Abstract
Pyroptosis is a pro-inflammatory form of cell death resulting from the activation of gasdermins (GSDMs) pore-forming proteins and the release of several pro-inflammatory factors. However, inflammasomes are the intracellular protein complexes that cleave gasdermin D (GSDMD), leading to the formation of robust cell membrane pores and the initiation of pyroptosis. Inflammasome activation and gasdermin-mediated membrane pore formation are the important intrinsic processes in the classical pyroptotic signaling pathway. Overactivation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome triggers pyroptosis and amplifies inflammation. Current evidence suggests that the overactivation of inflammasomes and pyroptosis may further induce the progression of cancers, nerve injury, inflammatory disorders and metabolic dysfunctions. Current evidence also indicates that pyroptosis-dependent cell death accelerates the progression of diabetes and its frequent consequences including diabetic peripheral neuropathy (DPN). Pyroptosis-mediated inflammatory reaction further exacerbates DPN-mediated CNS injury. Accumulating evidence shows that several molecular signaling mechanisms trigger pyroptosis in insulin-producing cells, further leading to the development of DPN. Numerous studies have suggested that certain natural compounds or drugs may possess promising pharmacological properties by modulating inflammasomes and pyroptosis, thereby offering potential preventive and practical therapeutic approaches for the treatment and management of DPN. This review elaborates on the underlying molecular mechanisms of pyroptosis and explores possible therapeutic strategies for regulating pyroptosis-regulated cell death in the pharmacological treatment of DPN.
Introduction
The World Health Organization (WHO) states that diabetes mellitus (DM) is growing, especially in low and middle-income countries. In contrast, more DM deaths occur in those under 70 age than in high-income countries.Citation1,Citation2 The seventh most common cause of mortality worldwide was DM, which affected 422 million people in 2014 and 1.6 million in 2016. Current data indicate that the prevalence of DM is anticipated to reach 642 million by 2040.Citation3,Citation4 Type 2 diabetes mellitus (T2DM) is mainly characterized by increased chronic inflammation burden.Citation5,Citation6 In addition, diabetic microvascular complications including diabetic peripheral neuropathy (DPN) are also characterized by a high inflammatory burden.Citation7,Citation8 It is widely believed that DPN is a devastating complication of DM characterized by complex molecular pathogenesis.Citation9 DPN is closely related to a variety of clinical symptoms including nerve damage, paraesthesia and sensory loss and affects around 50% of adults diagnosed with diabetes.Citation10 However, effective therapies lack specificity and are not currently available. Thus, it is necessary to find efficient approaches against DPN based on the exact molecular mechanisms implicated in the pathogenesis. Therefore, effective therapies to alleviate DPN must be developed based on the underlying mechanisms. The pathogenesis of DPN is complex, involving chronic inflammation and dysfunction of Schwann cells as major contributing factors.Citation11 In addition, environmental and genetic factors including lifestyle factors such as detrimental nutrition, physical inactivity/sedentary lifestyle, obesity or overweight and alcohol and smoking consumption, have been proven to increase the risk of DPN and its complications. Numerous investigations indicate that hyperglycemia promotes AGEs and ROS overproduction, which further stimulate oxidative stress and chronic inflammation, resulting in a variety of complications.Citation12,Citation13 Current research shows that activation of pyroptosis-mediated cell death mediates the advancement of diabetic complications including DPN.Citation14–19
Cell death is typically classified as programmed cell death (PCD) or non-programmed cell death (non-PCD). Specific genes encode signals or actions that eliminate functionally dispensable, infected, or possibly malignant cells and characterize the types of PCD. The most common forms of PCD are pyroptosis, apoptosis and necroptosis.Citation20 Pyroptosis is a form of cell death that involves the creation of pores in the cytoplasmic membrane, cellular contraction, membrane denaturation and the release of inflammatory molecules.Citation21 Pyroptosis, also called gasdermins (GSDMs)-dependent programmed necrosis, is the most recently characterized form of PCD triggered by the disruption of extracellular and intracellular homeostasis that deteriorates the innate immunity system.Citation22–24 Emerging evidence indicates that pyroptosis, a form of PCD with hyperinflammation, typically leads to a significant infection and induces the progression of multiple diseases.Citation25,Citation26 Numerous studies have shown that excessive pyroptosis activation can lead to the progression of several neurological, cardiovascular and inflammatory diseases.Citation27–42 Pyroptosis exacerbates metabolic conditions including hyperglycemia by inducing persistent inflammation and insulin-resistant mediators.Citation43–46 Current studies indicate that the activation of inflammasome and pyroptosis plays contributory roles in developing diabetes complications, particularly in DPN.Citation15,Citation47–55 In addition, pyroptosis-mediated prolonged inflammation induces the development of depression, neurodegenerative disorders, ischemic strokes and intracranial hemorrhages andCitation56 promotes neuroinflammatory injury and pain and disrupts the regeneration and repair of peripheral nerves.Citation57–59
In this review, we first covered the underlying molecular mechanisms of pyroptosis and addressed various signaling pathways that may activate pyroptosis and their impact on the progression of DPN. This review finally presents an overview of prospective therapeutic compounds/drugs for targeting inflammasome and pyroptosis in the treatment and management of DPN.
Biological Features of Pyroptosis
Pyroptosis is mainly derived from the Greek roots “pyro” and “ptosis”, signifying fever and falling, respectively.Citation60,Citation61 This nomenclature is used to characterize a recently discovered form of programmed cell death (PCD) with inflammatory properties. Since 1990, scientists have identified that Shigella flexneri or Salmonella infection eradicates mouse macrophages or human monocytes.Citation62 Shigella dysenteriae was reported by Arturo Zychlinsky in 1997 to activate caspase-1 in host cells.Citation63 The Arturo Zychlinsky lab discovered in 1999 that restricting caspase-1 suppressed Salmonella-induced cell death.Citation64 The research teams of Lawrence H. Boise and Brad Cookson found in 2001 that bacterial infection resulted in macrophage death through the activation of Caspase-1-dependent programmed necrosis, a death mechanism distinct from apoptosis.Citation65,Citation66 Pyroptosis and apoptosis have common biological characteristics and functions such as DNA fragmentation and chromatin. Pyroptotic cells show swelling and the formation of bubble-like bulges on their cell membranes prior to rupture.Citation67 Apoptosis involves Caspase-3 for membrane blebbing. Pyroptosis features specific cellular characteristics that distinguish it from other forms of apoptosis.Citation68–70 Apoptosis is commonly regarded as a non-inflammatory form of cell death, while pyroptosis may trigger low-grade inflammation.Citation71 Pyroptosis is induced by extracellular and intracellular signals including bacterial and viral infections, exposure to toxins and specific chemotherapeutic agents/drugs.Citation72–74 Compared to necrosis, pyroptosis produces cytoplasmic flattening resulting from plasma membrane rupture against explosive rupture. Caspase activation or granzyme release induces gasdermin N-terminal oligomerization and pore creation (1–2 μm) in plasma membrane, facilitating a mature form of IL-1β/IL-18 (4.5 nm) and caspase-1 (7.5 nm) permeability.Citation75 The water infiltrating through pores promotes cell enlargement, osmotic lysis, plasma membrane rupture and IL-1β and IL-18 release.Citation25,Citation76 The low molecular weight of 7-amino actinomycin (7-AAD), propidium iodide (PI) and ethidium bromide (EtBr) allows pyroptotic cell permeability. In contrast to pyroptotic cells, apoptotic cells maintain the integrity of the membrane, eliminating the formation of these dyes.Citation77 Surprisingly comparable to apoptotic cells, Annexin V indicates pyroptotic cells and attaches to phosphatidyl serine (PS). Thus, Annexin V cannot distinguish between apoptotic and pyroptotic cells. Apoptotic bodies are produced in apoptosis, whereas pyroptotic bodies are produced in pyroptosis.Citation78 Pyroptotic bodies are 1–5 µm in diameter, similar to apoptotic bodies.Citation79 However, a novel gasdermin-D (GSDMD) protein has been identified and characterized, which is typically in an auto-inhibitory state.Citation80 Following caspase splitting, GSDMD produces the N-terminal fragment (GSDMD-NT), which further inflates and ruptures cells. Therefore, GSDMD serves as the effector molecule that regulates the execution of pyroptosis-dependent cell death. Similar to the GSDMD pore-forming protein, GSDMA, GSDMB, GSDMC, DFNA5/GSDME and DFNB59 induce pyroptosis and membrane denaturation.Citation81,Citation82 Recently, Wang and colleagues proved that the mechanism of pyroptosis induced by the GSDMD-NT is consistent with the N-terminal domain of GSDME interacting with 4, 5-diphosphate phosphatidylinositol [PI (4,5) P2], resulting in the perforation of liposomes and elimination of their phospholipid components.Citation74 In an article published in 1994 by Feng Shao and colleagues, pyroptosis was renamed gasdermin family-driven programmed necrosis in cells.Citation25 Recently, Chauhan and co-workers also observed that neutrophil elastase (NE) hydrolyzed GSDMD and triggered neutrophil pyroptosis.Citation83 Therefore, pyroptosis is another form of regulated cell death (RCD) that extensively depends on gasdermin protein family members for creating plasma membrane pores, often (but not typically) as a result of inflammatory caspase activation, according to the Nomenclature Committee on Cell Death (NCCD) in 2018.Citation21
Molecular Mechanisms of Pyroptosis
Classical and non-classical inflammasome pathways, apoptotic caspases-dependent and granzymes-dependent pathways have been recognized as the primary signaling pathways that induce pyroptosis-dependent cell death.Citation79 Gasdermin proteins are the end mediators in these signaling pathways and are required to be cleaved by precursor caspases or granzymes.Citation84 Caspases are classified into inflammatory and apoptotic bodies, depending on their specific role and function.Citation85 Caspases-1/4/5/11 are inflammatory caspases that trigger pyroptosis, inhibit the proliferation of pathogens and regulate the maturation and secretion of a variety of pro-inflammatory factors.Citation86 Activation of inflammatory caspases serves as the primary defense mechanism against infectious pathogens. Inflammasome is a multiprotein complex that initiates Caspase-1 activity downstream of the cell membrane.Citation87–90 The activation of inflammatory Caspase-4/5/11 does not require a molecular complex and has been demonstrated to bind LPS directly.Citation91 Apoptotic caspases primarily initiate and regulate the cellular mechanisms of apoptosis. Current investigations have shown the ability of proteases to cleave gasdermins, which results in triggering pyroptosis-driven cell death.Citation92
Classical Signaling Pathways
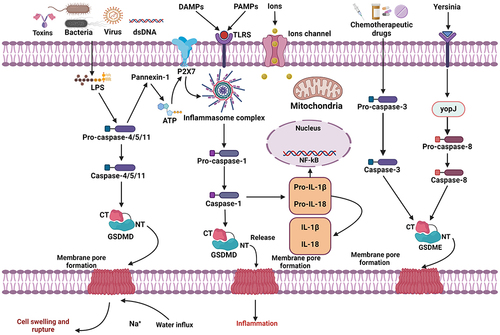
Classical pyroptotic cell death can be triggered by the formation of inflammasomes, leading to the cleavage of GSDMD and extensive release of pro-inflammatory factors including IL-1β and IL-18.Citation93,Citation94 Inflammasomes are multi-molecular complexes that stimulate the adaptive immune response and protect the host against microbial infections.Citation95–99 Inflammasomes can cause non-microbial diseases. Many studies indicate that inflammasomes and their cytokines are essential in oncogenesis including proliferation, metastasis and invasion.Citation100–103 Activated cytosolic PRRs recognize pathogen- and danger-associated molecular patterns (PAMPs and DAMPs) to form the inflammasome.Citation104,Citation105 The activation of PRRs further triggers the downstream signaling pathways, leading to the production of type I interferons and the release of several pro-inflammatory cytokines. PRRs interact with pro-caspase-1 and ASC to produce inflammasomes upon cellular stimulation by signal molecules including bacteria and viruses.Citation106–108 Nucleotide-binding oligomerization domain-like receptors (NLRs including NLRP1, NLRP3 and NLRC4), absent in melanoma 2 (AIM2) and pyrin are the most common PRRs.Citation109,Citation110 The N-terminal pyrin domain (PYD), nucleotide-binding oligomerization domain (NOD), LRR and CARD are components of NLRP1.Citation111 The PYD is essential for interacting with the ASC protein. NOD activates the signal by regulating the generation of ATP. LRR identifies and auto-inhibits ligands. CARD proteins then participate in the recruitment of pro-caspase-1. The anthrax fatal toxin, muramyl dipeptide and Toxoplasma gondii elements may trigger the activation of NLRP1.Citation112 NLRP3 includes N-terminal PYD, NOD and LRR without CRAD. Multiple factors including bacteria, viruses, fungi, uric acid, ROS, ATP and intrinsic damage signals activate the NLRP3 inflammasome axis.Citation113,Citation114 Extracellular ATP triggers the release of IL-1β and activation of Caspase-1 through the stimulation of the P2X7 receptor, which further increases the efflux of K+ ions.Citation115 The NLRC4 protein has an N-terminal caspase activation and recruitment domain (CARD), a central nucleotide-binding domain (NBD) and a C-terminal LRR domain. Flagellin and proteins of the type III endocrine system elicit a response from NLRC4.Citation116 PYD and HIN-200 domains in AIM2 may recognize bacteria- or virus-derived double-stranded nucleotides.Citation117 Pyrin protein comprises a PYD domain, two B-box domains and a C-terminal SPRY/PRY region. Pyrin mainly specifies bacterial toxins or effectors that inactivate host Rho guanosine triphosphatases.Citation118 PRRs recruit pro-caspase-1 directly or indirectly through ASC to form Caspase-1-dependent inflammasome, which self-cleaves to stimulate Caspase-1. Active Caspase-1 splits IL-1β and IL-18 precursors, releasing GSDMD-NT protein for pore creation and inducing inflammation and pyroptosis ().Citation107 The host protects against pathogens by regulating classical inflammasome-mediated pyroptosis in immune cells.
Figure 1 Cellular and molecular mechanisms of pyroptosis-related signaling pathways. Pyroptotic signaling pathways are mainly activated by the stimulation of damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), leading to the activation of a variety of inflammasome signals. The activated inflammasome proteins further activate the Caspase-1 signaling pathway. Then, activated Caspase-1 splits GSDMD protein molecules to produce GSDMD N-fragment and plasma membrane pores, resulting in pyroptosis-dependent cell death. Furthermore, the Caspase-1 pathway triggers the formation and maturation of IL-1β and IL-18 inflammatory factors. In addition, LPS binds to Caspase-4/5/11 precursor, which further activates pyroptosis-regulated cell death through the formation of GSDMD-dependent pores in the cytoplasmic region. Caspase-3/GSDME can also induce pyroptosis-mediated cell death. Furthermore, mitochondrial and death receptors can trigger the Caspase-3 pathway. The activated Caspase-3 splits GSDME to produce GSDME N-fragment, which further creates plasma membrane pores, cell contraction and rupture, resulting in pyroptosis-mediated cell death.
