending on the difference in the degree of disc degeneration defined by the Pfirrmann classification.Citation46 Current evidence suggests that the proportion of IL-1β-positive cells is particularly prominent in chondrocyte-like cells in inner AF tissue, and this immunopositivity increases with the severity of degeneration.Citation48 Beyond that, there is limited information that can be gleaned on cytological studies of the effects of pyroptosis on CEP. Increased expression of pyroptosis-related indicators, including NLRP3, Caspase-1 and IL-1β, has been documented in CEP tissues of IVDD patients and animal model.Citation27,Citation49 Accumulating in vitro experiments show that treatment with H2O2 or LPS can increase ROS levels in human NP cells, which in turn augments NLRP3, cleaved IL-1, cleaved IL-18, and PYCARD expression, leading to activation of pyroptosis in NP cells.Citation26,Citation46 These findings substantiate that IVD cells exhibit a pyroptosis phenotype during the pathogenesis of IVDD, suggesting pyroptosis may play an important role in disease progression.
Biological Roles of Pyroptosis of Different IVD Cells on the Pathological Phenotypes of IVDD
An increasing body of evidence suggests that pyroptosis of chondrocytes could promote apoptosis and autophagy in cartilages, production of inflammatory cytokines, and ECM metabolism homeostasis imbalance, resulting in osteoarthritis and other bone degenerative diseases.Citation20,Citation50–54 Noteworthy, chondrocyte pyroptosis has been associated with the growth of blood vessels and nerves, triggering severe pain reactions.Citation48 Similar with chondrocyte cells, IVD cell pyroptosis has been demonstrated to play an important role in IVDD progression. Nonetheless, the relationship between pyroptosis of IVD cells and other pathological changes of IVDD progression is still unclear, which leads us to speculate that pyroptosis of different IVD cells can accelerate the IVDD process by aggravating other pathological changes in multiple IVD compartments.
Pyroptosis and Production of Cytokines in IVDD
Inflammation occurs in response to tissue injury and is a key factor in the degenerative process of IVD and LBP.Citation2 Overwhelming evidence substantiates that pyroptosis is involved in inflammatory responses during IVDD. It has been reported that Propionibacterium acnes induces NP cell pyroptosis activation via the NLRP3-dependent pathway, which accounts for inflammation in IVDD.Citation44 In an ex vivo bovine IVDD model, IL-1β exposure increased the expression of IL-6, IL-8, monocyte chemoattractant protein-1, and prostaglandin E2 in IVDs.Citation55 Conversely, the binding of IL-6 with its soluble receptor promotes IL-1β-induced proteoglycan catabolism, suggesting a positive feedback loop involving IL-1β and IL-6 that could further amplify the IVD inflammatory response.Citation56 Another study showed that LPS treatment could dramatically enhance the expression of TNF-α, IL-6, and other inflammatory factors in NP cells by down-regulating microRNA-200C-3p.Citation57 IL-10 is a cytokine with anti-inflammatory properties that plays a central role during infection by limiting the immune response to pathogens and thereby preventing damage to the host.Citation58 The above literature substantiates that activation of pyroptosis in IVDD tissue induces inflammatory responses and aggravates disc degeneration by stimulating various cytokines, including IL-1β, IL-6, IL-8, IL-18, etc.
NP Cell Pyroptosis and NP Cell Apoptosis
Apoptosis is a form of programmed cell death distinguished from pyroptosis, whereby cells self-destruct and are involved in many biological events, including tissue homeostasis, removal of unwanted cells, and developmental sculpting.Citation59,Citation60 It has been shown that increased NP cell apoptosis contributes to IVDD progression. In some bioprocesses, the activation of pyroptosis can trigger apoptosis, although the relationship between pyroptosis and apoptosis in NP cells remains obscure.Citation26,Citation61 Pyroptosis is characterized by the release of inflammatory cytokines, while inflammatory inhibition reduces NP cell apoptosis, indicating that pyroptosis may be one of the main reasons for apoptosis in NP cells. Notably, IL-1β, an important downstream protein of pyroptosis, has been documented to induce apoptosis of NP cells by elevating pro-apoptotic proteins, including Caspase-3 and Bax, and reducing anti-apoptotic proteins.Citation62 A recent study showed that CY-09, an NLRP3 inflammasome-specific inhibitor, could reduce LPS-induced NP cell apoptosis in vitro, indicating that inhibition of NLRP3 inflammasome activation can protect against LPS-induced apoptosis of NP cells.Citation61
NP Cell Pyroptosis and NP Cell Autophagy
Autophagy is an evolutionarily conserved intracellular recycling system that delivers cytoplasmic content to lysosomes for degradation, thus maintaining metabolism and homeostasis.Citation3 Growing evidence suggests that autophagy could limit the activation of inflammasomes and alleviate the secretion of inflammatory cytokines.Citation63,Citation64 It is reasonable to assume that autophagy may regulate inflammasome activation and affect the outcome of cell pyroptosis. However, the relationship between NP cell pyroptosis and autophagy is complicated. Current evidence suggests that IVD cell autophagy can exert a protective effect when IVDs are subjected to oxidative stress, starvation, hypoxia, inflammation, and infection, triggering or exacerbating disc damage.Citation65 Meanwhile, it has been found that the expression of GSDMD and autophagy-related protein LC3 and ATG5 are increased in NP tissues of human IVDD patients and rat IVDD models.Citation66 Further analysis showed that treatment with autophagy inhibitor 3-MA resulted in increased expression of GSDMD, NLRP3, and Caspase-1 proteins, while autophagy inducer rapamycin significantly reversed these alterations.Citation46 The above studies substantiate a negative correlation between pyroptosis and autophagy in NP cells. Nonetheless, whether NP cell pyroptosis can influence autophagy warrants further investigation.
