Abstract
Macrophages belong to the innate immune system giving us protection against pathogens. However it is known that they are also involved in rheumatic diseases. Activated macrophages have two different phenotypes related to different stimuli: M1 (classically activated) and M2 (alternatively activated). M1 macrophages release high levels of pro-inflammatory cytokines, reactive nitrogen and oxygen intermediates killing microorganisms and tumor cells; while M2 macrophages are involved in resolution of inflammation through phagocytosis of apoptotic neutrophils, reduced production of pro-inflammatory cytokines, and increased synthesis of mediators important in tissue remodeling, angiogenesis, and wound repair. The role of macrophages in the different rheumatic diseases is different according to their M1/M2 macrophages phenotype.
Keywords:
Introduction
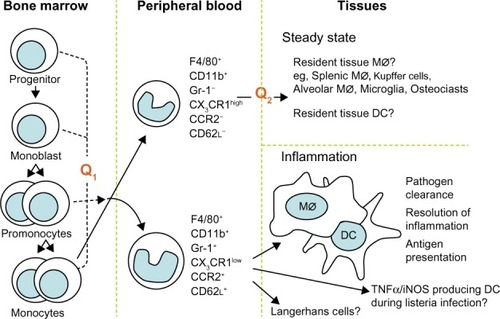
Macrophages are cells of the innate immune system involved in immunological response against pathogens but also in autoimmune disorders such as rheumatic diseases. They play the role of antigen-presenting cells and they release many inflammatory cytokines and chemokines that contribute to cartilage, bone, and tissue destruction. We want to present several aspects of macrophage function in autoimmune diseases: the development of the two monocyte subsets and of the two macrophage phenotypes. Macrophages are the main cells in most tissues. Their numbers increase massively in inflammation, in autoimmunity diseases, and in cancers. Their progenitor cell is CD34+ in the bone marrow, that differentiates into monoblasts and then into pro-monocytes; finally into monocytes (M0), that are released into the blood. Monocytes circulate for 1–3 days in the blood and then they enter tissues to differentiate into mature resident macrophages;Citation1 for example they become Kupffer cells in the liver, microglial cells in the brain, and Langerhans cells in the skin. All these resident macrophages, although different in some aspects, have capacity to influence normal cell turnover and tissue remodeling, to counteract microbial infections, and to facilitate repair in sites of injury.Citation2 The presence or absence of the Fc receptor CD16 identifies two populations of human monocytes, as demonstrated by Passlick et al.Citation3 It seems that CD16+ monocytes do not express the chemokine receptor CCR2 according to Weber et alCitation4 and these cells have an enhanced capacity for trans-endothelial migration.Citation5 It is known that there are two phenotypically and functionally distinct monocyte subsets: inflammatory and resident phenotype. The first one is characterized by CCR2+CD62L+ or CD14lowCD16+ phenotype, while the last one is characterized by CCR2−CD62L− or CD14+CD16−. The inflammatory phenotype is preferentially recruited to inflammatory lesions, while the resident one is hypothesized to be a source of tissue resident macrophage and dendritic cells ().Citation6
Figure 1 Schematic representation of the development of the monocyte subsets.
Abbreviation: DC, dendritic cells.
In the M1/M2 model described by Badylak and Gilbert, CD68 is a specific macrophage surface marker; CD80 and CCR7 identify pro-inflammatory and cytotoxic macrophage M1 phenotype, while CD163 is specific to M2 phenotype during the remodeling process.Citation7
When macrophages are recruited into tissues, they become “activated macrophages” and they can have two different phenotypes related to different stimuli: M1 (classically activated) and M2 (alternatively activated) ().Citation8,Citation9
Figure 2 Schematic representation of macrophage polarization.
Abbreviations: LPS, lipopolysaccharide; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor α; IL, interleukin; IL-1ra, interleukin 1 receptor antagonist; TGF-β, transformer growth factor β.