Non-Classical Signaling Pathways
Human Caspase-4/5 (mouse ortholog Caspase-11) is not associated with the downstream sensory complexes in the non-classical pyroptotic signaling pathway. Human Caspase-4/5 (mouse orthologs Caspase-11) can be triggered by attaching directly to intracellular LPS via the N-terminal CARD in the non-classical pyroptotic signaling pathway, which excludes upstream sensory complexes.Citation119 In contrast to dendritic cells, macrophages are sensitive to the oxidized phospholipid 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC, a TLR4 agonist), which inhibits the non-classical inflammasome.Citation120 Caspase-4/5/11 may also split GSDMD into GSDMD-NT, which further polymerizes and creates pores on the cytoplasmic membrane region.Citation90,Citation121 The NLRP3/Caspase-1 pathway is required for the maturation and secretion of IL-1/IL-18, while Caspase-4/5/11 cannot cleave pro-IL-1/pro-IL-18.Citation122,Citation123 In addition, Caspase-4/5/11 splits GSDMD pore-forming protein, effluxing K+ and triggering NLRP3 inflammasome activation and pyroptosis.Citation124,Citation125 Yang et al revealed that pannexin-1 is another essential protein that stimulates Caspase-11-dependent non-classical pyroptotic cell death.Citation124 LPS stimulates Caspase-11 to cleave and alter Pannexin-1, releasing cellular ATP and initiating pyroptosis through the activation of the P2X7 receptor.Citation126 Interestingly, pannexin-1-deficient murine BMDMs may also induce K+ efflux and NLRP3 inflammasome-driven Caspase-1 activation with P2X7 independence, as reported in 2011.Citation127 Furthermore, eradicating pannexin-1 in mice protects against endotoxin shock, indicating that specific potassium (K+) ion channels regulate the non-classical NLRP3 inflammasome pathway.Citation124 The activation of the Caspase-11 pathway stimulates the NLRP3 inflammasome in non-classical pyroptotic signaling.Citation128
Alternative Signaling Pathways
Gasdermin proteins exhibit a high degree of structural conservation within their family. All gasdermins, excluding DFNB59, incorporate C-terminal and N-terminal domains and the N-terminus results in the activation of pyroptosis.Citation84 Researchers have found that chemotherapeutic drugs can significantly induce Caspase-3-mediated cleavage of GSDME with elevated GSDME expression, resulting in the generation of N-GSDME termini in tumor cells.Citation74,Citation129 Apoptosis-mediated caspases Yersinia infection in mouse macrophages have been shown to impede TGF-β-activated kinase 1 (TAK1) and trigger Caspase-8-driven GSDMD cleavage.Citation130,Citation131 Caspases-3/8 were believed to be incapable of generating gasdermin to induce pyroptosis. Further research found that Yersinia infection releases YopJ, which restricts TAK1 and triggers Caspase-8-mediated GSDMD cleavage in mouse macrophages.Citation132 these findings contribute to the progress and expanding knowledge of pyroptosis-regulated cell death. Surprisingly, PD-L1 modulates TNF-induced apoptosis into pyroptosis in breast cancer cells.Citation133 In hypoxia, p-Stat3 enhances PD-L1 nuclear translocation and GSDMC transcription. LPS commonly triggers pyroptosis by activating the Caspase-4/5/11 signaling pathways.Citation134 Researchers have discovered that macrophages activated by LPS undergo Caspase-8-mediated pyroptosis.Citation133 TNF-α activation induces the stimulation of Caspase-8 to lyse GSDMC, generate N-GSDMC and create membrane pores, leading to pyroptosis.Citation133 Nuclear PD-L1, Caspase-8 and GSDMC are required for TNF-induced pyroptosis in macrophages. Furthermore, antibiotics and chemotherapeutics can significantly promote pyroptotic death of breast cancer cells through the activation of the Caspase-8/GSDMC signaling axis.Citation133 The regulation of the NLRP3/Caspase-1-dependent pyroptotic signaling axis involves Caspase-6, which facilitates the interaction between receptor-interacting serine/threonine protein kinase 3 and Z-DNA protein 1.Citation135 However, the specific roles and functions of the other caspases involved in pyroptosis must be further investigated.
It was reported in 2020 that CAR T cells release GzmB, which activates the Caspase-3 signaling pathway in target cells.Citation136 Subsequently, the pyroptotic mechanism was mediated by Caspase-3 and GSDME, resulting in extensive pyroptosis. Recent findings show that GzmB directly cleaves GSDME molecules and triggers pyroptosis, promoting the anti-tumor immune response and restricting tumor formation.Citation137 It was subsequently demonstrated that Cytotoxic T lymphocytes (CTLs) and natural killer cells (NK) eliminated GSDMB-positive cells through the activation of pyroptosis. The fatal effects were triggered by the cleavage of GSDMB at the Lys229/Lys244 site by GzmA, which is derived from lymphocytes. Some tissues, especially the epithelium of the digestive tract and tumors express high levels of GSDMB.Citation138 The work by Zhou et al presented novel findings indicating that gasdermin may undergo hydrolysis by GzmA at a place other than aspartic acid, resulting in the creation of cytoplasmic pores.Citation139 These findings challenge the prevailing notion that Caspases can only initiate pyroptosis.
Role of Pyroptosis-Related Signaling Pathways in the Progression of DPN
DPN is a prevalent and devastating complication of DM.Citation140–142 Extracellular matrix protein accumulation, excessive inflammation, axonal deterioration and unmyelinated fiber degeneration result in sensory conduction delays and long-term nerve injury in patients with DPN.Citation143 Multiple studies have indicated that the activation of pyroptosis and inflammasome are closely implicated in the pathogenesis and development of DPN.Citation144–146 GSDMD is a recently discovered pore-forming protein belonging to the NLRP3 inflammasome family.Citation76,Citation147–150 Mastrocola et al found that the activation of the Caspase-1 pathway requires the cleavage of GSDMDC1 in the inflammasome complex.Citation151 Recently, Sun and colleagues performed Western blot assay to detect the protein expression level of GSDMDC1 in SN tissue under chronic hyperglycemia conditions.Citation144 It was found that the diabetic group exhibited elevated GSDMDC1 protein expression compared to the standard control group. More importantly, the authors indicated that GSDMD-dependent pyroptosis plays a progressive role in DPN. Thus, GSDMD-dependent pyroptosis induces peripheral neuropathy and nerve damage, leading to the development of DPN. GSDMD could be a pathophysiological biomarker for detecting the progression of DPN. However, further studies are extensively required to explore the contributory roles and functions of GSDMD-dependent pyroptosis in the pathogenesis and progression of DPN.
Inflammasomes are complexes comprising a sensor protein, an adaptor protein and an effector protein pro-caspase-1.Citation95,Citation152 Inflammasomes are categorized into two families such as the nucleotide-binding domain, leucine-rich repeat-containing proteins (NLRs) and the absence in melanoma 2 (AIM2)-like receptors (ALRs).Citation153 The activation of pro-caspase-1 results in the creation of cleaved-caspase-1, which cleaves pro-IL-1β into IL-1β, causing abnormal pain.Citation154 NLRP3 is a key molecule within the inflammasome, which can be activated by thioredoxin-interacting protein (TXNIP) and has attracted much attention in the field of pain.Citation155–157 Upregulation of NLRP3 inflammasome in the sciatic nerve and dorsal root ganglion (DRG) is reportedly implicated in the pathogenesis and progression of DPN.Citation158 However, the epigenetic regulatory mechanisms underlying the modulation of NLRP3-dependent pyroptosis remain to be elucidated.
TXNIP is a multifunctional protein commonly called Vitamin D3 enhancing protein 1 or protein 2.Citation159,Citation160 Growing evidence indicates that TXNIP regulates cell proliferation, apoptosis, glucose and lipid metabolism.Citation159–162 In addition, TXNIP regulates oxidative stress in the TRX system.Citation163 Elevated oxidative stress decreases the activity of TRX and promotes the expression and activation of TXNIP. Zhou et al observed the relationship between the NLRP3 and TXNIP.Citation164 ROS triggered TXNIP to dissociate from TRX and attach to the NLRP3 protein. TXNIP facilitates NLRP3 inflammasome activation and IL-1β generation in T2DM progression.Citation165 P2RX7 is a purinergic type 2 receptor, which regulates ligand-gated ion channels in the membrane region. High levels of millimolar ATP further activate the P2RX7 receptor during pathological conditions. Inflammasome activation is triggered by P2RX7 stimulation that facilitates membrane K+ efflux. Activation of P2RX7 by ATP leads to a prolonged elevation in intracellular Ca2+, resulting in the assembly of the inflammasome and subsequent activation of Caspase-1 signaling.Citation166 Excessive P2RX7 and NLRP3 inflammasome activation promote IL-1β release in depression and diabetic complications including DPN.Citation167 Mustofa et al discovered that IL-1β maturation and release are required to stimulate P2RX7 in LPS-primed mouse Schwann cells.Citation168 Caspase-1 splits GSDMD into the N-terminal proteolytic fragment (GSDMD-NT), creating cell membrane pores and inducing cell death by producing pro-inflammatory cytokines including IL-1β and IL-18 that trigger an extensive inflammatory reaction. Caspase-1 pathway activation promotes GSDMD cleavage, GSDMD-NT production and IL-1β release, leading to pyroptosis in high glucose-stimulated Schwann cells.Citation19 Rogers et al discovered that Caspase-3 effectively triggered apoptosis and cleaved GSDME, resulting in the generation of N-terminal fragments (N-GSDME) and the initiation of pyroptosis.Citation169 Wang et al found that Caspase-3 cleavage formed N-GSDME, which interacted with 4, 5-diphosphate phosphatidylinositol (PI[4,5]P2) and perforated liposomes to release their contents.Citation74,Citation170 GSDME cleavage generates N-GSDME, which leads to the creation of membrane pores and pyroptosis. Moreover, GSDME-NT induces the permeabilization of the mitochondrial membrane, resulting in apoptosis through the mitochondrial intrinsic signaling pathways. Recent evidence explores that Caspase-3-dependent apoptosis can turn into pyroptosis-regulated cell death by cleaving the GSDME protein molecule.Citation171 The GSDME-dependent pyroptosis signaling pathway is implicated in a variety of physiological and pathological processes.Citation18,Citation172–178 Li and colleagues have reported that GSDME-dependent pyroptosis contributes to inducing renal cell death and prolonged inflammation in diabetic neuropathy.Citation52 The actual function and molecular mechanism of GSDME-dependent pyroptosis in neuropathic pain during DPN have remained unknown. Therefore, further studies are highly required to explore the pathophysiological roles of apoptosis-driven pyroptosis in DPN. However, SH-3 and WW domains interact with NLRP3 inflammasome.Citation72 The NLRP3 inflammasome comprises three proteins: ASC, NLRP3 and pro-caspase-1. The NLRP3 inflammasome activates pro-Caspase-1, which further facilitates the generation of pro-inflammatory cytokines IL-1β and IL-18 and triggers pyroptosis.Citation179
Inflammation exceeds the capacity of the hosts to combat pathogens effectively and hyperglycemia frequently results in sterile inflammation, which occurs with viral or bacterial pathogens. Recent studies indicate that inflammation significantly affects metabolic and hemodynamic dysfunction associated with diabetes.Citation180,Citation181 The activation of NLRP3 inflammasome further aggravates this perturbation, consistent with mounting evidence that OS induces prolonged inflammation.Citation182–184 Metabolic disorders such as obesity induce the activation of NLRP3 by creating high amounts of glucose, palmitate and ceramide, which activate Caspase-1 and cause the cleavage of IL-1β molecules.Citation185,Citation186 Inflammatory reactions such as IL-1β release from the NLRP3 inflammasome, lead to the advancement of neuropathic pain. Therefore, NLRP3 inflammasome-mediated pyroptosis and inflammation induce the progression of DPN. A growing body of research suggests that decreasing the NLRP3 inflammasome provides a promising therapeutic approach for the treatment and management of DPN and pain.Citation157,Citation187,Citation188
P2X4 regulates endogenous DAMPs and stimulates NLRP3 inflammasome, which promotes the production and secretion of several pro-inflammatory cytokines including IL-1β and IL-18. P2X4 receptors and downstream inflammatory cytokines trigger neuropathic pain in gliocytes. Interestingly, Kang and co-workers revealed that elevated P2X4 and NLRP3 expression further induces the progression of diabetic neuropathic pain in rat models.Citation187 NF-κB pathway modulates cell proliferation and differentiation, morphogenesis, apoptosis and inflammasome activation, thereby triggering inflammation via enhancing the release of several cytokines, chemokines and adhesion molecules.Citation189 Several stimuli trigger NF-κB and plays a regulatory role in the inflammatory response, stress response, pyroptosis and apoptosis. NF-kB/NLRP3 inflammasome activation is a key factor in the development of DM.Citation2 The correlation between NLRP3 inflammasome activation and pyroptosis in DPN, which offered a theoretical foundation for regulating pyroptosis and exerting a protective effect by inhibiting the NF-κB/NLRP3 signaling axis. NLRP3 inflammasomes have been profoundly attributed to the pathogenic mechanisms that induce T2DM and its related complications.Citation49 Ding et al revealed that NLRP3 inflammasome modulates endoplasmic reticulum stress, which regulates glucose tolerance, insulin resistance, inflammation and apoptosis in adipose tissue in DM.Citation190 It seems that the activation of NLRP3 inflammasome leads to the maturation and the release of pro-inflammatory mediator IL-1β in the sciatic nerve of diabetic rats. Furthermore, blood biochemistry demonstrates an increase in K+ and a decrease in Ca2+ in the plasma. The change in K+ concentration is considered an essential factor in NLRP3 inflammasome activation, because K+ efflux alone can potentiate IL-1β maturation.Citation191,Citation192 The trends indicate a reduction in the Tnfr and CX3CR1 expression, although the effects were not significant in the sciatic nerve, probably due to the interindividual variability. Therefore, NLRP3-dependent pyroptosis and inflammation are a key player in inducing the progression of diabetic complications including DPN.
NF-κB is a key regulator of HDAC2, which is implicated in triggering neuropathic pain.Citation193 Furthermore, HDAC2 significantly affects dorsal horn development after peripheral nerve injury.Citation194 Previous research has revealed that miR-183 mediates CCI-induced neuropathic pain.Citation195 Further microarray study indicated that miR-183 activates the TXNIP/NLRP3 inflammasome axis in peripheral nerve injury, further triggering neuropathic pain. The upregulation of the NLRP3 inflammasome has been reported to further aggravate neuropathic pain.Citation188 Mechanistically, Miao and colleagues investigated the contribution of NF-κB p65-induced HDAC2 inflammatory response in neuropathic pain through the miR-183/TXNIP/NLRP3 signaling axis, which may aid in comprehending the pathophysiology of neuropathic pain in DM.Citation196 Zhou et al found that TXNIP/NLRP3 inflammasome activation may contribute to insulin resistance and hyperglycemic progression and regulate neurological pain through miR-23a in spinal glial cells.Citation197 Hao and colleagues proposed that the elevated TXNIP/NLRP3 complex may further promote the up-regulation of IL-1β levels and accelerate inflammation.Citation198 In addition, Chen and colleagues have revealed that the TET2-TXNIP-NLRP3 inflammasome axis contributes to the progression of DNP.Citation199 The authors also showed that the secretion of Caspase-1-mediated IL-1β pro-inflammatory cytokine triggers neuroinflammation during diabetic conditions in mouse models.Citation199 Therefore, TXNIP may produce a promising therapeutic target for the prevention and management of peripheral neuropathy in patients with diabetes. Zhang et al aimed to explore the presence of brain microglia activation in neuropathic pain and to analyze the effectiveness and underlying molecular mechanisms of glucagon-like peptide-1 receptor agonist (GLP-RA) on DPN through regulating microglia.Citation197 The authors revealed that downregulation of GLP-1RA could significantly induce inflammation by the activation of NLRP3 inflammasome in DPN, suggesting that NLRP3-dependent pyroptosis is a key contributor to the progression of DPN. Interestingly, a pioneering research reports that the GLP-1RA agonist mitigates the progression of diabetic neuropathic pain by obstructing the activation of NLRP3 inflammasome in brain microglia.Citation197
The inflammatory response is one of the essential pathologic features that link to the onset and progression of DPN.Citation200,Citation201 Inflammasome-driven inflammation contributes to a wide range of inflammatory reactions and targeting the NLRP3 inflammasome pathway is vital for the treatment of inflammation-associated diseases such as pulmonary disease, asthma, coronavirus disease 2019 and DPN.Citation73,Citation202–204 NLRP3 inflammasome is activated after stimulation, followed by a series of immune responses such as NLRP3 inflammasome proteins (molecule NLRP3, the adaptor molecule ASC and Caspase-1) production, Caspase 1-dependent release of the pro-inflammatory cytokines and pyroptotic cell death.Citation205 Additionally, Schwann cell loss or apoptosis commonly occurred in both clinic DPN patients and experimental animals and inhibition of Schwann cell dysfunction ameliorated the progression of DPN. Therefore, the suppression of inflammasome activation and Schwann cell apoptosis might be a potential therapeutic approach for the treatment and management of DPN.