Pyroptosis and Matrix Degradation
The imbalance between ECM synthesis and degradation is an important pathological process in IVDD. Col2 and proteoglycan (predominantly aggrecan) content is crucial to proper disc function, particularly in NP, while MMP (MMP1, 3, 9, 10 and 13) and ADAMTS (ADAMTS-4 and −5) are the primary enzymes that degrade Col2 and Aggrecan, respectively. Interestingly, a study demonstrated that pyroptosis and its downstream protein could stimulate the production of MMP and ADAMTS during IVDD in vitro and in vivo. Moreover, it has been shown that IL-1β or LPS-stimulation regulates ECM anabolic and catabolic homeostasis by upregulating MMP1, 3, 9, 10 and decreasing Col2 and Aggrecan, thereby accelerating IVDD progression.Citation67–73 Conversely, hyperbaric oxygen reduces MMP3 amounts in degenerated human NP cells by suppressing of IL-1β.Citation74 Interestingly, lactoferricin, a cationic antimicrobial peptide, has been reported to exert a significant anti-inflammatory effect.Citation75 A study reported that lactoferricin could block IL-1β-induced expression of ADAMTS-4 and ADAMTS-5 in bovine IVD cells.Citation67
Moreover, it has been shown that inhibiting NLRP3 inflammasome activity could suppress ECM degradation and slow the process of IVDD. Bromodomain-containing protein 4 is an important upstream regulator of NLRP3 inflammasome activity. It has been reported that JQ1, a bromodomain-containing protein 4 inhibitor, could significantly inhibit bromodomain-containing protein 4-mediated activation of the NLRP3 inflammasome, thereby inhibiting the expression of MMP3, 13, ADAMTS-4, −5 and upregulating Col2 and proteoglycan levels, ultimately slowing IVDD.Citation76 As a focal adhesion protein, Kindlin-2 inhibits inflammatory signals to maintain IVD homeostasis. Recent evidence from clinical samples and abnormal mechanical stress-induced mouse coccygeal IVDD model showed that Kindlin-2 is significantly decreased in NP cells in severe IVDD patients and aged mice. In contrast, Kindlin-2 loss activates the NLRP3 inflammasome and stimulates IL-1β expression in NP cells, further downregulating Kindlin-2 expression. Importantly, this vicious cycle promotes ECM catabolism by increasing MMP13 and decreasing Col2 expression.Citation77,Citation78
These findings lead us to speculate that NP pyroptosis contributes to the synthesis of IVD MMPs and ADAMTS, thereby aggravating the loss of ECM and the development of IVDD. Importantly, targeting pyroptosis may be a promising direction for inhibiting ECM degradation of IVDD tissues.
Pyroptosis and CEP Degeneration and Tearing
As one of the initiating factors of IVDD, CEP degeneration and tearing participate in or accelerate IVDD by disrupting the nutrient supply of IVD. Current evidence suggests that in IVDD patients, the avulsed CEP is characterized by multiple defects, apparent inflammation, and nucleus invasion, as well as activated NLRP3 inflammasome with upregulation of NLRP3, Caspase-1, and IL-1β, suggesting that pyroptosis is activated in CEP tissues of IVDD patients with Modic alterations on MRI, causing the progression of LBP.Citation49,Citation79 Meanwhile, CEP cells induced by LPS can activate the cGAS/STING molecular pathway, resulting in cartilage tear damage with irregular structure and loss of hyaline cartilage and its cells.Citation70 In line with these findings, our recent study using a lumbar instability surgery-induced IVDD mouse model indicated that the expression level of NLRP3 in the CEP ectopic bone formation area was significantly increased in IVDD mice.Citation27 These findings substantiate that pyroptosis of CEP tissues is closely related to CEP degeneration and tearing. Nonetheless, the specific mechanism remains to be further studied, especially ectopic bone formation in CEP in IVDD disease.
Pyroptosis, Vascularization and Nerve Ingrowth
It is well known that IVD is the largest avascular tissue in the organism with branches of the sinuvertebral nerve in the posterior aspect of the outer AF.Citation80,Citation81 Paradoxically, IVD tissues gradually become vascularized and innervated during IVDD progression and positively correlate with the severity of IVDD.Citation80,Citation81 It is widely believed that innervation follows vascular ingrowth.Citation82 In vivo and in vitro evidence from IVDD patient NP tissues showed a positive correlation between IL-1β and expression of VEGF as well as neurotrophic factors, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in human IVD tissues. At the same time, IL-1β generated during the degeneration of IVDs could further stimulate the expression of VEGF, NGF, and BDNF, suggesting pyroptosis may be associated with vascularization and nerve ingrowth in IVDD disease. Blocking pyroptosis may be an alternative way to impede vascularization and nerve ingrowth.Citation29
Vascular endothelial growth factor (VEGF) is the most important pro-angiogenesis factor that stimulates the proliferation and migration of endothelial cells, thus contributing to the formation of neovessels. By using a rat IVD degeneration model with anterior limbs and a tail-removing method, Liu et al reported that positive expression of VEGF in degenerative IVD tissue is responsible for neovascularization and infiltration in IVD, which eventually accelerates the IVDD process.Citation83 Consistently, a study reported that IL-1β could stimulate NP cells to express more VEGF under hypoxic conditions, whereas treatment with IL-1β antibody reduced the production of VEGF in NP cells.Citation84,Citation85 In conclusion, the above findings suggest that pyroptosis-mediated inflammation may stimulate neovascular ingrowth within the IVD by promoting the production of VEGF.
Neurotrophic factors such as NGF and BDNF secreted by blood vessels can promote the survival and differentiation of neurons, expressed in AF and NP cells of IVD, influencing and enhancing the innervation and pain in degenerative IVD.Citation86 A previous study documented that high molecular weight hyaluronic acid hydrogels could attenuate the expression of IL-1R, NGF, and BDNF in IL-1β induced inflammation model of NP cells, which may have a therapeutic effect on inflammation-associated pain in discs.Citation87 Beyond that, Netrin-1 also plays a positive role in promoting peripheral nerve regeneration.Citation88 Further evidence from clinical samples showed increased netrin-1 immunopositive IVD cells in degenerative IVD, which is closely related to the progression of neurovascular growth.Citation89 These pieces of evidence allow us to conjecture that pyroptosis may affect Netrin-1 and induce nerve ingrowth, although the specific mechanism warrants further study.