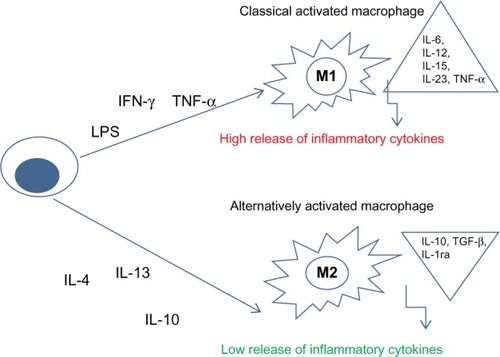
M1 macrophages are important in killing microorganisms and tumor cells; they release high levels of pro-inflammatory cytokines, reactive nitrogen and oxygen intermediates.
M2 macrophages are subdivided into three subpopulations in response to different cytokines and chemokines. IL-4 or IL-13 activates M2a; immune complexes (ICs) in combination with IL-1β or LPS activate M2b phenotype, while IL-10, TGF-β or glucocorticoids induce M2c macrophages. M2 macrophages are involved in resolution of inflammation through phagocytosis of apoptotic neutrophils, reduced production of pro-inflammatory cytokines, and increased synthesis of mediators important in tissue remodeling, angiogenesis, and wound repair ().Citation8,Citation9
Macrophages and rheumatoid arthritis
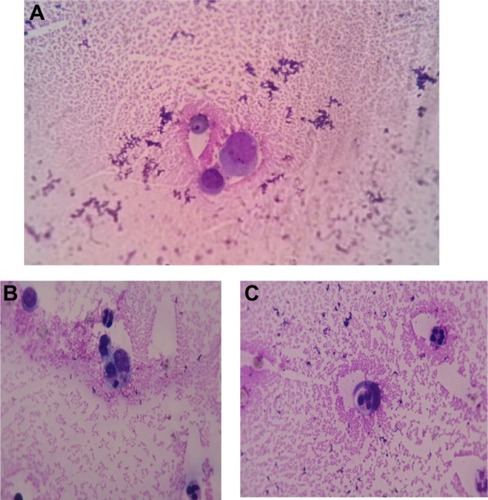
Macrophages play a key role in the pathogenesis of rheumatoid arthritis (RA). They produce many pro-inflammatory cytokines and chemokines and then contribute to the cartilage and bone destruction.Citation10 An increased number of macrophages are found in the synovial tissue; these cells can be activated to produce inflammatory cytokines. In we show an activated macrophage from synovial fluid of an RA patient, while illustrate macrophages and neutrophil granulocytes during phagocytosis of a small lymphocyte in the same patient.
Figure 3 Wright stain images of a patient with rheumatoid arthritis.
Notes: (A) Activated macrophage in synovial fluid of rheumatoid arthritis. wright stain, synovial fluid smear from a patient with rheumatoid arthritis 1,000×; (B) and (C) macrophages phagocytizing a small lymphocyte and neutrophil granulocytes. wright stain 1,000× (rheumatoid arthritis).
Synovial sublining macrophage number can be used as a biomarker for disease severity as well as a predictor of responsiveness to disease-modifying antirheumatic drug (DMARD) therapy.Citation11 It has demonstrated a strong correlation between number of macrophages, the mean change in the disease activity score,Citation12 and the degree of joint erosion.Citation13,Citation14 M1 macrophages have a TNFhighIL-12highIL-10lowiNOS2high pro-inflammatory phenotype, while M2 macrophages have a TNFlowIL-12lowIL-10high-arginasehigh anti-inflammatory phenotype.Citation15
From an in vitro polarization study using human monocytes, it was found that CD80 is the main phenotypic marker for human M1 macrophages polarized by IFN-γ, whereas CD200R is upregulated and CD14 is specifically downregulated on M2 macrophages polarized by IL-4, while CD163 and CD16 were found to be specific markers for macrophages polarized by IL-10 ().Citation16
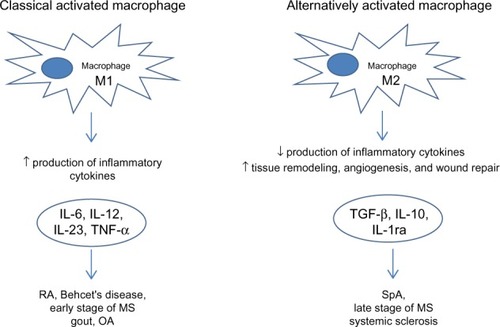
Figure 4 Macrophages in rheumatic diseases.