CXC motif chemokines have been implicated in neuronal injury and inflammatory reactions.Citation206 Zhang et al performed a bioinformatics analysis utilizing data from the Gene Expression Omnibus (GEO) database to identify chemokine ligands (CXCLs) motifs associated with DPN.Citation207 It was found that the expression level of CXCL2 was remarkably elevated in STZ-induced DPN rat sciatic nerve and HG-stimulated RSC96 cells.Citation207 CXCL2 knockdown alleviates hyperglycemia-induced pyroptosis and inflammation by decreasing Caspase-3 activity in vitro and in vivo. In HG-treated RSC96 cells, CXCL2 knockdown increased cell viability and reduced apoptosis due to cleaved Caspase 3–9 expression. Furthermore, CXCL2 knockdown restricted the activation of NLRP3 inflammasome and mitigated the release of pro-inflammatory cytokines such as IL-1β and IL-18. Under the HG condition, NLRP3 inflammasome activator nigericin enhances inflammasome activation by abolishing the inhibitory effects of CXCL2 knockdown. Therefore, NLRP3 inflammasome activation and prolonged inflammation further induce the pathogenesis and progression of DPN.
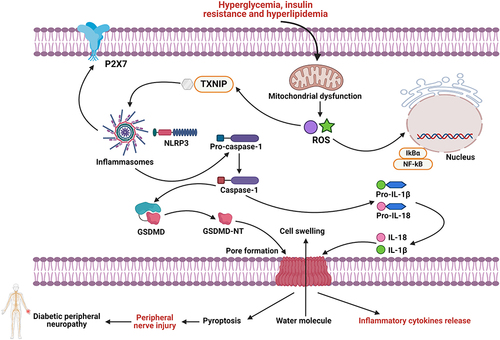
Glycogen synthesis kinase-3β (GSK3β) is a serine/threonine kinase that is consistently active and primarily regulated through phosphorylation at the serine residue.Citation208–210 The activity of GSK3β is elevated in the spinal cord of an animal model of neuropathic pain.Citation211 Accumulating studies have revealed that inhibiting the activation of GSK3β alleviates the generation of pro-inflammatory cytokines and triggers the production of anti-inflammatory cytokines in cortical microglia stimulated with LPS in vitro.Citation209,Citation210,Citation212 GSK3β can play an important role in activating NLRP3 inflammasome-mediated pyroptosis. Therefore, GSK3β/NLRP3-dependent pyroptosis further induces the development of diabetic neuropathic pain (DNP). NLRP3 inflammasome may recognize ROS generated by normal or malfunctioning mitochondria in the same cell. It has been suggested that elevated levels of ROS are detected by a complex containing TRX and TXNIP, which results in the complex dissociation.Citation213 Accumulating evidence has shown that ROS generation stimulates tissue inflammation and induces NLRP3 inflammasome overactivation, leading to the progression of a variety of diseases.Citation214,Citation215 Recently, Wang et al showed that ROS overproduction further contributes to the progression of DNP by activating the TXNIP-NLRP3-NR2B signaling axis.Citation216 In summary, these findings suggest that the activation of pyroptosis-regulated signaling pathways plays a contributory role in the pathogenesis and progression of DPN (). Therefore, a more comprehensive investigation of the role and underlying molecular mechanisms of the pyroptosis-driven signaling pathway in the progression and pathogenesis of DPN is highly warranted.
Figure 2 Pathophysiological roles of pyroptosis-dependent cell death in the course of the pathogenesis of DPN. Hyperglycemia, insulin resistance and hyperlipidemia stimulate NLRP3/ASC inflammasome signals, further activating pro-caspase-1 into Caspase-1 form. Then, activated Caspase-1 induces the generation and maturation of inflammatory factors including IL-1β and IL-18, resulting in low-grade inflammation and peripheral nerve injury, eventually advancing DPN.
ROS is known to play a prominent role in the pathogenesis of a variety of diseases including DM.Citation217 Hyperglycemia, metabolic disorders, increased oxidative stress and mitochondrial dysfunction may exacerbate peripheral nerve damage in people with diabetes. NLRP3 is an inflammasome activated by ROS, which acts as a second messenger in the activation of the inflammasome and is believed to stimulate pyroptosis by activating the NLR/Caspase-1 signaling complex.Citation192,Citation218 Under normal conditions, the anti-oxidant enzymes can eliminate ROS during cell metabolism to maintain the balance of ROS generation and elimination.Citation219 The accumulation of ROS results in oxidative stress and cellular disorders such as upregulation of lipid peroxidation and cell apoptosis when endogenous anti-oxidant defense cannot eliminate it in time.Citation219 The overproduction of ROS can further activate the expression of NF-κB, which is a crucial transcription factor in inflammation, stress response and cell growth and survival.Citation220 The hyperglycemia, hyperinsulinemia and insulin resistance of diabetes enhanced oxidative stress, leading to excessive cytokine generation in DPN.Citation221 It has been found that ROS-dependent NLRP3 inflammasome activation induces downstream pro-inflammatory responses, aggravating chronic inflammatory nerve damage.Citation222 Emerging studies have shown that inflammatory factors infiltrate peripheral nerves and chronic inflammatory reactions impair the normal function of peripheral nerves.Citation223,Citation224 Under inflammatory conditions, the cascade release of pro-inflammatory factors such as IL-6, IL-1 and TNF-α activates glial cells, which further express receptors for pro-inflammatory mediators and participate in inflammatory immune responses. Second, pro-inflammatory factors produce a cascade-like reaction, resulting in inflammation. Finally, the release of substance P and excitatory amino acids continues to depolarize dorsal horn neurons, leading to pain sensitivity and persistent pain.Citation225–227 Therefore, ROS-dependent inflammasome activation and inflammatory reactions may further contribute to the pathogenesis and progression of DPN.
Inhibition of Pyroptosis-Dependent Signaling Pathways for the Therapeutic Regulation of DPN
Jinmaitong (JMT) is a traditional Chinese compound with a long history of use and has shown significant clinical effectiveness in the prevention and treatment of DPN.Citation228 Previous studies have indicated that JMT lowers blood glucose and lipid metabolism, reduces nerve conduction velocity in DPN patients, alleviates numbness, cold and pain and enhances nerve transmission.Citation229 Further studies reveal that JMT inhibits oxidative stress and alleviates DNA damage to sciatic nerves (SNs) in STZ-induced diabetic rats.Citation230 Prior research has demonstrated that JMT targets peripheral neuronal apoptotic genes such as Bcl-2 and Caspase-3.Citation231 Xie et al reported that JMT exerts promising anti-oxidative effects to protect against SNs injury in STZ-induced diabetes.Citation232 Recent evidence suggests that overactivation of NLRP3 inflammasome plays an essential role in the pathogenesis of DM and its complications.Citation49,Citation123,Citation190,Citation233,Citation234 The NLRP3 inflammasome includes the apoptosis-associated speck-like adaptor protein (ASC), NLRP3 and pro-caspase-1. Upon activation, NLRP3 becomes ligated to ASC and then binds to pro-caspase-1. This binding promotes cleavage and transformation of pro-caspase-1 to Caspase-1, which further facilitates the generation and maturation of pro-inflammatory factors IL-1β and IL-18 and activates pyroptosis.Citation73,Citation235 TXNIP has been reported to be upstream of NLPP3 and the complexes of these two proteins are necessary for inflammasome activation.Citation164 Intriguingly, Sun et al demonstrated that JMT alleviates the pathogenesis and progression of DPN by inhibiting the activation of the TXNIP/NLRP3 inflammasome axis and mitigating pyroptosis-mediated inflammatory reactions in STZ-induced diabetic rats.Citation144 JMT could suppress the expression level of TXNIP and NLRP3 inflammasome proteins, as demonstrated by immunostaining and Western blot analysis of SNs in diabetic rats. In addition, the protein expression Cleaved-Caspase-1 was higher and the Caspase-1 precursor level was downregulated in the diabetic rats compared to the control rats, indicating the activation of the Caspase-1-dependent pyroptosis is a key contributor to the progression of DPN. It was also demonstrated that JMT decreased the protein expression level of GSDMDC1 and suppressed the activation of the Caspase-1 signaling pathway in the STZ-induced diabetic rat model. Therefore, JMT could be a new ant-pyroptotic drug candidate for alleviating DPN (). This research supports the therapeutic application of JMT as a traditional Chinese medicine in DPN treatment. However, further studies are highly required to investigate the primary active components of JMT targeting other pyroptosis-related signaling pathways in future research endeavors.
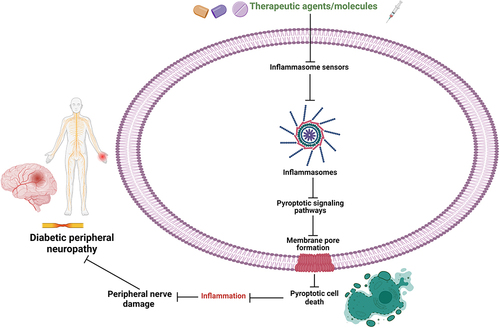
Figure 3 Schematic illustration of therapeutic implications by several experimental compounds/drugs targeting inflammasome and pyroptosis-related signaling pathways for novel therapeutic strategies in the treatment and management of DPN.
Loganin (LGN) is an iridoid glycoside obtained from the fruit of Cornus officinalis. A number of investigations have confirmed the promising anti-oxidant, anti-inflammatory and hypoglycemic actions of LGN, a compound traditionally applied in the treatment of DN.Citation236–238 Past research indicates that LGN can remarkably alleviate diabetes-mediated anxiety and depression by lowering blood glucose and mitigating pro-inflammatory cytokines.Citation239 Further investigation revealed that LGN stimulates the release of neurotrophic factors, which reduce mesencephalic neuronal death, ameliorate neurite damage and inhibit the activation of oxidative stress.Citation240 Wang and co-workers have shown that LGN exerts remarkable neuroprotective actions by alleviating neuronal pyroptosis in rats with cerebral hemorrhage.Citation241 Recently, Kong et al reported that LGN ameliorates diabetic renal injury by obstructing the activation of NLRP3 inflammasome-dependent pyroptosis.Citation242 Li and co-workers also indicated that LGN treatment attenuated OGD/R-induced cardiomyocyte pyroptosis by alleviating cell membrane damage and inhibiting the pyroptosis-related protein expression level of Cleaved Caspase-1, IL-1β and IL-18.Citation243 Furthermore, LG intervention blocked GLP-1R/NLRP3 pathway activation in OGD/R-stimulated H9C2 cardiomyocytes by promoting GLP-1R expression and obstructing NLRP3 inflammasome stimulation.Citation243 Intriguingly, LGN treatment mitigates ROS production, inhibits NF-κB–P2RX7–TNXIP protein expression and alleviates NLRP3 inflammasome-mediated RSC96 cell damage. Previous research has indicated that LGN mitigates the generation of inflammatory mediators including IL-1β by restricting the activation of NF-κB signaling pathway in the spinal cord tissue of PDN rat models.Citation244 In terms of neuroprotection, LGN attenuates mesencephalic neuronal apoptosis, neurite nerve damage and oxidative stress through the enhancement of neurotrophic factors.Citation240 Furthermore, LGN has been demonstrated to mitigate neuropathic pain by ameliorating Schwann cell demyelination in rats with chronic contraction injury.Citation245 High glucose levels adversely affect apoptosis, metabolism, proliferation and migration of Schwann cells.Citation246 Accumulating evidence suggests that the overproduction of ROS caused by high glucose induces the activation of oxidative stress and inflammation, a recognized mechanism in the molecular pathogenesis of DPN.Citation168,Citation247 The study by Cheng and colleagues provides the first evidence that LGN treatment inhibits the activation of NLRP3 inflammasomes and subsequent pyroptosis by inhibiting the formation of ROS in high-glucose-treated RSC96 Schwann cells.Citation19 Intriguingly, the authors revealed that LG treatment attenuates pyroptosis-driven inflammatory reactions and downregulates NF-κB-P2RX7-TNXIP protein expression, protecting RSC96 cells against NLRP3 inflammasome overactivation.Citation19 More importantly, the authors showed that LGN treatment could significantly suppress the mRNA and protein expression level of NLRP3, ASC, Caspase-1 and GSDMD and pro-inflammatory factors IL-1β and IL-18 in high-glucose-stimulated Schwann RSC96 cells.Citation19 It was proved that LGN can alleviate hyperglycemia-induced peripheral nerve injury by suppressing pyroptosis-dependent cell death (). Therefore, LGN could be a novel pharmacological drug candidate for suppressing NLRP3 inflammasome-dependent pyroptosis and inflammation in the treatment of DPN.
Table 1 Compounds/Agents Targeting Pyroptosis-Related Signaling Pathways for the Therapeutic Regulation of DPN
Curcumin (CUR), a bioactive compound found in turmeric, exhibits wide-ranging therapeutic actions in the treatment of a variety of diseases.Citation256–260 Numerous pieces of evidence indicate that CUR possesses promising anti-inflammatory, anti-oxidant and neuroprotective properties that attenuate the pathogenesis and progression of diabetic complications including DPN.Citation261–264 Recently, Zhang and co-workers explored that CUR alleviates the progression of DPN by enhancing the expression of NGF in rat models.Citation265 Dwivedi et al evaluated the pharmacological effects of nCUR combined with long-acting subcutaneous insulin (INS) in STZ-induced rats.Citation248 Current studies have explored that apoptosis-regulated signaling pathways further lead to the activation of pyroptosis.Citation71,Citation171,Citation266 Pioneering research performed by Elsayed and co-workers showed that CUR alleviated apoptosis and inhibited glial activation with modulation of Nrf2/HO-1 and NF-kB signaling in STZ-induced diabetic spinal cord central neuropathy. Therefore, CUR has possible therapeutic targets attenuating Caspase-3-dependent pyroptosis to alleviate and treat DPN. More specifically, the authors revealed that nCUR alone or in combination with insulin alleviates neuropathic pain by obstructing the activation of NLRP3 inflammasome and mitigating the release of inflammatory factors, indicating that nCUR exerts promising pharmacological targets suppressing inflammasome-mediated cell death for ameliorating the progression of DPN. Therefore, CUR could be a potent anti-pyroptotic drug candidate in alleviating DPN. However, the pharmacological effects and underpinning molecular mechanisms of CUR targeting Caspase-1/GSDMD-dependent pyroptosis and inflammation in ameliorating DPN remain elusive. Further studies must be executed to explore the pharmacological target of CUR in other pyroptosis-related signaling pathways in the pharmacological treatment of DPN.