Pyroptosis and Lymphatic Involvement
The lymphatic system is part of the immune system that protects the body from infection and has long been considered to play an essential impact in immune-related diseases by modulating the inflammatory response to lesions or pathogens, and disrupting the lymphatic system by affecting its normal development or function can lead to severe consequences.Citation90 The quantification of specific lymphatic endothelial cell markers podoplanin and LYVE-1 in clinical vertebral samples has revealed no lymphatics in intact normal IVDs or spinal vertebrae of children or adults.Citation91 Unexpectedly, in infected and displaced degenerate disc tissues, scattered small vessels lined by LYVE-1+/podoplanin+ endothelial cells were observed, demonstrating the presence of de novo lymphatic vessels in the outer periosteum, paraspinal ligaments, and surrounding connective tissue.Citation13,Citation91 Additionally, monocytes/macrophages are cells of the innate immune system that regulate tissue homeostasis and host defense during pathogen infection by controlling the formation of lymphatic vessels.Citation92–95 Increasing evidence from human and rodent IVDD model samples indicates that monocytes/macrophages recruited from the peripheral blood infiltrate into IVDs and produce proinflammatory cytokines, contributing to the degenerative process.Citation96 In the LPS-induced rat IVDD model, abundant local production of pro-inflammatory cytokines, including IL-1β, is accompanied by the increased expression of M1 macrophages, as evidenced by elevated specific markers iNOS and CD68.Citation70 Meanwhile, IL-1β stimulation or IVD injury could increase chemoattractant protein-1 expression in macrophages, which may indirectly contribute to inflammation through monocyte recruitment from blood during IVD injury.Citation97 Therefore, we speculate that pyroptosis-induced inflammation is related to lymphatic ingrowth into IVD tissues.
Potential Therapeutic Strategies for IVDD via Targeting Pyroptosis
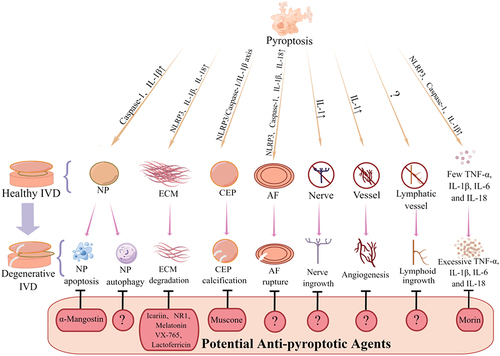
Given the importance of pyroptosis in many aspects of IVDD, various anti-pyroptosis strategies have been applied in animal models or clinical trials to treat IVDD. The agents targeting pyroptosis for the treatment of IVDD include natural small molecular compounds, such as icariin, morin, notoginsenoside R1, and protein inhibitors, such as NLRP3 inhibitors, Caspase-1 inhibitors, GSDMD inhibitors, and IL-1 inhibitors (). Therefore, cell pyroptosis might be an effective therapeutic target for IVDD.
Table 2 Therapeutic Agents for Treating IVDD by Targeting Pyroptosis
Natural Small Molecular Compounds
Icariin
Icariin represents a class of prenylated flavonoids and a principal active component of the Traditional Chinese Medicine herb, Epimedium grandiflorum. It is well-established that the pharmacological effects of icariin include anti-osteoporosis, neuroprotective effects, protective effects of cardiovascular disease, anti-inflammation, improvement effects on the reproductive system, antioxidant stress effect, anti-depression, and anti-tumor effects,Citation105 which has been harnessed for the management of hypertension, coronary heart disease, osteoporosis, menopausal syndrome, rheumatism, neurasthenia, bronchitis, and hypogonadism.Citation106 A previous study reported that icariin treatment inhibits rat chondrocyte pyroptosis by inhibiting NLRP3-mediated inflammasome and production of IL-1β, thereby alleviating rat osteoarthritis progression.Citation98 In line with this finding, emerging evidence from an in vitro NP cell inflammation model suggested that icariin exerts a strong protective effect against IL-1β-induced inflammatory response in NP cells.Citation107
Morin
Morin is a natural polyphenol originally isolated from members of the Mulberry family and can be extracted from the leaves, fruits, stems, and branches of many plants.Citation108 Growing evidence suggests that morin has antioxidant, anti-inflammatory, and anti-pyroptosis properties.Citation109–111 A mechanistic study showed that thioredoxin (TRX)-interacting protein (TXNIP) could interact with NLRP3 and participate in NLRP3 inflammasome-dependent pyroptosis, while TXNIP deficiency impaired activation of the NLRP3 inflammasome and subsequent secretion of IL-1β.Citation99,Citation112 Importantly, morin, an inhibitor of TXNIP, can attenuate NP cell pyroptosis and ameliorate IVDD via inhibition of the TXNIP/NLRP3/Caspase-1/IL-1β signaling pathway.Citation99,Citation112
Notoginsenoside R1
Notoginsenoside R1 (NR1) is one of the main bioactive compounds in the Chinese herb Sanchi root, which plays a certain role in bone metabolism regulation, cardiovascular protection, kidney protection, and anti-cancer effects.Citation79 Over the years, NR1 has been widely applied to treat IVDD.Citation113 Further mechanistic study suggested that NR1 significantly reduces the increased pyroptosis markers, such as NLRP3, cleaved-Caspase-1, IL-1β, and GSDMD-N and ECM degradation induced by TNF-α in AF puncture-induced rat IVDD model, indicating that NR1 may protect against IVDD via inhibiting NF-κB/NLRP3 pathway.Citation100
Protein Inhibitors
NLRP3 Inhibitor
Muscone
Muscone (known as 3-methylcyclopentadecanone), one of the macrocyclic musk compounds, is primarily responsible for the characteristic odor of musk, and can relieve pain.Citation101 In China, muscone is widely used to treat neck pain.Citation101 A previous experiment indicated that muscone could significantly downregulate the levels of LPS-induced inflammatory cytokines and inhibit NF-κB-mediated activation of the NLRP3 inflammasome, thereby improving cardiac function in myocardial infarction mice.Citation114 Moreover, muscone has been shown to restore the structure of degenerative discs in cervical spine instability surgery-induced rat IVDD model (excision of the paraspinal muscles and supra- and inter-spinous ligaments from C2-C7). Further mechanistical analysis showed that muscone reversed IL-1β and reduced the viability of CEP chondrocytes and the corresponding upregulation of various proinflammatory cytokines, including prostaglandin E2, 6-keto-prostaglandin F1α, IL-1β, TNF-α, cyclooxygenase 2, inducible nitric oxide synthase, etc.Citation101