Abbreviations: RA, rheumatoid arthritis; OA, osteoarthritis; MS, multiple sclerosis; SpA, spondyloarthritis; TNF-α, tumor necrosis factor α; IL, interleukin; IL-1ra, interleukin 1 receptor antagonist; TGF-β, transformer growth factor β.
Polarization of RA synovial tissue or synovial fluid macrophages in M1 or M2 phenotype depends on “flare” of rheumatic disease. In fact, patients with highly active RA show a prevalence of M1 phenotype; on the other hand, RA patients with low disease activity score or clinical remission show a M2 phenotype. Furthermore, glucocorticoids induce the M2 state,Citation8 and this “switching” is one of the pharmacological effects of this drugs class on RA. Other DMARDs (such as methotrexate and leflunomide) also have this mechanism of action on macrophage populations; moreover they inhibit cell replication and recruitment of immature and inflammatory monocytes to sites of inflammation.Citation15 Elevated levels of GM-CSF and M-CSF are seen in synovial fluid from RA patients. GM-CSF has many actions on neutrophils, eosinophils, macrophages, and dendritic cells, whereas M-CSF acts more specifically on the macrophage lineage. It has been proposed that M-CSF in the absence of inflammation maintains steady-state levels of M2 macrophages, while GM-CSF induces pro-inflammatory M1 macrophages that release other inflammatory cytokines, such as TNF-α, IL-12, and IL-23. During development of arthritis, TNF-α and IL-1 stimulate fibroblasts and chondrocytes to release GM-CSF and M-CSF.Citation17 CD68 and CD163 are two commonly used markers for identification of synovial macrophages in RA synovium. CD68 is a scavenger receptor that binds to oxidized low-density lipoprotein and may also be involved in the cell–cell interaction.Citation17 It is localized on both cell surface and lysosomal membranes. Changes in the number of synovial sublining CD68+ macrophages can be used as a sensitive biomarker to predict the possible efficacy of anti-rheumatic therapy because this variation’s number correlates with disease activity score.Citation17 CD163 is a type I trans-membrane protein that belongs to the group B scavenger receptor cysteine-rich superfamily. The major role of CD163 is its capacity to bind and internalize hemoglobin–haptoglobin complexes leading to the release of IL-10 and carbon oxide, which exert strong anti-inflammatory effects.Citation17 CD163 has major advantages as a macrophage marker, as compared with CD68, in RA synovium. In fact CD163 discriminates between synovial macrophages and synovial intimal fibroblasts, which also stain positive for CD68 in diseased tissue.Citation17 Moreover, soluble CD163 in sera is a promising diagnostic marker for untreated new-onset systemic juvenile idiopathic arthritis and macrophage activation syndrome, which is characterized by excessive expansion of T cells and hemophagocytic macrophages that induce a strong inflammatory reaction.Citation18 Macrophage markers can help rheumatology specialists to choose the best biological DMARD for rheumatoid patients; in fact it is known in literature that in baseline synovial myeloid, but not lymphoid, gene signature expression is higher in patients with good clinical response to anti-TNF-α therapy.
Dennis et al observed that some serum molecules can predict clinical response to different biological DMARDs. In particular, high baseline serum sICAM1, associated with the myeloid phenotype, is associated with the highest American College of Rheumatologists (ACR) 50 response rate to anti-TNF-α treatment; while high serum CXCL13, associated with the lymphoid phenotype, is associated with greater ACR50 response to anti-IL6R treatment.Citation19
Macrophages and angiogenesis
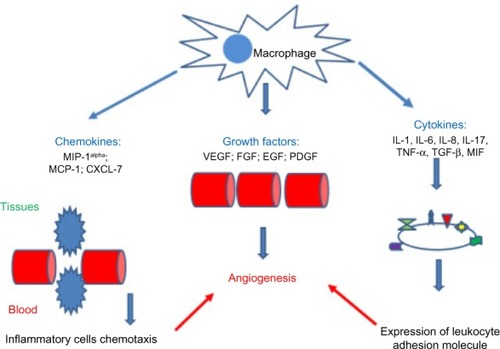
Angiogenesis has a key role in pathogenesis of several rheumatic diseases, such as RA, osteoarthritis (OA), ankylosing spondylitis, systemic sclerosis (SSc), systemic lupus erythematosus (SLE), and vasculitides. Among major cell types involved in angiogenesis, macrophages are known to produce numerous angiogenic factors, including VEGF, FGF, TGF-β, PDGF, TNF-α, MCP-1, IL-6, IL-8, and IL-18 ().Citation20,Citation21
Figure 5 Chemokines, growth factors, and cytokines involved in the angiogenic activity of macrophages.