Vincamine (VIN) is a monoterpenoid indole alkaloid obtained from Catharanthus roseus, commonly called Vinca rosea. Growing evidence suggests that VIN exerts multiple biological functions including anti-coagulant, memory-enhancing, nootropic, hypoglycemic, hypolipidemic, vasodilatory and anti-oxidant properties.Citation267 Previous studies have shown that VIN possesses practical antidiabetic activities by elevating serum insulin and C-peptide levels in streptozotocin (STZ)-induced diabetic rats.Citation267–269 Du et al report that GPR40 agonist VIN enhances glucose homeostasis in T2 diabetic mice.Citation270 The author and co-workers found that VIN could protect the function of INS-832/13 cells by regulating G-protein-coupled receptor 40 (GPR40)/cAMP/Ca2+/IRS2/PI3K/Akt signaling pathways, while increasing glucose-stimulated insulin secretion (GSIS) by modulating GPR40/cAMP/Ca2+/CaMKII signaling pathway, which reveals a novel mechanism underlying GPR40-mediated cell protection and GSIS in INS-832/13 cells.Citation270 Xu and colleagues found that VIN intervention impeded sciatic nerve myelin sheath injury and improved foot skin IENF density in DPN mice.Citation249 The authors showed that VIN administration significantly ameliorated neurological dysfunctions in DPN mice. The authors showed that VIN administration improved the blood flow velocities and perfusion areas of foot pads and sciatic nerve tissues in DPN mice.Citation249 Moreover, VIN administration suppressed NLRP3 inflammasome activation through either β-Arrestin2 or β-Arrestin2/IκBα/NF-κB signaling, improved mitochondrial dysfunction through CaMKKβ/AMPK/SIRT1/PGC-1α signaling and alleviated oxidative stress through Nrf2 signaling pathway in the sciatic nerve tissues of DPN mice and LPS/ATP-treated RSC96 cells.Citation249 The beneficial effects of VIN were abolished by GPR40-specific knockdown in dorsal root ganglia and sciatic nerve tissues. This pioneering review supports that VIN could treat DPN by pharmacologically activating GPR40. A pharmacological treatment for DPN may be achieved by targeting GPR40 activation through the suppression of the NLRP3 inflammasome. Therefore, VIN may be an effective pharmacological drug candidate in inhibiting the activation of NLRP3 inflammasome-dependent pyroptosis for the alleviation of DPN. However, further research is highly warranted to explore the anti-pyroptotic effects of VIN in the treatment and management of DPN.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that regulates a wide range of biological functions through a specific receptor named the GLP-1 receptor (GLP-1R).Citation271 GLP-1RAs such as exendin-4 and liraglutide are common drugs used for the treatment of T2D.Citation272,Citation273 GLP-1RA administration has been demonstrated to alleviate diverse CNS disorders including Alzheimer’s disease, Parkinson’s disease and cerebral ischemia by attenuating microglial activation.Citation274–276 New research by Wang and colleagues found that activating microglial GLP-1R in the spinal cord alleviates inflammatory reactions, neuropathic pain and bone cancer.Citation277 Pioneering research also showed that intracerebroventricular GLP-1RA administration suppressed Iba-1 expression and microglia activation in the brain and thalamus of DNP rats.Citation250 A previous study by Mohiuddin et al showed that GLP-1 signaling protects peripheral nervous system neurons from oxidative insult in DPN. The therapeutic potential of GLP-1RAs on DPN was investigated in depth using the cellular oxidative insult model applied to the dorsal root ganglion (DRG) neuronal cell line. Interestingly, Zhang et al. GLP-1RA administration suppressed the classical pyroptosis-regulated mRNA and protein expression levels of IL-1β, NLRP3 and Cleaved Caspase-1 in LPS-stimulated BV2 microglia.Citation250 The authors showed that microglia were activated in the cortex and thalamus of diabetic rats.Citation250 Intracerebral administration of GLP-1RA or minocycline alleviated heat and mechanical allodynia in rats.Citation250 Moreover, the activation of brain microglia was attenuated in DNP rats by intracerebroventricular administration of GLP-1RA. The expression of NLRP3 in brain microglia, found by RNA sequencing, was reduced in DNP rats by administration of GLP-1RA. These findings suggest that GLP-1RAs have neuroprotective potential, which is achieved by their direct actions on DRG neurons by inhibiting the activation of inflammasome signaling pathways. Therefore, GLP-1RA could be a potential therapeutic avenue for attenuating diabetic neuropathic pain via suppressing the activation of NLRP3 inflammasome.
The fruit of the South American acai palm (Euterpe oleracea Mart), which is abundant in anti-oxidants, has recently gained interest as a functional diet. Açai berry has been applied in several studies as a potential anti-hyperglycemic and anti-inflammatory agent that prevents various injuries to physiological systems.Citation278–282 Multiple evidence indicates that açai berries consist of a variety of polyphenolic compounds including pelargonidin, cyanidin, malvidin, delphinidin, peonidin, dihydrokaempferol, quercetin, luteolin and chrysoerial.Citation283,Citation284 Many studies reveal that carotenoids such as carotene, lycopene, astaxanthin, lutein and zeaxanthin are also enriched in açai fruit pulp.Citation283,Citation285,Citation286 Açaí extract also presented beneficial effects against age-related oxidative stress in a d-Gal-induced aging model in human erythrocytes. Since aging of these cells is related to oxidative stress and E. oleracea presents anti-oxidant properties, this functional food could improve the functions of erythrocytes. The homeostasis of the organism as a whole counteracts age-related changes.Citation287 The bioactive components of açai berry extract possess multiple beneficial effects through modulation of OS, inflammation, autophagy, Nrf2 activity in the hippocampus and frontal brain and NLRP3 inflammasome activation.Citation288,Citation289 Cadona et al report that açaí extract exerts remarkable anti-neuroinflammatory actions by modulating the ROS/NLRP3/Caspase-1 inflammasome signaling axis.Citation290 Pioneering research by Impellizzeri and co-workers suggests that administration of 500 mg/kg dose of açai berry extract ameliorates cognitive impairment by restricting the activation of NLRP3/ASC/CASP signaling axis in both sciatic nerve and spinal cord tissues of STZ-induced DPN mouse models.Citation251 The authors clearly showed that açaí berry reduces mast cell degranulation and histological damage in diabetic neuropathy, improves physiological defense against ROS, modulates the NLRP3/ASC/CASP signaling axis, mitigates inflammation and inhibits oxidative stress. Thus, suppressing the NLRP3/ASC/CASP signaling pathway could be a potential therapeutic target in the treatment of DPN. Therefore, açai berry could be a novel therapeutic agent suppressing pyroptosis-dependent cell death for the treatment and management of DPN.
Swertiamarin (SWN) is an iridoid compound mainly derived from Enicostemma littorale Blume, a plant with decades of traditional application in the treatment of diabetes, swelling, rheumatism and abdominal ulcers.Citation291 A growing body of evidence indicates that SWN exerts promising anti-oxidant, hepatoprotective, anti-hyperlipidaemic, anti-nociceptive, anti-edematogenic and free radical scavenging properties.Citation292 SWN has recently been shown to decrease hyperinsulinemia and hyperglycemia in rat models of diabetes and obesity.Citation293,Citation294 Previous studies have indicated that SWN can significantly improve the insulin resistance effect of patients with T2DM by activating the AMPK pathway, thereby restoring and enhancing the insulin sensitivity of hepatocytes.Citation295 Furthermore, SWN significantly mitigates NF-κB-driven inflammation in arthritis animal models.Citation295 Saravanan et al demonstrated that SWN suppresses inflammation in adjuvant-induced arthritis by restricting NF-κB/IκB and JAK2/STAT3 transcription factors.Citation296 Recently, Wang and co-workers have revealed that SWN effectively attenuated DPN in rats by restricting the NOXS/ROS/NLRP3 signaling pathway.Citation252 The expressions of NOXS, ROS, NLRP3 and inflammatory factors in DPN rats were detected using ELISA and the protein expressions of NOXS, ROS and NLRP3 were also detected with Western blotting.Citation252 It was shown that SWN downregulated the protein expressions of NOXS, ROS and NLRP3, maintained the balance of inflammatory factors, increased the pain threshold and nourished nerves to treat DPN in the rat models.Citation252 Therefore, SWN could be a promising therapeutic drug candidate for attenuating the pathogenesis and progression of DPN by suppressing the activation of NLRP3-dependent pyroptosis. However, further investigations are highly required to clarify the pharmacological targets of other pyroptosis-driven signaling pathways that alleviate DPN. Additional studies are also needed to explore the advantages and disadvantages of the two based on equivalent efficacy and the underlying molecular mechanisms to provide active and effective prevention and treatment and new low-toxic and harmless drug targets for DPN patients.
The P2X4 receptor and pro-inflammatory cytokines in gliocyte-mediated signaling pathways contribute to the progression of neuropathic pain.Citation297 DAMPs downstream of P2X4 activate the NLRP3 inflammasome, which further matures and secretes cytokines, disrupting cells and tissues.Citation298,Citation299 P2X4 could modulate the production of proinflammatory cytokines such as IL-1β by activating NLRP3 inflammasome via endogenous DAMPs. Dexmedetomidine (DEX) is a recently developed α2-adrenergic receptor agonist that selectively ameliorates sympathetic nervous system functions and delivers significant anxiolytic effects.Citation300 Past studies have indicated that DEX alleviates pain and slightly reduces breathing rate.Citation301,Citation302 Recently, pioneering research by Lin and co-workers has shown that administration of DEX mitigates DPN by suppressing the activation of oxidative stress and dysfunction of the mitochondria via regulating the microRNA-34a/SIRT2/S1PR1 axis.Citation303 DEX has been revealed to alleviate spinal nerve damage by modulating the activation of the P2X4 receptor.Citation304 In addition, Zhang and co-workers have demonstrated that the administration of DEX can effectively DPN-related behaviors and nerve cell damage by suppressing Caspase-3/9-dependent cell death and ROS production in diabetic rats.Citation253 Previous studies have demonstrated that DEX exhibits potential anti-inflammatory activity, suggesting its potential to alleviate diabetes-induced inflammation.Citation305,Citation306 Inflammatory cytokines play an important role in neuronal damage, especially in painful neuropathy. The elevated levels of P2X4 and NLRP3 in STZ-treated rats suggested that cytokines are involved in the pathogenesis and progression of neuropathy.Citation307 The exact mechanism of high glucose-induced P2X4 expression is not fully understood. It has been reported that hyperglycemic stimulation can activate the JAK/STAT signaling pathway by promoting the activity of transcription factor STAT1, thereby increasing the expression of P2X4.Citation308 Numerous evidence indicates that overactivation of NLRP3 inflammasome may regulate the inflammation process of DM and related complications.Citation49,Citation309 In addition, P2X4 receptors could mediate the secretion of pro-inflammatory cytokine IL-1β.Citation310 Kang et al investigated the impacts of P2X4/NLRP3 signaling on the development of DNP in rats.Citation187 The authors showed that the expression of P2X4 and NLRP3 in DNP rats was markedly reduced after treatment with DEX.Citation187 This finding is consistent with the involvement of the P2X4 receptor in the development of DNP. The NLRP3 and IL-1β upregulation findings suggest they intervene in inflammatory factor activation. The authors revealed that DEX administration suppressed the expression levels of P2X4, NLRP3 and IL-1β, suggesting that DEX modulates inflammasome overactivation and mitigates inflammation by blocking the activation of P2X4 receptor.Citation187 Therefore, DEX is more likely to target P2X4/NLRP3-dependent pyroptosis in alleviating DNP. However, the pharmacological target and underlying molecular mechanisms of DEX suppressing the Caspase-1/4/5/11 inflammasome-dependent pyroptosis are unknown. Therefore, more studies are highly required to analyze the pharmacological targets of DEX in suppressing pyroptosis-dependent cell death for promising therapeutic avenues in the treatment of DPN.
Salidroside (SLD), a phenylpropanoid glycoside molecule, is the active component in the root of Rhodiola rosea, which has been employed to alleviate high-altitude sickness for decades.Citation311,Citation312 Previous studies have shown that SLD exerts significant anti-oxidant, anti-inflammation and stress reducer and anti-cancer, cardioprotection and immune enhancer properties.Citation313,Citation314 Accumulating studies have demonstrated that SLD significantly improves glucose homeostasis in diabetic animals by alleviating inflammation and increasing cellular metabolic flux.Citation314,Citation315 Current research indicates that SLD exerts remarkable anti-pyroptotic effects in the treatment of multiple disorders including Parkinson’s diseases, Alzheimer’s diseases, etc.Citation46,Citation316–320 Prior investigation suggests that SLD alleviates neural injury in STZ-induced T2D in rats.Citation321 Moreover, Hu and co-workers have shown that SLD alleviates chronic constriction injury-induced neuropathic pain and inhibits the activation of TXNIP/NLRP3 signaling pathway.Citation254 Recently, Liu et al reported that SLD mitigates ulcerative colitis by suppressing macrophage pyroptosis and restoring Th17/Treg balance.Citation322 Chai et al also demonstrated that SLD ameliorates depression by suppressing NLRP3-mediated pyroptosis via inhibiting the activation of P2X7/NF-κB/NLRP3 signaling axis.Citation318 In addition, Wang and colleagues have revealed that SLD mitigates neuroinflammation and enhances functional recovery following spinal cord injury by regulating microglia polarization.Citation323 Intriguingly, Zheng and co-workers also demonstrated that SLD administration remarkably improved hyperglycemia, reduced insulin resistance and mitigated peripheral nerve injury and neuropathic pain in diabetic rats.Citation324 Mechanistically, it was shown that SLD mediated AMPK pathway activation and restricted NLRP3 inflammasome activation in DRGs.Citation324
Numerous studies have revealed the relevance of several ATP receptor subtypes to inflammation and neuropathic pain.Citation325–329 In particular, P2X7 receptors (P2X7Rs) are important cell surface regulators of several key inflammatory molecules including TNF-α, IL- 1β, IL-18 and IL-6. Moreover, P2X7Rs are upregulated in inflammation and neuropathic pain states.Citation325 Therefore, antagonists or modulators of P2X7Rs may have therapeutic potential as novel anti-inflammatory and anti-nociceptive agents. P2X7Rs could be among the pivotal targets of SLD for its anti-nociceptive and anti-inflammatory actions. Ni and colleagues have found that the effects of SLD on the altered pain behaviors, pro-inflammatory cytokines and the levels of P2X7 receptors in DM rats had a similar dose-response relationship, showing significant effects.Citation255 The notion that inhibition of P2X7 Rs may play an important role in conveying antinociceptive and anti-inflammatory effects of SLD is strongly bolstered by previous studies, demonstrating the crucial roles of P2X7 receptors in triggering inflammation and neuropathic pain. Knocking out the P2X7 gene in mice results in the absence of inflammatory and neuropathic behavioral hypersensitivities.Citation326 P2X7 Rs are up-regulated in injured nerves in patients with neuropathic pain and the gain-of-function in the P2X7 receptors is associated with pain hypersensitivity in osteoarthritis, post-mastectomy pain and diabetic neuropathic patients.Citation329 Past studies confirmed that selective P2X7R antagonists have therapeutic potential for the treatment of both inflammation and peripheral pain.Citation330 Therefore, SLD attenuated nociception in diabetes probably through inhibiting both expression and activation of P2X7Rs and subsequently reducing the release of pro-inflammatory cytokines. These findings suggest that SLD could be an effcetivepharmacological drug suppressing pyroptosis for a novel therapeutic avenue in the treatment and management of DPN.