α-Mangostin
α-Mangostin is a natural xanthone product isolated from the pericarps of the mangosteen tree as a secondary metabolite, representing one of the most studied chemopreventive agents.Citation115 Substantial evidence suggests that α-mangostin can interfere with the major stages of carcinogenesis (initiation, promotion, and progression).Citation116,Citation117 Moreover, α-Mangostin exhibits a novel biological function against inflammation in various inflammatory diseases.Citation116,Citation117 A recent study has demonstrated that α-mangostin treatment inhibits LPS-induced increase of NLRP3, ASC, and pro-Caspase-1 expression, as well as IL-1β and IL-18 production, suggesting α-mangostin exerts a protective effect on LPS-induced NLRP3 inflammasome of NP cells.Citation102
Melatonin
Melatonin is a methoxyindole principally synthesized and secreted by the pineal gland at night under normal light/dark conditions.Citation118 It is well established that melatonin can treat schizophrenia, primary headache disorders, and sleep disturbances.Citation119–121 Interestingly, melatonin was found to delay the progression of IVDD and relieve IVDD-related LBP in a needle puncture-induced IVDD rat model.Citation103 Subsequent in vitro and in vivo mechanistical analysis showed that melatonin impairs the IL-1β/NF-κB-NLRP3 inflammasome activation positive feedback loop by decreasing NLRP3, p20 and IL-1β levels.Citation103 These findings suggest that melatonin might be a potential therapeutic agent for IVDD.
Caspase-1 Inhibitor
VX-765
VX-765 is a newly developed selective small-molecule Caspase-1 inhibitor that can cross the blood–brain barrier and reduce inflammation in vitro and in vivo.Citation122 It can be orally taken for the treatment of epilepsy.Citation123 A recent study performed in a needle puncture-induced rat disc IVDD model has shown that Caspase-1 was significantly increased during IVDD progression, and VX-765 efficiently reduced Caspase-1 and GSDMD-N expression, thus inhibiting cell pyroptosis.Citation66 Moreover, VX-765 could dramatically retard degenerative phenotypes of NP tissues in IVDD mice, such as loss of ECM proteins replaced by fibrous tissues.Citation66
GSDMD Inhibitor
Disulfiram
Disulfiram is the most frequently used alcohol-aversive drug by American physicians in treating alcohol dependency disorders.Citation124 Moreover, many preclinical studies have shown the safety profile of disulfiram, which can target various cancers.Citation125 Interestingly, it has been found that disulfiram blocks pyroptosis and cytokine release in cells and LPS-induced septic death in mice by inhibiting the formation of GSDMD pore by targeting Cys191 on GSDMD, thereby preventing IL-1β release and pyroptosis.Citation104 However, disulfiram does not affect inflammatory Caspase cleavage or other upstream events in GSDMD.Citation104 Importantly, the inhibitory effect of disulfiram on GSDMD offers new therapeutic indications for repurposing this safe drug against inflammation associated with many diseases.
IL-1β Inhibitor
Lactoferricin
Lactoferricin was originally identified as an antimicrobial peptide derived from the digestion of lactoferrin (a multifunctional innate-defense protein in milk) by pepsin.Citation126 There is rich literature available substantiating that lactoferricin inhibits catabolic and inflammatory mediators in articular cartilage.Citation127 Currently available research suggests that lactoferricin significantly attenuates catabolic factor-induced stimulation of oxidative and inflammatory factors, such as iNOS, IL-6, and toll-like receptor-2 (TLR-2) and TLR-4, and disrupts IL-1β-mediated inflammation and suppression of proteoglycan production and synthesis, thus restoring proteoglycan accumulation and ECM formation in IVD.Citation67 Meanwhile, lactoferricin has been reported to antagonize IL-1 and LPS-mediated suppression of proteoglycan in an en bloc intradiscal microinjection model followed by ex vivo organ culture using both mouse and rabbit IVD tissue, suggesting a potential therapeutic benefit of lactoferricin against IVDD in the future.Citation67
Conclusions and Prospects
IVDD is an intricate disease involving many types of pathological alterations. Accruing evidence from recently published studies demonstrate that IVD cell pyroptosis is activated in IVDD progression, suggesting its potential role in disease pathogenesis. Importantly, pyroptosis can affect the composition, structure, and functions of IVD tissues and facilitates IVDD progression by promoting inflammation, NP cell loss, disc matrix catabolism, AF rupture, and CEP calcification (). Our previous study on the LSI surgery-induced mouse IVDD model found obvious pathological changes in the CEP 1 week after modeling, which were much earlier than those of NP and AF, indicating that the lesions of CEP may be more important. However, compared with NP and AF, the mechanism of pyroptosis in CEP remains largely unclear, which may hinder our understanding of pathogenesis. Further research on the relationship between CEP and pyroptosis may provide novel insights into the transport function of CEP, the compression of NP and the overall progress of IVDD. In addition, the mechanisms by which pyroptosis participates in sensory nerve and lymphoid ingrowth in the pathogenesis of IVDD remains obscure. The importance of sensory nerves and the lymphoid system in IVDD and LBP highlights the need for further research to understand the underlying mechanisms.