Abbreviations: MIP-1alpha, macrophage inflammatory protein-1 alpha; MCP-1, monocyte chemotactic protein 1; CXCL-7, CXC chemokine ligand 7; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; EGF, epidermal growth factor; PDGF, platelet derived growth factor; TNF-α, tumor necrosis factor α; IL, interleukin; TGF-β, transformer growth factor β; MIF, macrophage migration inhibitory factor.
Macrophages and SLE
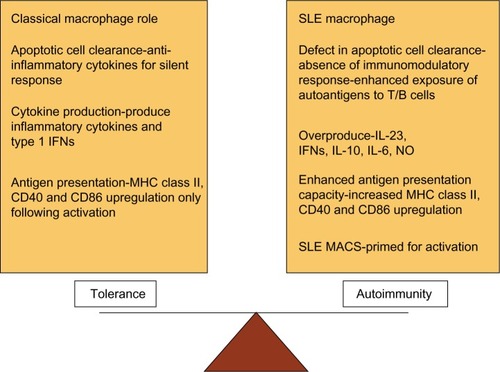
SLE is an autoimmune disease with a broad spectrum of clinical symptoms. Autoreactive B and T lymphocytes,Citation21 together with the innate immune system play a role in the pathogenesis of SLE. SLE macrophages are unable to clear apoptotic cells. In SLE, there is an altered pro-inflammatory/anti-inflammatory macrophage status that leads to an overproduction of inflammatory cytokines such as TNF-α, IL-6, IL-10, and antiviral type I IFNs ().Citation22,Citation23 In an inflammatory context, SLE monocytes and macrophages present self-antigens to autoreactive T cells rather than the immunosilent presentation normally associated with material from apoptotic cells.Citation22 Moreover, myeloid cells (including dendritic cells) induce overproduction of type I IFNs that lead to an overproduction of antibodies (classes IgG, IgA, IgM) and class switching from B cells.Citation22 Recent studies focused attention on innate immune system as promoter of autoimmunity.
Figure 6 Dysregulation of macrophage function in systemic lupus erythematosus (SLE).
Abbreviation: MHC, major histocompatibility complex; INFs, Interferons; MHC, major histocompatibility complex; CD40, cluster of differentiation 40; CD86, cluster of differentiation 86; IL, interleukin; NO, nitrous oxide; SLE MACS, SLE macrophages.
PRPs, present on immune cells, recognize microbes during the normal immune responses against infectious agents.Citation24 TLRs, members of PRPs, control immune responses detecting common molecular motifs, including RNA ligands by TLR3, TLR7 and TLR8, DNA ligands by TLR9, and bacterial cell surface proteins such as LPS or endotoxin that is a ligand for TLR4.
After the link between molecular motifs and TLRs on immune cells (dendritic cells, monocytes/macrophages, and B cells), many pro-inflammatory cytokines are released.