Conclusions, Current Challenges and Future Prospectives
Understanding the regulatory mechanisms of inflammasome activation is essential to regulate the prolonged inflammatory response following nerve injury, enhance peripheral nerve regeneration and alleviate inflammatory reactions in the patients with DM. Numerous research investigations have revealed that NLRP3 inflammasome activation results in peripheral nerve injury-induced pain in peripheral nerve injury models. The comprehension of the function and regulatory mechanisms of NLRP3 inflammasome in peripheral nerve regeneration during DM is currently constrained. The underlying molecular mechanisms of Schwann cell-macrophage coordination in the inflammatory response, nerve development and regeneration are also undefined. Further investigation must be conducted to analyze the contributory role of pyroptosis in Schwann cells and macrophages. Inhibiting excessive activation of the NLRP3 inflammasome and pyroptosis can enhance peripheral nerve injury repair based on its roles and regulatory mechanisms in the central and peripheral nervous systems. Several existing inhibitors have unanticipated adverse effects due to non-specificity. Therefore, further research is necessary to improve the selectivity of pyroptotic inhibitors. Researchers have recently concentrated on the effective experimental compounds/drugs targeting inflammasomes and pyroptotic signaling pathways. Natural bioactive compounds, traditional medicines and other natural products may present new therapeutic perspectives and guidance for the treatment and management of DPN. The therapeutic effects of these compounds/drugs are currently confined to the pre-clinical and clinical research stages. More pharmacological agents, particularly non-coding RNAs, require immediate investigation to evaluate and confirm their effectiveness and pharmacological actions for the treatment and management of DPN. Therefore, further research is necessary for a deeper comprehension of the mechanisms underpinning pyroptosis, which will contribute to our understanding of the role of pyroptosis-mediated cell death in the pathogenesis of DPN and facilitate the development of effective therapeutic agents that target pyroptotic signaling pathways.
Disclosure
The authors declare no competing interests in this work.
Additional information
Funding
References
- Galaviz KI, Narayan KMV, Lobelo F, Weber MB. Lifestyle and the Prevention of Type 2 Diabetes: a Status Report. Am J Lifestyle Med. 2018;12(1):4–20. doi:10.1177/1559827615619159
- De Silva AP, De Silva SHP, Haniffa R, et al. Inequalities in the prevalence of diabetes mellitus and its risk factors in Sri Lanka: a lower middle income country. Int j Equity Health. 2018;17(1):45. doi:10.1186/s12939-018-0759-3
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
- Aktas G. Association between the Prognostic Nutritional Index and Chronic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J Clin Med. 2023;12(18):5952. doi:10.3390/jcm12185952
- Zhang Y, Liu H. Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients. BMC Endocr Disord. 2024;24(1):42. doi:10.1186/s12902-024-01564-x
- Aktas G, Yilmaz S. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgraduate Medicine. 2023;135(5):519–523. doi:10.1080/00325481.2023.2214058
- Aktas G. Serum C-reactive protein to albumin ratio as a reliable marker of diabetic neuropathy in type 2 diabetes mellitus. Biomolecules & Biomedicine. 2024. doi:10.17305/bb.2024.10426
- Gandhi M, Fargo E, Prasad-Reddy L, Mahoney KM, Isaacs D. Diabetes: how to manage diabetic peripheral neuropathy. Drugs in Context. 2022;11. doi:10.7573/dic.2021-10-2
- Bönhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging Biomarkers, Tools, and Treatments for Diabetic Polyneuropathy. Endocrine Reviews. 2019;40(1):153–192. doi:10.1210/er.2018-00107
- Baum P, Toyka KV, Blüher M, Kosacka J. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)-New Aspects. J Mol Sci. 2021;22(19):10835.
- Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi:10.3390/biom5010194
- Lu S, Li Y, Qian Z, et al. Role of the inflammasome in insulin resistance and type 2 diabetes mellitus. Front Immunol. 2023;14:1052756. doi:10.3389/fimmu.2023.1052756
- Wei H, Cui D. Pyroptosis and Insulin Resistance in Metabolic Organs. Int J Mol Sci. 2022;23(19):11638. doi:10.3390/ijms231911638
- Cao Z, Huang D, Tang C, et al. Pyroptosis in diabetes and diabetic nephropathy. Int j clin chem. 2022;531:188–196. doi:10.1016/j.cca.2022.04.011
- Zeng C, Wang R, Tan H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int J Bio Sci. 2019;15(7):1345–1357. doi:10.7150/ijbs.33568
- Zheng F, Ma L, Li X, Wang Z, Gao R. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes. 2022;71(12):2739–2750. doi:10.2337/db22-0153
- Wang N, Ding L, Liu D, et al. Molecular investigation of candidate genes for pyroptosis-induced inflammation in diabetic retinopathy. Front Endocrinol. 2022;13:918605. doi:10.3389/fendo.2022.918605
- Cheng YC, Chu LW, Chen JY, et al. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells. 2020;9(9):1948. doi:10.3390/cells9091948
- Wang Y, Kanneganti TD. From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021;19:4641–4657. doi:10.1016/j.csbj.2021.07.038
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi:10.1038/s41418-017-0012-4
- Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi:10.1111/imr.12287
- He X, Fan X, Bai B, Lu N, Zhang S, Zhang L. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res. 2021;165:105447. doi:10.1016/j.phrs.2021.105447
- Wu KJ, Wang WR, Cheng QH, et al. Pyroptosis in neurodegenerative diseases: from bench to bedside. Cell Biol Toxicol. 2023;39:2467–2499. doi:10.1007/s10565-023-09820-x
- Shi J, Gao W, Shao F. Pyroptosis: gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
- Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. doi:10.1016/j.semcdb.2014.02.006
- Hu X, Zhang H, Zhang Q, Yao X, Ni W, Zhou K. Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J Neuroinflammation. 2022;19(1):242. doi:10.1186/s12974-022-02602-y
- Moonen S, Koper MJ, Van Schoor E, et al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 2023;145(2):175–195. doi:10.1007/s00401-022-02528-y
- Han C, Yang Y, Guan Q, et al. New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J Cell & Mol Med. 2020;24(14):8078–8090. doi:10.1111/jcmm.15439
- Qiu Z, Zhang H, Xia M, et al. Programmed Death of Microglia in Alzheimer’s Disease: autophagy, Ferroptosis, and Pyroptosis. J Prev Alzheimer’s Dis. 2023;10(1):95–103. doi:10.14283/jpad.2023.3
- McKenzie BA, Dixit VM, Power C. Fiery Cell Death: pyroptosis in the Central Nervous System. Trends Neurosci. 2020;43(1):55–73. doi:10.1016/j.tins.2019.11.005
- Yang B, Zhong W, Gu Y, Li Y. Emerging Mechanisms and Targeted Therapy of Pyroptosis in Central Nervous System Trauma. Front Cell Develop Biol. 2022;10:832114. doi:10.3389/fcell.2022.832114
- Yin Y, Chen F, Wang W, Wang H, Zhang X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol Vision. 2017;23:242–250.
- Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Proliferation. 2019;52(2):e12563. doi:10.1111/cpr.12563
- Toldo S, Abbate A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. 2024;21(4):219–237. doi:10.1038/s41569-023-00946-3
- Yarovinsky TO, Su M, Chen C, Xiang Y, Tang WH, Hwa J. Pyroptosis in cardiovascular diseases: pumping gasdermin on the fire. Semin Immunopathol. 2023;69:101809. doi:10.1016/j.smim.2023.101809
- Liu X, Luo P, Zhang W, Zhang S, Yang S, Hong F. Roles of pyroptosis in atherosclerosis pathogenesis. Biomed Pharmacothe. 2023;166:115369. doi:10.1016/j.biopha.2023.115369
- He B, Nie Q, Wang F, et al. Role of pyroptosis in atherosclerosis and its therapeutic implications. J Cell Physiol. 2021;236(10):7159–7175. doi:10.1002/jcp.30366
- Rao Z, Zhu Y, Yang P, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12(9):4310–4329. doi:10.7150/thno.71086
- Wei X, Xie F, Zhou X, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–992. doi:10.1038/s41423-022-00905-x
- Liang F, Zhang F, Zhang L, Wei W. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflammation Res. 2020;69(2):159–166. doi:10.1007/s00011-020-01315-3
- You R, He X, Zeng Z, Zhan Y, Xiao Y, Xiao R. Pyroptosis and Its Role in Autoimmune Disease: a Potential Therapeutic Target. Front Immunol. 2022;13:841732. doi:10.3389/fimmu.2022.841732
- Lin Y, Hu Y, Hu X, et al. Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis. Adipocyte. 2020;9(1):302–312. doi:10.1080/21623945.2020.1778826
- Zhao P, Yue Z, Nie L, et al. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol. 2021;48(10):1379–1392. doi:10.1111/jcpe.13517
- Zong Y, Chen W, Zhao Y, Suo X, Yang X. Salmonella Infection Causes Hyperglycemia for Decreased GLP-1 Content by Enteroendocrine L Cells Pyroptosis in Pigs. Int J Mol Sci. 2022;23(3):1217. doi:10.3390/ijms23031272
- Zhou J, Yan S, Guo X, et al. Salidroside protects pancreatic β-cells against pyroptosis by regulating the NLRP3/GSDMD pathway in diabetic conditions. Int Immunopharmacol. 2023;114:109543. doi:10.1016/j.intimp.2022.109543
- Mamun AA, Wu Y, Nasrin F, et al. Role of Pyroptosis in Diabetes and Its Therapeutic Implications. J Inflamm Res. 2021;14:2187–2206. doi:10.2147/jir.s291453
- Feng X, Yang X, Zhong Y, Cheng X. The role of ncRNAs-mediated pyroptosis in diabetes and its vascular complications. Cell Biochem Funct 2024;42(2):e3968. doi:10.1002/cbf.3968
- Li X, Xiao GY, Guo T, Song YJ, Li QM. Potential therapeutic role of pyroptosis mediated by the NLRP3 inflammasome in type 2 diabetes and its complications. Front Endocrinol. 2022;13:986565. doi:10.3389/fendo.2022.986565
- Zuo Y, Chen L, Gu H, et al. GSDMD-mediated pyroptosis: a critical mechanism of diabetic nephropathy. Expert Rev Mol Med. 2021;23:e23. doi:10.1017/erm.2021.27
- Cheng Q, Pan J, Zhou ZL, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2021;42(6):954–963. doi:10.1038/s41401-020-00525-z
- Li W, Sun J, Zhou X, Lu Y, Cui W, Miao LM-R. GSDME-Mediated Pyroptosis in Diabetic Nephropathy. Front Pharmacol. 2021;12:780790. doi:10.3389/fphar.2021.780790
- Zhang L, Ai C, Bai M, Niu J, Zhang Z. NLRP3 Inflammasome/Pyroptosis: a Key Driving Force in Diabetic Cardiomyopathy. Int J Mol Sci. 2022;23(18):10632. doi:10.3390/ijms231810632
- Wang G, Ma TY, Huang K, Zhong JH, Lu SJ, Li JJ. Role of pyroptosis in diabetic cardiomyopathy: an updated review. Front Endocrinol. 2023;14:1322907. doi:10.3389/fendo.2023.1322907
- Xu J, Cai S, Zhao J, et al. Advances in the Relationship Between Pyroptosis and Diabetic Neuropathy. Front Cell Develop Biol. 2021;9:753660. doi:10.3389/fcell.2021.753660
- Yu Q, Zhao T, Liu M, et al. Targeting NLRP3 Inflammasome in Translational Treatment of Nervous System Diseases: an Update. Front Pharmacol. 2021;12:707696. doi:10.3389/fphar.2021.707696
- Alcocer-Gómez E, Ulecia-Morón C, Marín-Aguilar F, et al. Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol Neurobiol. 2016;53(7):4874–4882. doi:10.1007/s12035-015-9408-7
- Iwata M, Ota KT, Li XY, et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry. 2016;80(1):12–22. doi:10.1016/j.biopsych.2015.11.026
- Menachem-Zidon O B, Goshen I, Kreisel T, et al. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33(9):2251–2262. doi:10.1038/sj.npp.1301606
- Vande Walle L, Lamkanfi MP. Current biology: CB. Curr Biol. 2016;26(13):R568–r572. doi:10.1016/j.cub.2016.02.019
- Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi:10.1038/358167a0
- Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15(15):3853–3860. doi:10.1002/j.1460-2075.1996.tb00759.x
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96(5):2396–2401. doi:10.1073/pnas.96.5.2396
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends in Microbiol. 2001;9(3):113–114. doi:10.1016/s0966-842x(00)01936-3
- Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends in microbiology. Trends Microbiol. 2001;9(2):64–67. doi:10.1016/s0966-842x(00)01937-5
- Chen X, He WT, Hu L, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26(9):1007–1020. doi:10.1038/cr.2016.100
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276(5318):1571–1574. doi:10.1126/science.276.5318.1571
- Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi:10.1038/nri3607
- Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. doi:10.1186/s13045-020-00946-7
- Bertheloot D, Latz E. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. J Hematol Oncol. 2021;18(5):1106–1121. doi:10.1038/s41423-020-00630-3
- Swanson KV, Deng M. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews. Immunology. 2019;19(8):477–489. doi:10.1038/s41577-019-0165-0
- He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi:10.1016/j.tibs.2016.09.002
- Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
- Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in Cell Death, Inflammation, and Pyroptosis. Ann Rev Immunol. 2020;38:567–595. doi:10.1146/annurev-immunol-073119-095439
- Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi:10.1038/nature18629
- Purnama CA, Meiliana A, Barliana MI, Lestari K. Update of cellular responses to the efferocytosis of necroptosis and pyroptosis. Cell Division. 2023;18(1):5. doi:10.1186/s13008-023-00087-6
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243(1):206–214. doi:10.1111/j.1600-065X.2011.01044.x
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi:10.1038/nrmicro2070
- Devant P, Kagan JC. Molecular mechanisms of gasdermin D pore-forming activity. Nature Immunol. 2023;24(7):1064–1075. doi:10.1038/s41590-023-01526-w
- Liu X, Xia S. Channelling inflammation: gasdermins in physiology and disease. Nature Reviews. Drug Discovery. 2021;20(5):384–405. doi:10.1038/s41573-021-00154-z
- Ouyang X, Zhou J, Lin L, Zhang Z, Luo S, Hu D. Pyroptosis, inflammasome, and gasdermins in tumor immunity. Innate Immunity. 2023;29(1–2):3–13. doi:10.1177/17534259221143216
- Chauhan D, Demon D, Vande Walle L, et al. GSDMD drives canonical inflammasome-induced neutrophil pyroptosis and is dispensable for NETosis. EMBO Reports. 2022;23(10):e54277. doi:10.15252/embr.202154277
- Zou J, Zheng Y, Huang Y, Tang D, Kang R, Chen R. The Versatile Gasdermin Family: their Function and Roles in Diseases. Front Immunol. 2021;12:751533. doi:10.3389/fimmu.2021.751533
- Van Opdenbosch N, Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50(6):1352–1364. doi:10.1016/j.immuni.2019.05.020
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological Reviews. 2017;277(1):61–75. doi:10.1111/imr.12534
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
- Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265(1):6–21. doi:10.1111/imr.12296
- Boucher D, Monteleone M, Coll RC. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215(3):827–840. doi:10.1084/jem.20172222
- Wang K, Sun Q, Zhong X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180(5):941–955.e20. doi:10.1016/j.cell.2020.02.002
- Matikainen S, Nyman TA. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204(12):3063–3069. doi:10.4049/jimmunol.2000373
- Heilig R, Dilucca M, Boucher D. Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life science alliance. 2020;3(6):73.