Ferroptosis is another newly discovered cell death that occurs via an iron-catalyzed process of lipid peroxidation initiated through non-enzymatic (Fenton reactions) and enzymatic mechanisms (lipoxygenases).Citation128 Like pyroptosis, it belongs to a genetically regulated type of non-apoptotic cell death, called regulatory necrosis,Citation129 characterized by the increased rupture of mitochondrial membrane increases.Citation130 An increasing body of evidence shows that oxidative stress could reduce phospholipid hydroperoxides on NP and AF cells by inhibiting glutathione peroxidase 4 (Gpx4), thus triggering ferroptosis and aggravating IVDD progression.Citation131–133 Another study using the kidney tissue of selenium-deficient broilers has reported that inhibiting Gpx4 could increase the release of ROS, activate the NLRP3 inflammasome, and release the inflammatory factors IL-1β and IL-18 to trigger pyroptosis,Citation134 whether a similar relationship exists in the context of IVDD progression remains obscure, which needs to be further clarified.
Nevertheless, our understanding of the role of pyroptosis in IVDD remains to be improved. Considering that the pathogenesis in IVDD also includes the thoracolumbar fascia and vertebrae, zygapophyseal joints, ligaments, and muscles.Citation135 The IVDD processes may be usually the consequence of the biomechanical imbalance of the spinal axis caused by these tissue structural and functional disorders. But studies on pyroptosis or pyroptotic-related molecules involving the above tissue are limited, the involved mechanisms may be diverse and multifaceted, and a systematic investigation of the increased disorders of these tissues caused by pyroptosis and the IVDD progression is urgently needed.
It should be borne in mind that current studies on the treatment of IVDD are based on the mechanism of pyroptosis participating in IVDD. In vitro and in vivo studies substantiated that some of the developed anti-pyroptosis methods have good therapeutic effects on IVDD. Unfortunately, no IVDD-related preclinical studies have been conducted to further explore the efficacy and side effects of these drugs as well as other drugs that have been put into clinical use to treat other diseases by targeting pyroptosis. Indeed, these findings highlight the need to develop novel drugs that target the pyroptosis of IVD cells to improve IVDD treatment. Importantly, our future studies will assess treatment efficacy with Caspase-1, GSDMD, and IL-1 antibodies on IVDD. Moreover, we will also further explore the efficacy and side effects of other drugs that have been put into clinical use to treat other diseases by regulating pyroptosis.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
- Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(104):20141191. doi:10.1098/rsif.2014.1191
- Gong CY, Zhang HH. Autophagy as a potential therapeutic target in intervertebral disc degeneration. Life Sci. 2021;273:119266. doi:10.1016/j.lfs.2021.119266
- Devereaux MW. Anatomy and examination of the spine. Neurol Clin. 2007;25(2):331–351. doi:10.1016/j.ncl.2007.02.003
- Dou Y, Sun X, Ma X, Zhao X, Yang Q. Intervertebral disk degeneration: the microenvironment and tissue engineering strategies. Front Bioeng Biotechnol. 2021;9:592118. doi:10.3389/fbioe.2021.592118
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20(2):107–121. doi:10.1016/s0945-053x(01)00125-1
- Fields AJ, Ballatori A, Liebenberg EC, Lotz JC. Contribution of the endplates to disc degeneration. Curr Mol Biol Rep. 2018;4(4):151–160. doi:10.1007/s40610-018-0105-y
- Wang Y, Kang J, Guo X, et al. Intervertebral disc degeneration models for pathophysiology and regenerative therapy -benefits and limitations. J Invest Surg. 2022;35(4):935–952. doi:10.1080/08941939.2021.1953640
- Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. doi:10.1016/j.biopha.2020.110660
- Binch A, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158–175. doi:10.1038/s41584-020-00568-w
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi:10.1038/ncomms14128
- Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat. 2018;233(1):86–97. doi:10.1111/joa.12817
- Kliskey K, Williams K, Yu J, Jackson D, Urban J, Athanasou N. The presence and absence of lymphatic vessels in the adult human intervertebral disc: relation to disc pathology. Skeletal Radiol. 2009;38(12):1169–1173. doi:10.1007/s00256-009-0770-2
- Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
- Liu L, Sun B. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol Life Sci. 2019;76(11):2031–2042. doi:10.1007/s00018-019-03060-1
- Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi:10.1016/j.cca.2017.11.005
- Zheng Z, Mechanisms LG. Therapeutic regulation of pyroptosis in inflammatory diseases and cancer. Int J Mol Sci. 2020;21(4). doi:10.3390/ijms21041456
- Wang S, Yuan YH, Chen NH, Wang HB. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol. 2019;67:458–464. doi:10.1016/j.intimp.2018.12.019
- Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
- Yu H, Yao S, Zhou C, et al. Morroniside attenuates apoptosis and pyroptosis of chondrocytes and ameliorates osteoarthritic development by inhibiting NF-κB signaling. J Ethnopharmacol. 2021;266:113447. doi:10.1016/j.jep.2020.113447
- Hu J, Zhou J, Wu J, et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 2020;247:112261. doi:10.1016/j.jep.2019.112261
- McKenzie BA, Mamik MK, Saito LB, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A. 2018;115(26):E6065–E6074. doi:10.1073/pnas.1722041115
- Liu Z, Gan L, Xu Y, et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63(1). doi:10.1111/jpi.12414
- Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging. 2020;12(10):9405–9426. doi:10.18632/aging.103215
- Wu LM, Wu SG, Chen F, et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis. 2020;293:26–34. doi:10.1016/j.atherosclerosis.2019.11.033
- Zhang J, Zhang J, Zhang Y, et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020;24(20):11742–11754. doi:10.1111/jcmm.15784
- Fu F, Bao R, Yao S, et al. Aberrant spinal mechanical loading stress triggers intervertebral disc degeneration by inducing pyroptosis and nerve ingrowth. Sci Rep. 2021;11(1):772. doi:10.1038/s41598-020-80756-6
- Fu F, Shao J, Tong P, Xiao L, Wu C, Ruan H. Establishment and evaluation of a mouse model of disc degeneration with spinal axial mechanical instability. Chin J Tradition Med Traumatol Orthoped. 2020;28(01):1–7.