TLRs are activated not only during response against host, but it is also known that they contribute to the pathogenesis of SLE.Citation25 TLRs (in particular TLR7, TLR8, and TLR9) of SLE patients recognize the serum ICs formed by autoantibodies to nucleic acids or nucleic acid binding proteins.Citation26 Dendritic and B cells can internalize these nucleic acid-containing ICs through Fc and surface immunoglobulin receptors, respectively.Citation27,Citation28 After the ICs’ internalization, nucleic acids activate TLRs present on endosomal compartment (RNA and DNA activate TLR7 and TLR9, respectively) and then dendritic cells are stimulated to produce IFN and B cells to undergo maturation. Plasmacytoid dendritic cells are the major source of IFN-α, under TLR7 or TLR9 activation. From these observations, it seems clear that innate immunity regulates key aspects of autoimmunity playing a key role in the pathogenesis of SLE but also in other autoimmunity diseases such as SSc. Moreover, it is known that specific B cell subtype in the marginal zone of the spleen, so-called marginal zone B cells (MZBs), are involved in SLE pathogenesis.Citation29 MZBs are essential for early responses against pathogens and they are phenotypically characterized by high IgM and complement receptor expression.Citation30 However, MZBs are also involved in self-reactivity.Citation31 Autoreactive MZBs produce autoantibodies to self-antigens derived from apoptotic cells.Citation32 In the marginal zone, the MZBs are in close contact with highly phagocytic macrophages called marginal zone macrophages (MZMs).Citation33 The MZMs are characterized by high expression of the class A scavenger receptor MARCO and SR-A.Citation33 MARCO and SR-A bind a variety of self- and foreign ligands, and through them MZMs play an important role in MZB activation.Citation29
Macrophages and SSc
SSc is an autoimmune disease characterized by inflammation, endothelial damage, and fibrosis. Type I IFNs have been implicated in B cell maturationCitation34,Citation35 and they ameliorate fibrosis of SSc by blocking effect of TGF-β. Upon TLRs’ activation, dendritic cells and macrophages release cytokines such as IL-1, TNF, and IL-6 that stimulate inflammation and fibrosis in SSc. Maturation of autoreactive B lymphocytes is induced by TLR activation, also. It seems that TLR activation leads to fibrosis through TGF-β.Citation36 Moreover data from literature suggest that TLR activation might directly stimulate fibroblast conversion to pro-fibrotic myofibroblasts through TLR3Citation37 or TLR9.Citation38 Macrophages, in the dermis of scleroderma patients, become pro-fibrotic under activation of IL-13Citation39 and they release many pro-fibrotic mediators, including PDGF and TGF-β. Th1 cytokine IFN-γ induces “classically activated” monocytes whereas Th2 cytokines IL-4 and IL-13 stimulate “alternatively activated” macrophages.Citation40 Alternatively activated macrophages are proposed to be pro-fibrotic, possibly by activating TGF-β. We need future studies to explain this paradigm, to date not fully explored in SSc.
Macrophages and gout
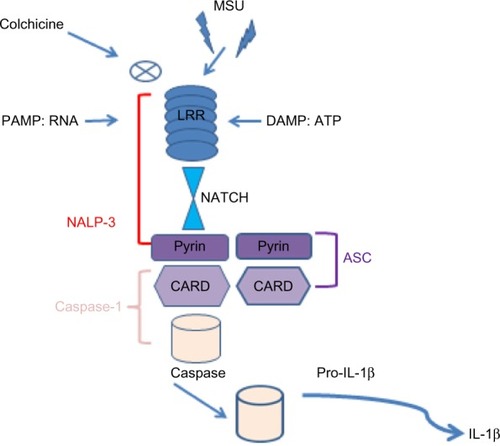
Hyperuricemia can induce precipitation of monosodium urate monohydrate crystals in joints and soft tissues leading to release of many pro-inflammatory cytokines such as TNF, IL-1, and IL-6.Citation41 It is well known that IL-1 plays a key role in the pathogenesis of gout. It is secreted in the molecular platform called inflammasome,Citation41 that promotes the activation of CASP1, which then cleaves Pro-IL-1 (). There are many different inflammasomes, but NALP3 inflammasome is the most important in gout. Monosodium urate monohydrate or calcium pyrophosphate dihydrate crystals or pathogen-associated molecular patterns or danger-associated molecular patterns bind NALP3 through an LRR domain (that is capable of recognizing microbial pathogen-associated molecular patterns).Citation41 NALP3 possesses a NACHT domain to bind ATP and a pyrin domain, which is critical for homotypic protein–protein interactions (). ASC, an adopter molecule, links NALP3 with CASP1 ().Citation42 These interactions lead to activation of CASP1 molecules, becoming capable of cleaving or processing Pro-IL-1 ().Citation41 So, it is clear that macrophage-derived IL-1β (M1-associated phenotype in gout) is a pivotal mediator of acute gout and could become a potential therapeutic target.Citation9 In this way, colchicine is a drug used to treat gout, able to block the activation of inflammasomes and then the production of macrophagic activated IL-1β ().
Figure 7 Schematic representation of the role of the inflammasome in processing Pro-IL-1β.