- He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
- Schneider KS, Groß CJ, Dreier RF, et al. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 2017;21(13):3846–3859. doi:10.1016/j.celrep.2017.12.018
- Rathinam VA, Fitzgerald KA. Inflammasome Complexes: emerging Mechanisms and Effector Functions. Cell. 2016;165(4):792–800. doi:10.1016/j.cell.2016.03.046
- Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11(3):213–220. doi:10.1038/nri2936
- Jacobs SR, Damania B. NLRs, inflammasomes, and viral infection. J Leukocyte Biol. 2012;92(3):469–477. doi:10.1189/jlb.0312132
- Zhang Y, Chi Z, Cui Z, Chang S, Wang Y, Zhao P. Inflammatory response triggered by avian hepatitis E virus in vivo and in vitro. Front Immunol. 2023;14:1161665. doi:10.3389/fimmu.2023.1161665
- Tsuchiya K, Hara H. The inflammasome and its regulation. Critical Rev Immunol. 2014;34(1):41–80. doi:10.1615/critrevimmunol.2013008686
- Deng Z, Lu L, Li B, Shi X, Jin H, Hu W. The roles of inflammasomes in cancer. Front Immunol. 2023;14:1195572. doi:10.3389/fimmu.2023.1195572
- Lillo S, Saleh M. Inflammasomes in Cancer Progression and Anti-Tumor Immunity. Front Cell Develop Biol. 2022;10:839041. doi:10.3389/fcell.2022.839041
- Faria SS, Costantini S, De lima VCC, et al. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci. 2021;28(1):26. doi:10.1186/s12929-021-00724-8
- Jang J-H, Kim D-H, Surh Y-J. Dynamic roles of inflammasomes in inflammatory tumor microenvironment. NPJ Precision Oncol. 2021;5(1):18. doi:10.1038/s41698-021-00154-7
- Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front Immunol. 2018;9:2379. doi:10.3389/fimmu.2018.02379
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–175. doi:10.1111/j.1600-065X.2012.01146.x
- Jorgensen I, Zhang Y, Krantz BA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113–2128. doi:10.1084/jem.20151613
- Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi:10.1038/ni.1960
- Hara H, Seregin SS, Yang D, et al. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell. 2018;175(6):1651–1664.e14. doi:10.1016/j.cell.2018.09.047
- Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J clin immunol. 2010;30(5):693–702. doi:10.1007/s10875-010-9425-2
- Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–241. doi:10.1038/nature13449
- Ting JP, Lovering RC, Alnemri ES, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28(3):285–287. doi:10.1016/j.immuni.2008.02.005
- Huang M, Zhang X. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. 2021;592(7856):773–777. doi:10.1038/s41586-021-03320-w
- Sandstrom A, Mitchell PS, Goers L, Mu EW. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science. 2019;364(6435):1330. doi:10.1126/science.aau1330
- Mitchell PS, Sandstrom A, Vance RE. The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries. Curr Opinion Immunol. 2019;60:37–45. doi:10.1016/j.coi.2019.04.015
- Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286(5):C1100–8. doi:10.1152/ajpcell.00494.2003
- Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev. 2015;265(1):85–102. doi:10.1111/imr.12293
- Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi:10.1038/nature07725
- Aubert DF, Xu H, Yang J, et al. A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation. Cell Host Microbe. 2016;19(5):664–674. doi:10.1016/j.chom.2016.04.004
- Smith C, Wang X, Yin H. Caspases come together over LPS. Trends in Immunol. 2015;36(2):59–61. doi:10.1016/j.it.2014.12.007
- Chu LH, Indramohan M, Ratsimandresy RA, et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat Commun. 2018;9(1):996. doi:10.1038/s41467-018-03409-3
- Aglietti RA, Dueber EC. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends in Immunol. 2017;38(4):261–271. doi:10.1016/j.it.2017.01.003
- Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: an Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20(13):3328. doi:10.3390/ijms20133328
- Zheng X, Wan J, Tan G. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front Immunol. 2023;14:1151185. doi:10.3389/fimmu.2023.1151185
- Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43(5):923–932. doi:10.1016/j.immuni.2015.10.009
- He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. doi:10.1038/nature16959
- Crespo Yanguas S, Willebrords J, Johnstone SR, et al. Pannexin1 as mediator of inflammation and cell death. Biochim. Biophys. Acta, Mol. Cell. Res. 2017;1864(1):51–61. doi:10.1016/j.bbamcr.2016.10.006
- Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi:10.1038/nature10558
- Downs KP, Nguyen H, Dorfleutner A, Stehlik C. An overview of the non-canonical inflammasome. Mol Aspect Med. 2020;76:100924. doi:10.1016/j.mam.2020.100924
- Zhang CC, Li CG, Wang YF, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–325. doi:10.1007/s10495-019-01515-1
- Orning P, Weng D, Starheim K, Ratner D. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science (New York, N.Y.). 2018;362(6418):1064–1069. doi:10.1126/science.aau2818
- Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA. 2018;115(46):E10888–e10897. doi:10.1073/pnas.1809548115
- Zheng Z, Deng W, Bai Y, et al. The Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-mediated Pyroptosis by Yersinia. Science. 2021;372(6549). doi:10.1126/science.abg0269
- Hou J, Zhao R, Xia W, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nature Cell Biology. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
- Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi:10.1038/nature13683
- Zheng M, Kanneganti TD. Newly Identified Function of Caspase-6 in ZBP1-mediated Innate Immune Responses, NLRP3 Inflammasome Activation, PANoptosis, and Host Defense. J Cellular Immunol. 2020;2(6):341–347. doi:10.33696/immunology.2.064
- Liu Y, Fang Y. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Science Immunol. 2020;5(43):7969.
- Zhang Z, Zhang Y, Xia S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415–420. doi:10.1038/s41586-020-2071-9
- Zhong X, Zeng H, Zhou Z, Su Y, Cheng H, Hou Y. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023;616(7957):598–605. doi:10.1038/s41586-023-05872-5
- Zhou Z, He H. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:6494. doi:10.1126/science.aaz7548
- Galiero R, Caturano A. Peripheral Neuropathy in Diabetes Mellitus: pathogenetic Mechanisms and Diagnostic Options. Int J Mol Sci. 2023;24(4):3554. doi:10.3390/ijms24043554
- D’Souza M, Kulkarni V, Bhaskaran U, et al. Diabetic Peripheral Neuropathy and its Determinants among Patients Attending a Tertiary Health Care Centre in Mangalore, India. J Public Health Res. 2015;4(2):450. doi:10.4081/jphr.2015.450
- Zhang X, Huang S, Zhuang Z, et al. Lipin2 ameliorates diabetic encephalopathy via suppressing JNK/ERK-mediated NLRP3 inflammasome overactivation. Int Immunopharmacol. 2023;118: 109930. doi:10.1016/j.intimp.2023.109930
- Yang C, Zhao X, An X, et al. Axonal transport deficits in the pathogenesis of diabetic peripheral neuropathy. Front Endocrinol. 2023;14:1136796. doi:10.3389/fendo.2023.1136796
- Sun Q, Wang C, Yan B, Shi X. Jinmaitong Ameliorates Diabetic Peripheral Neuropathy Through Suppressing TXNIP/NLRP3 Inflammasome Activation In The Streptozotocin-Induced Diabetic Rat Model. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;12:2145–2155. doi:10.2147/dmso.s223842
- Wang QQ, Wang M, Li Y, Liu YH, Sun LQ. Attenuation of Oxidative Stress-Induced Cell Apoptosis and Pyroptosis in RSC96 Cells by Salvianolic Acid B. Chin J Integr Med. 2022;28(3):243–248. doi:10.1007/s11655-021-3507-2
- Li W, Liang J, Li S, et al. Research progress of targeting NLRP3 inflammasome in peripheral nerve injury and pain. Int Immunopharmacol. 2022;110:109026. doi:10.1016/j.intimp.2022.109026
- Dai Z, Liu WC, Chen XY, Wang X, Li JL, Zhang X. Gasdermin D-mediated pyroptosis: mechanisms, diseases, and inhibitors. Front Immunol. 2023;14:1178662. doi:10.3389/fimmu.2023.1178662
- Broz P, Pelegrín P. The gasdermins, a protein family executing cell death and inflammation. Nature Reviews. Immunology. 2020;20(3):143–157. doi:10.1038/s41577-019-0228-2
- Kovacs SB, Miao EA. Gasdermins: effectors of Pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi:10.1016/j.tcb.2017.05.005
- Ramos-Junior ES, Morandini AC. Gasdermin: a new player to the inflammasome game. Biomedical Journal. 2017;40(6):313–316. doi:10.1016/j.bj.2017.10.002
- Mastrocola R, Penna C. Pharmacological Inhibition of NLRP3 Inflammasome Attenuates Myocardial Ischemia/Reperfusion Injury by Activation of RISK and Mitochondrial Pathways. Oxidative Medicine and Cellular Longevity. 2016;2016:5271251. doi:10.1155/2016/5271251
- Yerramothu P, Vijay AK, Willcox MDP. Inflammasomes, the eye and anti-inflammasome therapy. Eye (London, England). 2018;32(3):491–505. doi:10.1038/eye.2017.241
- Karki R, Man SM, Kanneganti TD. Inflammasomes and Cancer. Cancer Immunol Res. 2017;5(2):94–99. doi:10.1158/2326-6066.cir-16-0269
- Matsuoka Y, Yamashita A, Matsuda M, Kawai K, Sawa T, Amaya F. NLRP2 inflammasome in dorsal root ganglion as a novel molecular platform that produces inflammatory pain hypersensitivity. Pain. 2019;160(9):2149–2160. doi:10.1097/j.pain.0000000000001611
- Chen H, Zhou C, Xie K, Meng X, Wang Y, Yu Y. Hydrogen-rich Saline Alleviated the Hyperpathia and Microglia Activation via Autophagy Mediated Inflammasome Inactivation in Neuropathic Pain Rats. Neuroscience. 2019;421:17–30. doi:10.1016/j.neuroscience.2019.10.046
- Chen SP, Zhou YQ, Wang XM, et al. Pharmacological inhibition of the NLRP3 inflammasome as a potential target for cancer-induced bone pain. Pharmacol Res. 2019;147:104339. doi:10.1016/j.phrs.2019.104339
- Liu CC, Huang ZX, Li X, et al. Upregulation of NLRP3 via STAT3-dependent histone acetylation contributes to painful neuropathy induced by bortezomib. Exp Neurol. 2018;302:104–111. doi:10.1016/j.expneurol.2018.01.011
- Thakur V, Sadanandan J. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Endocrine Connections. 2020;21(3).
- Yoshihara E. TXNIP/TBP-2: a Master Regulator for Glucose Homeostasis. Antioxidants. 2020;9(8):765. doi:10.3390/antiox9080765
- Alhawiti NM, Al Mahri S, Aziz MA, Malik SS, Mohammad S. TXNIP in Metabolic Regulation: physiological Role and Therapeutic Outlook. Curr Drug Targets. 2017;18(9):1095–1103. doi:10.2174/1389450118666170130145514
- Lei Z, Chen Y, Wang J, et al. Txnip deficiency promotes β-cell proliferation in the HFD-induced obesity mouse model. Endocrine Connections. 2022;11(4):641.doi:10.1530/ec-21-0641
- Ao H, Li H, Zhao X, Liu B, Lu L. TXNIP positively regulates the autophagy and apoptosis in the rat Müller cell of diabetic retinopathy. Life Sci. 2021;267:118988. doi:10.1016/j.lfs.2020.118988
- Choi EH, Park SJ. TXNIP: a key protein in the cellular stress response pathway and a potential therapeutic target. Experimental & Molecular Medicine. 2023;55(7):1348–1356. doi:10.1038/s12276-023-01019-8
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi:10.1038/ni.1831
- Xu L, Lin X, Guan M. Verapamil Attenuated Prediabetic Neuropathy in High-Fat Diet-Fed Mice through Inhibiting TXNIP-Mediated Apoptosis and Inflammation. Oxidative Medicine and Cellular Longevity. 2019;2019:1896041. doi:10.1155/2019/1896041
- Yue N, Huang H, Zhu X, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. Journal of Neuroinflammation. 2017;14(1):102. doi:10.1186/s12974-017-0865-y
- Wang D, Wang H, Gao H, et al. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020;10(1):1–9. doi:10.1186/s13578-020-00388-1
- Moustafa PE, Abdelkader NF. Liraglutide ameliorated peripheral neuropathy in diabetic rats: involvement of oxidative stress, inflammation and extracellular matrix remodeling. Journal of Neurochemistry. 2018;146(2):173–185. doi:10.1111/jnc.14336
- Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689. doi:10.1038/s41467-019-09397-2
- Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2018;495(1):1418–1425. doi:10.1016/j.bbrc.2017.11.156
- Tsuchiya K. Switching from Apoptosis to Pyroptosis: gasdermin-Elicited Inflammation and Antitumor Immunity. Int J Mol Sci. 2021;22(1):426. doi:10.3390/ijms22010426
- Shen X, Wang H, Weng C, Jiang H, Chen J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021;12(2):186. doi:10.1038/s41419-021-03458-5
- Li Y, Yuan Y, Huang ZX, et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death and Differentiation. 2021;28(8):2333–2350. doi:10.1038/s41418-021-00755-6
- Mai FY, He P, Ye JZ, et al. Caspase-3-mediated GSDME activation contributes to cisplatin- and doxorubicin-induced secondary necrosis in mouse macrophages. Cell Proliferation. 2019;52(5):e12663. doi:10.1111/cpr.12663
- Wei Y, Lan B, Zheng T. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nature Communications. 2023;14(1):929. doi:10.1038/s41467-023-36614-w
- Tan G, Huang C, Chen J, Chen B, Zhi F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 2021;35(11):109265. doi:10.1016/j.celrep.2021.109265
- Neel DV, Basu H, Gunner G, et al. Gasdermin-E mediates mitochondrial damage in axons and neurodegeneration. Neuron. 2023;111(8):1222–1240.e9. doi:10.1016/j.neuron.2023.02.019
- Chen Y, Lian N, Chen S, et al. GSDME deficiency leads to the aggravation of UVB-induced skin inflammation through enhancing recruitment and activation of neutrophils. Nature. 2022;13(10):841.doi:10.1038/s41419-022-05276-9
- Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell. 2016;164(5):896–910. doi:10.1016/j.cell.2015.12.057
- Barbu E, Popescu MR, Popescu AC. Inflammation as A Precursor of Atherothrombosis, Diabetes and Early Vascular Aging. International Journal of Molecular Sciences. 2022;23(2). doi:10.3390/ijms23020963
- Kugathasan L, Sridhar VS, Tommerdahl KL, et al. Minireview: understanding and targeting inflammatory, hemodynamic and injury markers for cardiorenal protection in type 1 diabetes. Metabolism. 2024;153:155785. doi:10.1016/j.metabol.2024.155785
- Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, de Haan JB. Oxidative Stress and NLRP3-Inflammasome Activity as Significant Drivers of Diabetic Cardiovascular Complications: therapeutic Implications. Front Physiol. 2018;9:114. doi:10.3389/fphys.2018.00114
- Petrosino S, Campolo M, Impellizzeri D, et al. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front Pharmacol. 2017;8:308. doi:10.3389/fphar.2017.00308
- Fusco R, Siracusa R, Peritore AF, et al. The Role of Cashew (Anacardium occidentale L) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants. 2020;9(6):511. doi:10.3390/antiox9060511
- von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–652. doi:10.1016/j.neuron.2012.02.008
- Bullón P, Alcocer-Gómez E, Carrión AM, et al. AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome. Antioxid. Redox Signaling. 2016;24(3):157–170. doi:10.1089/ars.2014.6120
- Kang L, Yayi H. Dexmedetomidine attenuates P2X4 and NLRP3 expression in the spine of rats with diabetic neuropathic pain. Acta cirurgica brasileira. 2019;34(11):e201901105. doi:10.1590/s0102-865020190110000005
- Jia M, Wu C, Gao F, et al. Activation of NLRP3 inflammasome in peripheral nerve contributes to paclitaxel-induced neuropathic pain. Molecular Pain. 2017;13:1744806917719804. doi:10.1177/1744806917719804
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(17023):17023. doi:10.1038/sigtrans.2017.23
- Ding S, Xu S, Ma Y, Liu G. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Mol Psychiatry. 2019;9(12):548.