- Song Y, Wang Y, Zhang Y, et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J Cell Mol Med. 2017;21(7):1373–1387. doi:10.1111/jcmm.13067
- Tian Y, Bao Z, Ji Y, Mei X, Yang H. Epigallocatechin-3-gallate protects H(2)O(2)-induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation. Drug Des Devel Ther. 2020;14:2113–2122. doi:10.2147/DDDT.S251623
- Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature18590
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi:10.1111/j.1462-5822.2006.00751.x
- Fink SL, Cookson BT. Pillars article: caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol . 2006;8(11):1812–1825.
- Lei Q, Yi T, Chen C. NF-κB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–6052. doi:10.12659/MSM.908529
- He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
- Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941–955.e20. doi:10.1016/j.cell.2020.02.002
- Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157. doi:10.1038/s41577-019-0228-2
- Shao F. Gasdermins: making pores for pyroptosis. Nat Rev Immunol. 2021;21(10):620–621. doi:10.1038/s41577-021-00602-2
- Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–1121. doi:10.1038/s41423-020-00630-3
- Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177(7):1682–1699. doi:10.1016/j.cell.2019.05.026
- Cao W, Li J, Yang K, Cao D. An overview of autophagy: mechanism, regulation and research progress. Bull Cancer. 2021;108(3):304–322. doi:10.1016/j.bulcan.2020.11.004
- Wu DJ, Adamopoulos IE Autophagy and autoimmunity. Clin Immunol. 2017;176:55–62. doi:10.1016/j.clim.2017.01.007
- Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5(1):108. doi:10.1038/s41392-020-00216-5
- Huang L, Luo R, Li J, et al. β-catenin promotes NLRP3 inflammasome activation via increasing the association between NLRP3 and ASC. Mol Immunol. 2020;121:186–194. doi:10.1016/j.molimm.2020.02.017
- Chao-Yang G, Peng C, Hai-Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29(6):793–801. doi:10.1016/j.joca.2021.02.204
- Bai Z, Liu W, He D, et al. Protective effects of autophagy and NFE2L2 on reactive oxygen species-induced pyroptosis of human nucleus pulposus cells. Aging. 2020;12(8):7534–7548. doi:10.18632/aging.103109
- Chen ZH, Jin SH, Wang MY, et al. Enhanced NLRP3, caspase-1, and IL- 1β levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. Anat Rec. 2015;298(4):720–726. doi:10.1002/ar.23059
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732–745. doi:10.1186/ar1732
- Tang P, Zhu R, Ji WP, et al. The NLRP3/Caspase-1/Interleukin-1β axis is active in human lumbar cartilaginous endplate degeneration. Clin Orthop Relat Res. 2016;474(8):1818–1826. doi:10.1007/s11999-016-4866-4
- Yang J, Hu S, Bian Y, et al. Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front Cell Dev Biol. 2021;9:789948. doi:10.3389/fcell.2021.789948
- Li Z, Huang Z, Zhang H, et al. Moderate-intensity exercise alleviates pyroptosis by promoting autophagy in osteoarthritis via the P2X7/AMPK/mTOR axis. Cell Death Discov. 2021;7(1):346. doi:10.1038/s41420-021-00746-z
- Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185–196. doi:10.1016/j.clim.2012.12.011
- Fosang AJ, Beier F. Emerging Frontiers in cartilage and chondrocyte biology. Best Pract Res Clin Rheumatol. 2011;25(6):751–766. doi:10.1016/j.berh.2011.11.010
- Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi:10.1186/ar4133
- Teixeira GQ, Pereira CL, Ferreira JR, et al. Immunomodulation of human mesenchymal stem/stromal cells in intervertebral disc degeneration: insights from a proinflammatory/degenerative ex vivo model. Spine. 2018;43(12):E673–E682. doi:10.1097/BRS.0000000000002494
- Studer RK, Vo N, Sowa G, Ondeck C, Kang J. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-α. Spine. 2011;36(8):593–599. doi:10.1097/BRS.0b013e3181da38d5
- Cao J, Jiang M, Ren H, Xu K. MicroRNA‑200c‑3p suppresses intervertebral disc degeneration by targeting RAP2C/ERK signaling. Mol Med Rep. 2021;24(6). doi:10.3892/mmr.2021.12505
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi:10.1038/nri2711
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/01926230701320337
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4(7):552–565. doi:10.1038/nrm1150
- Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol Immunol. 2020;64(4):252–269. doi:10.1111/1348-0421.12771
- Wang K, Chen T, Ying X, et al. Ligustilide alleviated IL-1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. Int Immunopharmacol. 2019;69:398–407. doi:10.1016/j.intimp.2019.01.004
- Claude-Taupin A, Bissa B, Jia J, Gu Y, Deretic V. Role of autophagy in IL-1β export and release from cells. Semin Cell Dev Biol. 2018;83:36–41. doi:10.1016/j.semcdb.2018.03.012
- Takahama M, Akira S, Saitoh T. Autophagy limits activation of the inflammasomes. Immunol Rev. 2018;281(1):62–73. doi:10.1111/imr.12613
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi:10.1126/science.1099993
- Liao Z, Li S, Liu R, et al. Autophagic degradation of gasdermin D protects against nucleus pulposus cell pyroptosis and retards intervertebral disc degeneration in vivo. Oxid Med Cell Longev. 2021;2021:5584447. doi:10.1155/2021/5584447
- Kim JS, Ellman MB, Yan D, et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J Cell Physiol. 2013;228(9):1884–1896. doi:10.1002/jcp.24350