Abbreviations: MSU, monosodium urate monohydrate; LRR, leucine-rich repeat domain; PAMP, pathogen associated molecular pattern; DAMP, damage-associated molecular pattern; RNA, acid ribonucleic; ATP, adenosine triphosphate; NALP-3, PYD domains-containing protein 3; ASC, caspase activation and recruitment domain; NATCH, nucleotide-binding domain; CARD, caspase-recruitment domain; Pro-IL1β, pro-interleukin 1β.
Macrophages and spondyloarthritis
Spondyloarthritis (SpA) includes different forms of arthritis, comprises ankylosing spondylitis, psoriatic arthritis (PsA), reactive arthritis, enteropathic-related arthritis, and undifferentiated SpA. When we refer to lymphocytic cells we know the different origins of RA and SpA; but the synovial infiltrate of patients with PsA and RA is comparable with regard to numbers of fibroblast-like synoviocytes and macrophages.Citation43 Prominent swelling and inflammation of peripheral joints is a hallmark of both RA and SpA, with a preference for large joints of the lower extremities in SpA. Of interest, the total number of macrophages is similar in RA and SpA synovitis, but the subset expressing the M2 surface marker, CD163,Citation44,Citation45 is clearly increased in the latter.Citation46,Citation47 In accordance with this observation, it was recently described that synovial fluid from SpA patients promotes preferential expression of the M2 markers CD163 and CD200R in vitro. Interestingly, this was still observed even if synovial fluid levels of the prototypical M2-polarizing factors (IL-4, IL-13, and IL-10) were not increased as compared with those in RA synovial fluid.Citation48 In this respect RA macrophages are associated with an M1 phenotype whereas SpA macrophages with an M2 phenotype.
From these literature data, we can assume that SpA synovitis is characterized from overexpression of alternatively activated macrophages (M2 phenotype) responsible for pro-fibrotic state and for bone overgrowth (syndesmophytes) through abnormal tissue remodeling.
Macrophages and OA
OA is characterized by loss of articular cartilage and modification of subchondral bone. Fluid accumulation (joint effusion), bone overgrowth (osteophytes), and weakness of tendons and muscles can also result from the degenerative process. OA commonly affects the hands, feet, spine, and large weight-bearing joints, such as the hips and knees. The pathogenesis of OA is largely unknown and under current investigation. Biomechanical stress such as cartilage integrity, maintained by a balance obtained from cytokine-driven anabolic and catabolic processes, are involved in the pathogenesis of OA.Citation49 Cartilage erosion, induced by proteolytic enzymes that lead to a breakdown of the cartilage macromolecules, lead to synovial inflammation. Activated synoviocytes and mononuclear cells (eg, macrophages) release cytokines such as IL-1β and TNF-α and up-regulate MMP gene expression.Citation50,Citation51 Then, the synovial inflammation is implicated in many of the signs and symptoms of OA, including joint swelling and effusion.Citation52,Citation53
Histologically, the OA synovium shows hyperplasia with an increased number of lining cells and a mixed inflammatory infiltrate consisting mainly of macrophages.Citation54
Synovitis in OA is likely to contribute to disease progression, by releasing inflammatory cytokines and enzymes that degrade cartilage and induce progression of structural changes in OA.Citation55,Citation56 However, the levels of pro-inflammatory cytokines in the OA synovitis are lower than in RA. In particular, TNF-α and IL-1 have been suggested to be key players in OA pathogenesis, both in synovial inflammation and in activation of chondrocytes and they stimulate synovial cells and chondrocytes to produce IL-6, IL-8, and LIF as well as stimulate protease and prostaglandin production.Citation52,Citation57 It is clear that RA macrophage is the main promoter of disease activity and that macrophage-produced TNF-α is a major therapeutic target. The role of macrophages in OA is not well studied to date. OA synovial macrophages produce both pro-inflammatory cytokines and VEGFCitation52 and synovial macrophage differentiation differs between inflammatory and non-inflammatory OA.Citation55 In experimental OA the involvement of macrophages in this pathology has been demonstrated.Citation58,Citation59 van der Kraan et al demonstrated that macrophages mediate osteophyte formation and fibrosis in the early stages of experimental OA.Citation60 Moreover, these knee synovial macrophages were crucial in early MMP activity and appeared to mediate MMP production in synovium rather than cartilage.Citation61,Citation62 Blom et alCitation63 showed that Wnt signaling pathway is involved in OA pathogenesis.