- Walev I, Klein J, Husmann M, et al. Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2. J Immunol. 2000;164(10):5120–5124. doi:10.4049/jimmunol.164.10.5120
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi:10.1016/j.immuni.2013.05.016
- Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38(4):237–246. doi:10.1016/j.tins.2015.02.001
- Maiarù M, Morgan OB, Tochiki KK, et al. Complex regulation of the regulator of synaptic plasticity histone deacetylase 2 in the rodent dorsal horn after peripheral injury. Journal of Neurochemistry. 2016;138(2):222–232. doi:10.1111/jnc.13621
- Wen J, He T, Qi F, Chen H. MiR-206-3p alleviates chronic constriction injury-induced neuropathic pain through targeting HDAC4. Experimental Animals. 2019;68(2):213–220. doi:10.1538/expanim.18-0091
- Miao J, Zhou X, Ji T, Chen G. NF-κB p65-dependent transcriptional regulation of histone deacetylase 2 contributes to the chronic constriction injury-induced neuropathic pain via the microRNA-183/TXNIP/NLRP3 axis. J Neuroinflammation. 2020;17(1):225. doi:10.1186/s12974-020-01901-6
- Zhou Z, He M, Zhao Q, et al. Panax notoginseng Saponins Attenuate Neuroinflammation through TXNIP-Mediated NLRP3 Inflammasome Activation in Aging Rats. Current Pharm. Biotechnol. 2021;22(10):1369–1379. doi:10.2174/1389201021999201110204735
- Zhang X, Zhao S, Yuan Q, et al. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death & Disease. 2021;12(7):642. doi:10.1038/s41419-021-03930-2
- Chen W, Wang X, Sun Q, et al. The upregulation of NLRP3 inflammasome in dorsal root ganglion by ten-eleven translocation methylcytosine dioxygenase 2 (TET2) contributed to diabetic neuropathic pain in mice. J Neuroinflammation. 2022;19(1):302. doi:10.1186/s12974-022-02669-7
- Chen L, Li B, Chen B, et al. Thymoquinone Alleviates the Experimental Diabetic Peripheral Neuropathy by Modulation of Inflammation. Sci Rep. 2016;6(1):31656. doi:10.1038/srep31656
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi:10.1038/nrneurol.2011.137
- Freeman TL, Swartz TH. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front Immunol. 2020;11:1518. doi:10.3389/fimmu.2020.01518
- Wree A, McGeough MD, Inzaugarat ME, et al. NLRP3 inflammasome driven liver injury and fibrosis: roles of IL-17 and TNF in mice. Hepatology. 2018;67(2):736–749. doi:10.1002/hep.29523
- Takahashi M. Role of NLRP3 Inflammasome in Cardiac Inflammation and Remodeling after Myocardial Infarction. Biol. Pharm. Bull. 2019;42(4):518–523. doi:10.1248/bpb.b18-00369
- Liang Q, Cai W, Zhao Y, et al. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res. 2020;158:104884. doi:10.1016/j.phrs.2020.104884
- Li HL, Huang Y, Zhou YL, et al. C-X-C Motif Chemokine 10 Contributes to the Development of Neuropathic Pain by Increasing the Permeability of the Blood-Spinal Cord Barrier. Front Immunol. 2020;11:477. doi:10.3389/fimmu.2020.00477
- Zhang Y, Li C, Wang Z, Wang T, Zhou Y, Zheng L. Blocking CXC Motif Chemokine Ligand 2 Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Apoptosis and NLRP3 Inflammasome Activation. Biol. Pharm. Bull. 2023;46(5):672–683. doi:10.1248/bpb.b22-00680
- Mazzardo-Martins L, Martins DF, Stramosk J, Cidral-Filho FJ, Santos AR. Glycogen synthase kinase 3-specific inhibitor AR-A014418 decreases neuropathic pain in mice: evidence for the mechanisms of action. Neuroscience. 2012;226:411–420. doi:10.1016/j.neuroscience.2012.09.020
- Wang M, Wang K, Gao X, Zhao K, Chen H, Xu M. Anti-inflammatory effects of isoalantolactone on LPS-stimulated BV2 microglia cells through activating GSK-3β-Nrf2 signaling pathway. Int Immunopharmacol. 2018;65:323–327. doi:10.1016/j.intimp.2018.10.008
- Wang Y, Meng C, Zhang J, Wu J, Zhao J. Inhibition of GSK-3β alleviates cerebral ischemia/reperfusion injury in rats by suppressing NLRP3 inflammasome activation through autophagy. Int Immunopharmacol. 2019;68:234–241. doi:10.1016/j.intimp.2018.12.042
- Weng HR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Exp Neurol. 2014;252:18–27. doi:10.1016/j.expneurol.2013.11.018
- Cortés-Vieyra R, Silva-García O, Gómez-García A, Gutiérrez-Castellanos S, Álvarez-Aguilar C, Baizabal-Aguirre VM. Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front Immunol. 2021;12:675751. doi:10.3389/fimmu.2021.675751
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243(1):136–151. doi:10.1111/j.1600-065X.2011.01046.x
- An Y, Zhang H, Wang C, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB j. 2019;33(11):12515–12527. doi:10.1096/fj.201802805RR
- Amin FM, Abdelaziz RR, Hamed MF, Nader MA, Shehatou GSG. Dimethyl fumarate ameliorates diabetes-associated vascular complications through ROS-TXNIP-NLRP3 inflammasome pathway. Life Sci. 2020;256:117887. doi:10.1016/j.lfs.2020.117887
- Wang JW, Ye XY, Wei N, et al. Reactive Oxygen Species Contributes to Type 2 Diabetic Neuropathic Pain via the Thioredoxin-Interacting Protein-NOD-Like Receptor Protein 3- N -Methyl-D-Aspartic Acid Receptor 2B Pathway. Anesthesia Analg. 2022;135(4):865–876. doi:10.1213/ane.0000000000006117
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflammation. 2010;2010:453892. doi:10.1155/2010/453892
- Yu X, Lan P, Hou X, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol. 2017;66(4):693–702. doi:10.1016/j.jhep.2016.12.018
- Ding ZM, Jiao XF, Wu D, et al. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Biol. Interact. 2017;278:222–229. doi:10.1016/j.cbi.2017.10.030
- Park MH, Hong JT. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016;5(2):15. doi:10.3390/cells5020015
- Lee Y, Fluckey JD, Chakraborty S, Muthuchamy M. Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle. FASEB j. 2017;31(7):2744–2759. doi:10.1096/fj.201600887R
- Sho T, Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol. Appl. Biochem. 2019;66(1):4–13. doi:10.1002/bab.1700
- Dewanjee S, Das S, Das AK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018;833:472–523. doi:10.1016/j.ejphar.2018.06.034
- Dwir D, Giangreco B, Xin L, Tenenbaum L. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2020;25(11):2889–2904. doi:10.1038/s41380-019-0393-5
- Liu XS, Fan B, Szalad A, et al. MicroRNA-146a Mimics Reduce the Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes. 2017;66(12):3111–3121. doi:10.2337/db16-1182
- Cataño Cañizales YG, Uresti Rivera EE, García Jacobo RE, et al. Increased Levels of AIM2 and Circulating Mitochondrial DNA in Type 2 Diabetes. Iranian j Immunol. 2018;15(2):142–155.
- Singh S, Verma SK, Kumar S, et al. Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol Lett. 2018;196:1–10. doi:10.1016/j.imlet.2018.01.004
- Song W, Jiang W, Wang C, et al. Jinmaitong, a Traditional Chinese Compound Prescription, Ameliorates the Streptozocin-Induced Diabetic Peripheral Neuropathy Rats by Increasing Sciatic Nerve IGF-1 and IGF-1R Expression. Front Pharmacol. 2019;10:255. doi:10.3389/fphar.2019.00255
- Liang X, Cui L, Guo S. [Clinical study on jinmaitong composita on diabetic peripheral neuropathy]. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi. 1999;19(9):517–519. Chinese.
- Yin DH, Liang XC, Zhao LI, et al. Jinmaitong decreases sciatic nerve DNA oxidative damage and apoptosis in a streptozotocin-induced diabetic rat model. Exp Ther Med. 2015;10(2):778–786. doi:10.3892/etm.2015.2543
- Wang PY, Liang XC, Zhang H, et al. Effect of serum containing Jinmaitong Capsule on rats’ Schwann cell apoptosis induced by high glucose concentration. Chin J Integr Med. 2013;19(7):517–523. doi:10.1007/s11655-013-1506-7
- Xie J, Song W, Liang X, et al. Jinmaitong ameliorates diabetic peripheral neuropathy in streptozotocin-induced diabetic rats by modulating gut microbiota and neuregulin 1. Aging. 2020;12(17):17436–17458. doi:10.18632/aging.103750
- Rovira-Llopis S, Apostolova N, Bañuls C, Muntané J, Rocha M, Victor VM. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: new Therapeutic Targets. Antioxid. Redox Signaling. 2018;29(8):749–791. doi:10.1089/ars.2017.7313
- Yu ZW, Zhang J, Li X, Wang Y, Fu YH, Gao XY. A new research hot spot: the role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. doi:10.1016/j.lfs.2019.117138
- He X, Wang X, Fang J, et al. Bletilla striata: medicinal uses, phytochemistry and pharmacological activities. J Ethnopharmacol. 2017;195:20–38. doi:10.1016/j.jep.2016.11.026
- Ma W, Wang KJ, Cheng CS, et al. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 2014;153(3):840–845. doi:10.1016/j.jep.2014.03.051
- Chen Y, Jiao N, Jiang M, et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J Cell & Mol Med. 2020;24(11):6083–6095. doi:10.1111/jcmm.15198
- Chen Y, Chen J, Jiang M, et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020;252:117653. doi:10.1016/j.lfs.2020.117653
- Rajabi M, Mohaddes G, Farajdokht F, Nayebi Rad S, Mesgari M, Babri S. Impact of loganin on pro-inflammatory cytokines and depression- and anxiety-like behaviors in male diabetic rats. Physiol Int. 2018;105(3):199–209. doi:10.1556/2060.105.2018.1.8
- Tseng YT, Lin WJ, Chang WH, Lo YC. The novel protective effects of loganin against 1-methyl-4-phenylpyridinium-induced neurotoxicity: enhancement of neurotrophic signaling, activation of IGF-1R/GLP-1R, and inhibition of RhoA/ROCK pathway. Phytotherapy Research: PTR. 2019;33(3):690–701. doi:10.1002/ptr.6259
- Wang Y, Zhao M, Li J, Liu Y. Loganin exerts neuroprotective effect by inhibiting neuronal pyroptosis in rat with cerebral haemorrhage. Clin. Exp. Pharmacol. Physiol. 2024;51(6):e13858. doi:10.1111/1440-1681.13858
- Kong X, Zhao Y, Wang X, et al. Loganin reduces diabetic kidney injury by inhibiting the activation of NLRP3 inflammasome-mediated pyroptosis. Chem. Biol. Interact. 2023;382:110640. doi:10.1016/j.cbi.2023.110640
- Li W, Fan P, Wang X, Tang H. Loganin alleviates myocardial ischemia–reperfusion injury through GLP-1R / NLRP3 -mediated pyroptosis pathway. Environmental Toxicology. 2023;38(11):2730–2740. doi:10.1002/tox.23908
- Cheng YC, Chiu YM, Dai ZK. Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Biomolecules. 2021;10:48.
- Chu LW, Cheng KI, Chen JY, et al. Loganin prevents chronic constriction injury-provoked neuropathic pain by reducing TNF-α/IL-1β-mediated NF-κB activation and Schwann cell demyelination. Phytomedicine. 2020;67:153166. doi:10.1016/j.phymed.2019.153166
- Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. doi:10.1016/j.lfs.2020.117459
- Mrakic-Sposta S, Vezzoli A, Maderna L, Gregorini F, Montorsi M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid Med Cell Longev. 2018;2018:1767265. doi:10.1155/2018/1767265
- Dwivedi S, Gottipati A, Ganugula R. Oral Nanocurcumin Alone or in Combination with Insulin Alleviates STZ-Induced Diabetic Neuropathy in Rats. Molecular Pharmaceutics. 2022;19(12):4612–4624. doi:10.1021/acs.molpharmaceut.2c00465
- Xu JW, Xu X, Ling Y, et al. Vincamine as an agonist of G-protein-coupled receptor 40 effectively ameliorates diabetic peripheral neuropathy in mice. Acta Pharmacol. Sin. 2023;44(12):2388–2403. doi:10.1038/s41401-023-01135-1
- Zhang Q, Li Q, Liu S, et al. Glucagon-like peptide-1 receptor agonist attenuates diabetic neuropathic pain via inhibition of NOD-like receptor protein 3 inflammasome in brain microglia. Diabetes Res Clin Pract. 2022;186:109806. doi:10.1016/j.diabres.2022.109806
- Impellizzeri D, Siracusa R, D’Amico R, et al. Açai Berry Ameliorates Cognitive Impairment by Inhibiting NLRP3/ASC/CASP axis in STZ-Induced Diabetic Neuropathy in Mice. J Neurophysiol. 2023;130(3):671–683. doi:10.1152/jn.00239.2023
- Wang B, Yao J, Yao X, et al. Swertiamarin alleviates diabetic peripheral neuropathy in rats by suppressing NOXS/ ROS/NLRP3 signal pathway. Nan fang yi ke da xue xue bao = J South Med Univ. 2021;41(6):937–941. doi:10.12122/j.issn.1673-4254.2021.06.18
- Zhang YZ, Zhou ZC, Song CY, Chen X. The Protective Effect and Mechanism of Dexmedetomidine on Diabetic Peripheral Neuropathy in Rats. Front Pharmacol. 2020;11:1139. doi:10.3389/fphar.2020.01139
- Hu T, Sun Q, Gou Y, et al. Salidroside Alleviates Chronic Constriction Injury-Induced Neuropathic Pain and Inhibits of TXNIP/NLRP3 Pathway. Neurochemical Research. 2022;47(2):493–502. doi:10.1007/s11064-021-03459-y
- Ni GL, Cui R, Shao AM, Wu ZM. Salidroside Ameliorates Diabetic Neuropathic Pain in Rats by Inhibiting Neuroinflammation. Journal of Molecular Neuroscience: MN. 2017;63(1):9–16. doi:10.1007/s12031-017-0951-8
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi:10.1208/s12248-012-9432-8
- Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091–20112. doi:10.3390/molecules191220091
- Marton LT, Pescinini ESLM, Camargo MEC, et al. The Effects of Curcumin on Diabetes Mellitus: a Systematic Review. Front Endocrinol. 2021;12:669448. doi:10.3389/fendo.2021.669448
- Akaberi M, Sahebkar A, Emami SA. Turmeric and Curcumin: from Traditional to Modern Medicine. Adv Exp Med Biol. 2021;1291:15–39. doi:10.1007/978-3-030-56153-6_2
- Zhai K, Brockmüller A, Kubatka P. Curcumin’s Beneficial Effects on Neuroblastoma: mechanisms, Challenges, and Potential Solutions. Biomolecules. 2020;10(11):1469.