- Kim JH, Choi H, Suh MJ, Shin JH, Hwang MH, Lee HM. Effect of biphasic electrical current stimulation on IL-1β-stimulated annulus fibrosus cells using in vitro microcurrent generating chamber system. Spine. 2013;38(22):E1368–E1376. doi:10.1097/BRS.0b013e3182a211e3
- Shi C, Wu L, Lin W, et al. MiR-202-3p regulates interleukin-1β-induced expression of matrix metalloproteinase 1 in human nucleus pulposus. Gene. 2019;687:156–165. doi:10.1016/j.gene.2018.11.056
- Su Q, Cai Q, Li Y, et al. A novel rat model of vertebral inflammation-induced intervertebral disc degeneration mediated by activating cGAS/STING molecular pathway. J Cell Mol Med. 2021;25(20):9567–9585. doi:10.1111/jcmm.16898
- Zhan S, Wang K, Song Y, et al. Long non-coding RNA HOTAIR modulates intervertebral disc degenerative changes via Wnt/β-catenin pathway. Arthritis Res Ther. 2019;21(1):201. doi:10.1186/s13075-019-1986-8
- Tian Y, Yuan W, Fujita N, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182(6):2310–2321. doi:10.1016/j.ajpath.2013.02.037
- Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–39749. doi:10.1074/jbc.M111.264549
- Niu CC, Yuan LJ, Chen LH, et al. Beneficial effects of hyperbaric oxygen on human degenerated intervertebral disk cells via suppression of IL-1β and p38 MAPK signal. J Orthop Res. 2011;29(1):14–19. doi:10.1002/jor.21195
- Mader JS, Richardson A, Salsman J, et al. Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp Cell Res. 2007;313(12):2634–2650. doi:10.1016/j.yexcr.2007.05.015
- Hong J, Li S, Markova DZ, et al. Bromodomain-containing protein 4 inhibition alleviates matrix degradation by enhancing autophagy and suppressing NLRP3 inflammasome activity in NP cells. J Cell Physiol. 2020;235(7–8):5736–5749. doi:10.1002/jcp.29508
- Attwaters M. Kindlin-2 reduces IVD inflammation. Nat Rev Rheumatol. 2022;18(3):125. doi:10.1038/s41584-022-00753-z
- Chen S, Wu X, Lai Y, et al. Kindlin-2 inhibits Nlrp3 inflammasome activation in nucleus pulposus to maintain homeostasis of the intervertebral disc. Bone Res. 2022;10(1):5. doi:10.1038/s41413-021-00179-5
- Liu H, Yang J, Yang W, et al. Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des Devel Ther. 2020;14:551–565. doi:10.2147/DDDT.S240511
- Karamouzian S, Eskandary H, Faramarzee M, et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35(8):881–886. doi:10.1097/BRS.0b013e3181b9c986
- Miyagi M, Millecamps M, Danco AT, Ohtori S, Takahashi K, Stone LS. ISSLS Prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine. 2014;39(17):1345–1354. doi:10.1097/BRS.0000000000000334
- Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–292. doi:10.1002/path.1108
- Liu XW, Kang J, Fan XD, Sun LF. Expression and significance of VEGF and p53 in rat degenerated intervertebral disc tissues. Asian Pac J Trop Med. 2013;6(5):404–406. doi:10.1016/S1995-7645(13)60047-4
- Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10(4):R99. doi:10.1186/ar2487
- Kwon WK, Moon HJ, Kwon TH, Park YK, Kim JH. The role of hypoxia in angiogenesis and extracellular matrix regulation of intervertebral disc cells during inflammatory reactions. Neurosurgery. 2017;81(5):867–875. doi:10.1093/neuros/nyx149
- Binch AL, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16(5):416. doi:10.1186/s13075-014-0416-1
- Isa IL, Srivastava A, Tiernan D, et al. Hyaluronic acid based hydrogels attenuate inflammatory receptors and neurotrophins in interleukin-1β induced inflammation model of nucleus pulposus cells. Biomacromolecules. 2015;16(6):1714–1725. doi:10.1021/acs.biomac.5b00168
- Chen X, He WT, Hu L, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26(9):1007–1020. doi:10.1038/cr.2016.100
- Dun XP, Parkinson DB. Role of netrin-1 signaling in nerve regeneration. Int J Mol Sci. 2017;18(3). doi:10.3390/ijms18030491
- Klaourakis K, Vieira JM, Riley PR. The evolving cardiac lymphatic vasculature in development, repair and regeneration. Nat Rev Cardiol. 2021;18(5):368–379. doi:10.1038/s41569-020-00489-x
- Kashima TG, Dongre A, Athanasou NA. Lymphatic involvement in vertebral and disc pathology. Spine. 2011;36(11):899–904. doi:10.1097/BRS.0b013e3182050284
- Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi:10.1172/JCI23874
- Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12(2):230–234. doi:10.1038/nm1340
- Schledzewski K, Falkowski M, Moldenhauer G, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209(1):67–77. doi:10.1002/path.1942
- Yan J, Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020;30(12):979–989. doi:10.1016/j.tcb.2020.09.006
- Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine. 2001;26(14):1522–1527. doi:10.1097/00007632-200107150-00004
- Kawakubo A, Uchida K, Miyagi M, et al. Investigation of resident and recruited macrophages following disc injury in mice. J Orthop Res. 2020;38(8):1703–1709. doi:10.1002/jor.24590
- Zu Y, Mu Y, Li Q, Zhang ST, Yan HJ. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14(1):307. doi:10.1186/s13018-019-1307-6
- Zhou Y, Chen Z, Yang X, et al. Morin attenuates pyroptosis of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of the TXNIP/NLRP3/Caspase-1/IL-1β signaling pathway. Biochem Biophys Res Commun. 2021;559:106–112. doi:10.1016/j.bbrc.2021.04.090
- Tang K, Su W, Huang C, Wu Y, Wu X, Lu H. Notoginsenoside R1 suppresses inflammatory response and the pyroptosis of nucleus pulposus cells via inactivating NF-κB/NLRP3 pathways. Int Immunopharmacol. 2021;101(PtB):107866. doi:10.1016/j.intimp.2021.107866