WISP-1 expression was strongly increased in the synovium and cartilage of human and experimental OACitation64 regulating chondrocyte and macrophage MMP and aggrecanase expression (molecules capable of inducing articular cartilage damage).Citation64 In OA, macrophage-derived MMP and pro-inflammatory cytokines might contribute to cartilage destruction and are likely to play a crucial role in disease pathogenesis. To date it seems that M1 phenotype is involved in OA pathogenesis, but more future studies are necessary to define the role of macrophages, in particular, their phenotype.
Macrophages in Behcet’s disease and Sjogren’s syndrome
Behcet’s disease (BD) is a chronic, relapsing, inflammatory disorder which is clinically characterized by bipolar aphthosis and microvascular skin and ocular lesions.Citation65 The synovitis of patients with BD in terms of CD68+ macrophage numbers is similar in the intimal lining layer as well as the synovial sublining to the synovitis of PsA patients.Citation66 However, it has been reported that serum factor(s) are able to induce classical (pro-inflammatory) activation (M1 phenotype) of human peripheral blood macrophages in vitro in this diseaseCitation67 suggesting that serum factor(s) might be responsible for inflammatory changes in BD.
Sjogren’s syndrome (SS) is a systemic autoimmune disease that affects woman targeting exocrine glands primarily. Chronic focal inflammatory infiltration is constituted by CD3+ T and CD19+ B lymphocytes as well as M1 and M2 macrophages and it increases inflammatory cytokines’ release. Baban et al demonstrated that B cells and M2 macrophages are more prominent than T cells and M1 macrophages. This result could be explained by temporal relation of immune cell infiltrates in salivary glands in SS.Citation68,Citation69
Macrophages and anti-neutrophil cytoplasmic antibodies-related vasculitis
The primary small vessel systemic vasculitides are disorders that target small blood vessels, inducing vessel wall inflammation and are associated with development of anti-neutrophil cytoplasmic antibodies (ANCAs). From data literature it is known that antibodies activate neutrophils inappropriately, leading to endothelial and vascular damage through cytokines such as TNF. The role of monocyte/macrophages in small vessel systemic vasculitides remain unknown to date, although some studies continue to suggest that down-regulating their activities can be beneficial. To support this, van der Veen et al showed that the use of a p38 MAPK inhibitor reduced ANCA-activation of neutrophils but also reduced glomerular macrophage accumulation and crescent formation.Citation70 One feature of the accelerated apoptosis was perplexing. When a neutrophil becomes apoptotic, normally there is enhanced expression of phosphatidylserine molecules on its surface which allows it to be recognized and eaten by a phagocytic cell. ANCA-treated neutrophils did not show enhanced expression of phosphatidylserine on their surfaces. This suggested that the cell membrane changes were dissociated from the morphological and nuclear changes of apoptosis and, perhaps more importantly, that the apoptotic neutrophils might not be efficiently cleared by phagocytic cells such as macrophages. In vitro, human monocyte-derived macrophages were, as predicted, less efficient at removing ANCA-treated apoptotic neutrophils than untreated apoptotic neutrophils. The consequences of this failure of removal may be profound since late apoptotic neutrophils disintegrate with release of cell contents into the microenvironment. These observations may also explain the fragmentation of white blood cells seen in electron microscopy studies of early vasculitic lesions.Citation70
Macrophages and multiple sclerosis
Multiple sclerosis (MS), or experimental autoimmune encephalomyelitis (EAE) in an animal model, is a debilitating neurological disorder of the central nervous system (CNS). This disorder is defined by inflammatory infiltration of the CNS, constituted by leukocytes and dendritic cells. In the early stage of MS, peripheral macrophages infiltrate the CNS, where, together with residential microglia, they participate in the induction and development of disease. During the early phase, microglia/macrophages are immediately activated to become classically activated macrophages (M1 cells), releasing pro-inflammatory cytokines and damaging CNS tissue. During the later phase, alternatively activated macrophage phenotype (M2 cells) is predominant in CNS inflammation, releasing anti-inflammatory cytokines, and they are responsible for inflammation resolution and tissue repair.Citation71 The balance between activation and polarization of M1 cells and M2 cells in the CNS is important for disease progression. M1 cell polarization is driven by pro-inflammatory IFN-γ and IL-12, while IL-4 and IL-13 drive switch to M2 cell. Because macrophage polarization is reversible and dependent on cytokine environment, macrophage phenotypes in the CNS can be modulated by molecular intervention to obtain beneficial effects on disease progression.Citation72
In EAE, an animal model of MS, the therapeutic effect of fasudil, a selective ROCK inhibitor was observed.Citation72 Fasudil ameliorated the clinical severity of EAE with an improvement in demyelination and inhibition of inflammatory cells. One cause of its potential effect is due to induction of switch from inflammatory M1 to anti-inflammatory M2 macrophages. The polarization of M2 macrophages was associated with the decrease of inflammatory cytokine IL-1β, TNF-α, and MCP-1.Citation73
Effect of treatments on macrophage lineage numbers
Different conventional and biological DMARDs may play a role through macrophage inhibition.