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi:10.1007/978-0-387-46401-5_3
- Benameur T, Giacomucci G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules (Basel, Switzerland). 2021;27(1). doi:10.3390/molecules27010236
- Parsamanesh N, Moossavi M, Bahrami A, Butler AE, Sahebkar A. Therapeutic potential of curcumin in diabetic complications. Pharmacol Res. 2018;136:181–193. doi:10.1016/j.phrs.2018.09.012
- Ataei M, Gumpricht E, Kesharwani P, Jamialahmadi T, Sahebkar A. Recent advances in curcumin-based nanoformulations in diabetes. J Drug Targeting. 2023;31(7):671–684. doi:10.1080/1061186x.2023.2229961
- Zhang WX, Lin ZQ. Curcumin Ameliorates the Experimental Diabetic Peripheral Neuropathy through Promotion of NGF Expression in Rats. Chemistry & Biodiversity. 2022;19(6):e202200029. doi:10.1002/cbdv.202200029
- Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem. Biol. 2017;24(4):507–514.e4. doi:10.1016/j.chembiol.2017.03.009
- Nandini HS, Naik PR. Antidiabetic, antihyperlipidemic and antioxidant effect of Vincamine, in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2019;843:233–239. doi:10.1016/j.ejphar.2018.11.034
- Wang J, Lv X, Xu J, et al. Design, synthesis and biological evaluation of vincamine derivatives as potential pancreatic β-cells protective agents for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 2020;188:111976. doi:10.1016/j.ejmech.2019.111976
- Moini-Nodeh S, Rahimifard M, Baeeri M, Hodjat M, Haghi-Aminjan H, Abdollahi M. Vinpocetine Effect on the Juncture of Diabetes and Aging: an in-vitro study. Drug Res. 2021;71(8):438–447. doi:10.1055/a-1381-6625
- Du T, Yang L, Xu X, et al. Vincamine as a GPR40 agonist improves glucose homeostasis in type 2 diabetic mice. J Endocrinol. 2019;240(2):195–214. doi:10.1530/joe-18-0432
- Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–756. doi:10.1016/j.cmet.2018.03.001
- Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: crosstalk in Diabetes. Trend endocrinol metabol. 2017;28(2):88–103. doi:10.1016/j.tem.2016.10.001
- Insuela DBR, Carvalho VF. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur. J. Pharmacol. 2017;812:64–72. doi:10.1016/j.ejphar.2017.07.015
- Lee CH, Jeon SJ, Cho KS, et al. Activation of Glucagon-Like Peptide-1 Receptor Promotes Neuroprotection in Experimental Autoimmune Encephalomyelitis by Reducing Neuroinflammatory Responses. Mol Neurobiol. 2018;55(4):3007–3020. doi:10.1007/s12035-017-0550-2
- Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine. 2018;24(7):931–938. doi:10.1038/s41591-018-0051-5
- Tai J, Liu W, Li Y, Li L, Hölscher C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1678:64–74. doi:10.1016/j.brainres.2017.10.012
- Gong N, Xiao Q, Zhu B, et al. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci. 2014;34(15):5322–5334. doi:10.1523/jneurosci.4703-13.2014
- da Silva Cristino Cordeiro V, de Bem GF, da Costa CA, et al. Euterpe oleracea Mart. seed extract protects against renal injury in diabetic and spontaneously hypertensive rats: role of inflammation and oxidative stress. Eur J Nutr. 2018;57(2):817–832. doi:10.1007/s00394-016-1371-1
- de Bem GF, da Costa CA, da Silva Cristino Cordeiro V, et al. Euterpe oleracea Mart. (açaí) seed extract associated with exercise training reduces hepatic steatosis in type 2 diabetic male rats. J Nutr Biochem. 2018;52:70–81. doi:10.1016/j.jnutbio.2017.09.021
- Kim H, Simbo SY, Fang C, et al. Açaí (Euterpe oleracea Mart) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018;9(6):3097–3103. doi:10.1039/c8fo00595h
- Souza-Monteiro JR, Arrifano GPF, Queiroz A. Antidepressant and Antiaging Effects of Açaí (Euterpe oleracea Mart) in Mice. Oxidative Medicine and Cellular Longevity. 2019;2019:3614960. doi:10.1155/2019/3614960
- de Almeida Magalhães TSS, de Oliveira Macedo PC, Converti A, de Lima AA. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules. 2020;10(6):813. doi:10.3390/biom10060813
- Mn AL, AlSaadi AM, Whitby A, Kim DH. Acai Berry (Euterpe sp) Extracts Are Neuroprotective against L-Glutamate-Induced Toxicity by Limiting Mitochondrial Dysfunction and Cellular Redox Stress. Nature. 2023;13(4):1019. doi:10.3390/life13041019
- Lee JY, Kim N, Choi YJ, et al. Anti-inflammatory and Anti-tumorigenic Effects of Açai Berry in Helicobacter felis-infected mice. J Cancer Prev. 2016;21(1):48–54. doi:10.15430/jcp.2016.21.1.48
- Moura RS, Ferreira TS, Lopes AA, et al. Effects of Euterpe oleracea Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine. 2012;19(3–4):262–269. doi:10.1016/j.phymed.2011.11.004
- Earling M, Beadle T, Niemeyer ED. Açai Berry (Euterpe oleracea) Dietary Supplements: variations in Anthocyanin and Flavonoid Concentrations, Phenolic Contents, and Antioxidant Properties. Plant Foods for human Nutrition (Dordrecht, Netherlands). 2019;74(3):421–429. doi:10.1007/s11130-019-00755-5
- Remigante A, Spinelli S, Straface E. Açaì (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells. 2022;11(15). doi:10.3390/cells11152391
- de Moraes Arnoso BJ, Magliaccio FM, de Araújo CA, et al. Açaí seed extract (ASE) rich in proanthocyanidins improves cardiovascular remodeling by increasing antioxidant response in obese high-fat diet-fed mice. Chem. Biol. Interact. 2022;351:109721. doi:10.1016/j.cbi.2021.109721
- Laurindo LF, Barbalho SM. Açaí (Euterpe oleracea Mart) in Health and Disease: a Critical Review. Nutrients. 2023;15(4):989. doi:10.3390/nu15040989
- Cadoná FC, de Souza DV, Fontana T, et al. Açaí (Euterpe oleracea Mart) as a Potential Anti-neuroinflammatory Agent: NLRP3 Priming and Activating Signal Pathway Modulation. Molecular Neurobiology. 2021;58(9):4460–4476. doi:10.1007/s12035-021-02394-x
- Patel TP, Soni S, Parikh P, Gosai J, Chruvattil R, Gupta SS. An Active Lead from Enicostemma littorale Regulates Hepatic and Adipose Tissue Gene Expression by Targeting PPAR- γ and Improves Insulin Sensitivity in Experimental NIDDM Rat Model. Evidence Based Complementary Alternative Med. 2013;2013:358673. doi:10.1155/2013/358673
- Jaishree V, Badami S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J Ethnopharmacol. 2010;130(1):103–106. doi:10.1016/j.jep.2010.04.019
- Jaishree V, Narsimha S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Biomed Pharmacothe. 2020;130:110561. doi:10.1016/j.biopha.2020.110561
- Patel N, Tyagi RK, Tandel N, Garg NK, Soni N. The Molecular Targets of Swertiamarin and its Derivatives Confer Anti- Diabetic and Anti-Hyperlipidemic Effects. Curr Drug Targets. 2018;19(16):1958–1967. doi:10.2174/1389450119666180406113428
- Xu L, Li D, Zhu Y, et al. Swertiamarin supplementation prevents obesity-related chronic inflammation and insulin resistance in mice fed a high-fat diet. Adipocyte. 2021;10(1):160–173. doi:10.1080/21623945.2021.1906510
- Saravanan S, Islam VI, Babu NP, et al. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur j Pharm Sci. 2014;56:70–86. doi:10.1016/j.ejps.2014.02.005
- Ducza L, Gajtkó A, Hegedűs K, et al. Neuronal P2X4 receptor may contribute to peripheral inflammatory pain in rat spinal dorsal horn. Front Mol Neurosci. 2023;16:1115685. doi:10.3389/fnmol.2023.1115685
- Grace PM, Strand KA, Galer EL, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA. 2016;113(24):E3441–50. doi:10.1073/pnas.1602070113
- Biber K, Tsuda M, Tozaki-Saitoh H, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30(9):1864–1873. doi:10.1038/emboj.2011.89
- Rong H, Zhao Z, Feng J, et al. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun. 2017;64:195–207. doi:10.1016/j.bbi.2017.03.006
- Coursin DB, Coursin DB, Maccioli GAD. Current opinion in critical care. Current Opinion in Critical Care. 2001;7(4):221–226. doi:10.1097/00075198-200108000-00002
- Ayoglu H, Gul S, Hanci V, et al. The effects of dexmedetomidine dosage on cerebral vasospasm in a rat subarachnoid haemorrhage model. J clin Neurosci. 2010;17(6):770–773. doi:10.1016/j.jocn.2009.10.012
- Lin Y, Wei Y, Wei Y, et al. Dexmedetomidine alleviates oxidative stress and mitochondrial dysfunction in diabetic peripheral neuropathy via the microRNA-34a/SIRT2/S1PR1 axis. Int Immunopharmacol. 2023;117:109910. doi:10.1016/j.intimp.2023.109910
- Zhou TT, Wu JR, Chen ZY, Liu ZX, Miao B. Effects of dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in spinal microglia in rats with spared nerve injury. Brain Res. 2014;1568:21–30. doi:10.1016/j.brainres.2014.04.025
- Zhang Y, Jia S, Gao T, Zhang R, Liu Z, Wang Y. Dexmedetomidine mitigate acute lung injury by inhibiting IL-17-induced inflammatory reaction. Immunobiology. 2018;223(1):32–37. doi:10.1016/j.imbio.2017.10.017
- Yan X, Cheng X, Zhou L, He X, Zheng W, Chen H. Dexmedetomidine alleviates lipopolysaccharide-induced lung injury in Wistar rats. Oncotarget. 2017;8(27):44410–44417. doi:10.18632/oncotarget.17899
- Khan S, Choi RJ, Lee J, Kim YS. Attenuation of neuropathic pain and neuroinflammatory responses by a pyranocoumarin derivative, anomalin in animal and cellular models. Eur. J. Pharmacol. 2016;774:95–104. doi:10.1016/j.ejphar.2016.02.008
- Chen K, Zhang J, Zhang W, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45(5):932–943. doi:10.1016/j.biocel.2013.02.009
- Zhang J, Ma X, Liu F, et al. Role of NLRP3 inflammasome in diabetes and COVID-19 role of NLRP3 inflammasome in the pathogenesis and treatment of COVID-19 and diabetes NLRP3 inflammasome in diabetes and COVID-19 intervention. Front Immunol. 2023;14:1203389. doi:10.3389/fimmu.2023.1203389
- Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci. 2013;7:191. doi:10.3389/fncel.2013.00191
- Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293–302.
- Zhang X, Xie L, Long J, et al. Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties. Chem. Biol. Interact. 2021;339:109268. doi:10.1016/j.cbi.2020.109268
- Alotaibi NA, Slater NK, Rahmoune H. Salidroside as a Novel Protective Agent to Improve Red Blood Cell Cryopreservation. PLoS One. 2016;11(9):e0162748. doi:10.1371/journal.pone.0162748
- Wang M, Luo L, Yao L, et al. Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus. Sci Rep. 2016;6:25399. doi:10.1038/srep25399
- Zheng T, Yang X, Wu D, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br. J. Pharmacol. 2015;172(13):3284–3301. doi:10.1111/bph.13120
- Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging. 2020;12(10):9405–9426. doi:10.18632/aging.103215
- Cai Y, Chai Y, Fu Y, et al. Salidroside Ameliorates Alzheimer’s Disease by Targeting NLRP3 Inflammasome-Mediated Pyroptosis. Front Aging Neurosci. 2021;13:809433. doi:10.3389/fnagi.2021.809433
- Chai Y, Cai Y, Fu Y, et al. Salidroside Ameliorates Depression by Suppressing NLRP3-Mediated Pyroptosis via P2X7/NF-κB/NLRP3 Signaling Pathway. Front Pharmacol. 2022;13:812362. doi:10.3389/fphar.2022.812362
- Xing SS, Yang J, Li WJ, et al. Salidroside Decreases Atherosclerosis Plaque Formation via Inhibiting Endothelial Cell Pyroptosis. Inflammation. 2020;43(2):433–440. doi:10.1007/s10753-019-01106-x
- Shi S, Huang D, Wu Y, et al. Salidroside pretreatment alleviates PM(2.5) caused lung injury via inhibition of apoptosis and pyroptosis through regulating NLRP3 Inflammasome. Food Chem Toxicol. 2023;177:113858. doi:10.1016/j.fct.2023.113858
- Qu ZQ, Zhou Y, Zeng YS, et al. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012;7(1):e29641. doi:10.1371/journal.pone.0029641
- Liu X, Zhou M, Dai Z, et al. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytotherapy Res. 2023;37(2):367–382. doi:10.1002/ptr.7636
- Wang C, Wang Q, Lou Y, et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med. 2018;22(2):1148–1166. doi:10.1111/jcmm.13368
- Zheng T, Wang Q, Bian F, et al. Salidroside alleviates diabetic neuropathic pain through regulation of the AMPK-NLRP3 inflammasome axis. Toxicol Appl Pharmacol. 2021;416:115468. doi:10.1016/j.taap.2021.115468
- Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signalling. 2009;5(1):63–73. doi:10.1007/s11302-008-9110-6
- Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi:10.1016/j.pain.2005.01.002
- Lister MF, Sharkey J, Sawatzky DA, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflammation. 2007;4(1):5. doi:10.1186/1476-9255-4-5
- Liu S, Zou L, Xie J, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Molecular Brain. 2016;9(1):44. doi:10.1186/s13041-016-0226-2
- Ursu D, Ebert P, Langron E, et al. Gain and loss of function of P2X7 receptors: mechanisms, pharmacology and relevance to diabetic neuropathic pain. Molecular Pain. 2014;10:37. doi:10.1186/1744-8069-10-37
- Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br. J. Pharmacol. 2007;151(5):571–579. doi:10.1038/sj.bjp.0707265