- Liang QQ, Zhang M, Zhou Q, Shi Q, Wang YJ. Muscone protects vertebral end-plate degeneration by antiinflammatory property. Clin Orthop Relat Res. 2010;468(6):1600–1610. doi:10.1007/s11999-009-1079-0
- Chen J, Bian M, Pan L, Yang H. α-Mangostin protects lipopolysaccharide-stimulated nucleus pulposus cells against NLRP3 inflammasome-mediated apoptosis via the NF-κB pathway. J Appl Toxicol. 2022;42(9):1467–1476. doi:10.1002/jat.4306
- Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8:10. doi:10.1038/s41413-020-0087-2
- Hu JJ, Liu X, Xia S, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–745. doi:10.1038/s41590-020-0669-6
- He C, Wang Z, Shi J. Pharmacological effects of icariin. Adv Pharmacol. 2020;87:179–203. doi:10.1016/bs.apha.2019.10.004
- Zhang X, Sun H, Su Q, et al. Antidepressant-like activity of icariin mediated by group I mGluRs in prenatally stressed offspring. Brain Dev. 2017;39(7):593–600. doi:10.1016/j.braindev.2017.03.021
- Hua W, Zhang Y, Wu X, et al. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr Pharm Des. 2018;23(39):6071–6078. doi:10.2174/1381612823666170615112158
- Caselli A, Cirri P, Santi A, Paoli P. Morin: a promising natural drug. Curr Med Chem. 2016;23(8):774–791. doi:10.2174/0929867323666160106150821
- Mohammadi N, Asle-Rousta M, Rahnema M, Amini R. Morin attenuates memory deficits in a rat model of Alzheimer’s disease by ameliorating oxidative stress and neuroinflammation. Eur J Pharmacol. 2021;910:174506. doi:10.1016/j.ejphar.2021.174506
- Khamchai S, Chumboatong W, Hata J, Tocharus C, Suksamrarn A, Tocharus J. Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep. 2020;10(1):13379. doi:10.1038/s41598-020-70214-8
- Yu S, Liu X, Yu D, Changyong E, Yang J. Morin protects LPS-Induced mastitis via inhibiting NLRP3 inflammasome and NF-κB signaling pathways. Inflammation. 2020;43(4):1293–1303. doi:10.1007/s10753-020-01208-x
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi:10.1038/ni.1831
- Hu H, Chen Y, Huang F, et al. Panax notoginseng saponins attenuate intervertebral disc degeneration by reducing the end plate porosity in lumbar spinal instability mice. JOR Spine. 2021;4(4):e1182. doi:10.1002/jsp2.1182
- Du Y, Gu X, Meng H, et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J Transl Res. 2018;10(12):4235–4246.
- Chavan T, Muth A. The diverse bioactivity of α-mangostin and its therapeutic implications. Future Med Chem. 2021;13(19):1679–1694. doi:10.4155/fmc-2021-0146
- Zhang KJ, Gu QL, Yang K, Ming XJ, Wang JX. Anticarcinogenic effects of α-mangostin: a review. Planta Med. 2017;83(3–4):188–202. doi:10.1055/s-0042-119651
- Liu T, Duan W, Nizigiyimana P, et al. Alpha-mangostin attenuates diabetic nephropathy in association with suppression of acid sphingomyelianse and endoplasmic reticulum stress. Biochem Biophys Res Commun. 2018;496(2):394–400. doi:10.1016/j.bbrc.2018.01.040
- Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015;61(2–3):77–84. doi:10.1016/j.neuchi.2015.03.002
- Morera-Fumero AL, Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int J Mol Sci. 2013;14(5):9037–9050. doi:10.3390/ijms14059037
- Gelfand AA, Goadsby PJ. The role of melatonin in the treatment of primary headache disorders. Headache. 2016;56(8):1257–1266. doi:10.1111/head.12862
- Esposito S, Laino D, D’Alonzo R, et al. Pediatric sleep disturbances and treatment with melatonin. J Transl Med. 2019;17(1):77. doi:10.1186/s12967-019-1835-1
- Boxer MB, Quinn AM, Shen M, et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. Chem Med Chem. 2010;5(5):730–738. doi:10.1002/cmdc.200900531
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013;103(1):2–30. doi:10.1016/j.eplepsyres.2012.10.001
- Wright C, Moore RD. Disulfiram treatment of alcoholism. Am J Med. 1990;88(6):647–655. doi:10.1016/0002-9343(90)90534-k
- Lu C, Li X, Ren Y, Zhang X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother Pharmacol. 2021;87(2):159–172. doi:10.1007/s00280-020-04216-8
- Wakabayashi H, Takase M, Tomita M. Lactoferricin derived from milk protein lactoferrin. Curr Pharm Des. 2003;9(16):1277–1287. doi:10.2174/1381612033454829
- Ahmadinia K, Yan D, Ellman M, Im HJ. The anti-catabolic role of bovine lactoferricin in cartilage. Biomol Concepts. 2013;4(5):495–500. doi:10.1515/bmc-2013-0013
- Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi:10.1038/s41422-019-0164-5
- Peng JJ, Song WT, Yao F, et al. Involvement of regulated necrosis in blinding diseases: focus on necroptosis and ferroptosis. Exp Eye Res. 2020;191:107922. doi:10.1016/j.exer.2020.107922
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
- Yang RZ, Xu WN, Zheng HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. 2021;236(4):2725–2739. doi:10.1002/jcp.30039
- Bin S, Xin L, Lin Z, Jinhua Z, Rui G, Xiang Z. Targeting miR-10a-5p/IL-6R axis for reducing IL-6-induced cartilage cell ferroptosis. Exp Mol Pathol. 2021;118:104570. doi:10.1016/j.yexmp.2020.104570
- Wang W, Jing X, Du T, et al. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic Biol Med. 2022;190:234–246. doi:10.1016/j.freeradbiomed.2022.08.018
- Gu X, Wang Y, He Y, et al. MiR-1656 targets GPX4 to trigger pyroptosis in broilers kidney tissues by activating NLRP3 inflammasome under Se deficiency. J Nutr Biochem. 2022;105:109001. doi:10.1016/j.jnutbio.2022.109001
- Delitto A, George SZ, Van Dillen L, et al. Low back pain. J Orthop Sports Phys Ther. 2012;42(4):A1–A57. doi:10.2519/jospt.2012.42.4.A1