As expressed above, one other stimulus that favors the M2 state is glucocorticoids,Citation14 and this “switching” may form part of the action of this class of drugs at the macrophage level. This is one of the effects of DMARDs (such as methotrexate and leflunomide) on macrophage populations, in addition to inhibition of cell replication and recruitment of immature and inflammatory monocytes to sites of inflammation.Citation13
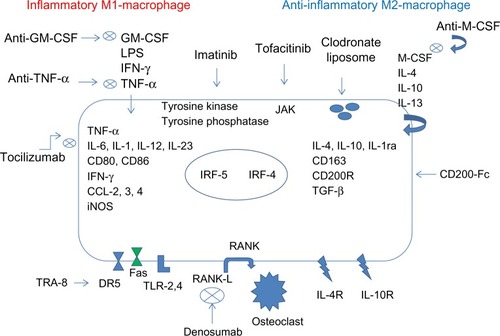
In RA, TNF-α acts as a positive feedback signal to further promote development and survival of macrophages. Under these circumstances, which are usually encountered in clinical practice, blockade of TNF-α signaling in macrophages can promote depletion of macrophages.Citation74 Etanercept and infliximab have been shown to induce apoptosis in monocytes and macrophages in both synovial fluid and peripheral blood in vivo.Citation75 Furthermore, treatment with TNF-α blockers reduces the number of infiltrating synovial granulocytes and macrophages, as well as reducing the expression of chemokines, IL-8, and MCP-1.Citation76 Anti-TNF-α therapy has also been shown to potentially ameliorate arthritis by upregulating TGF-β and reversing the functional defect of regulatory T cells in RA, which can then inhibit the activity of inflammatory macrophages.Citation77 Infliximab, a chimeric monoclonal antibody directed against TNF-α, in combination with methotrexate, leads to decreased synovial and skin VEGF expression in patients affected by PsA.Citation78 The development of other biological therapies, such as the anti-IL-6 receptor antibody, tocilizumab, exhibits the therapeutic effect, in part, by inhibition of macrophage development and function.Citation79 It also may reduce VEGF production in RA.Citation80,Citation81 Thalidomide, recently introduced in the treatment of RA and lupus, is responsible for angiogenesis and TNF-α inhibition.Citation82,Citation83 Blockade of GM-CSF or M-CSF inhibits the development of arthritis.Citation84,Citation85 The oral inhibitor of M-CSF receptor 27 reduced the arthritis progression by inactivating the tyrosine kinase, which can also be achieved by imatinib mesylate (Glivec®; Novartis International AG, Basel, Switzerland).Citation86 Since depletion of both M-CSF and GM-CSF is effective, several therapies are in Phase I or II clinical trials or preclinical studies using GM-CSF-specific antibodies, GM-CSF receptor-specific antibodies, or antibodies directed at M-CSF or its receptor.Citation87,Citation88 JAK inhibitors, including tofacitinib, may also act to effect macrophage development.Citation16,Citation88 Macrophage polarization and related therapeutic targets and novel agents are summarized in .
Figure 8 Macrophage polarization in RA synovium and related therapeutic targets.
Abbreviation: RA, rheumatoid arthritis; GM-CSF, Granulocyte-macrophage colony-stimulating factor; LPS, Lipopolysaccharide; TNF-α, Tumor necrosi factor; INF-γ, Interferon-γ; CD, cluster of differentation; iNOS, Inducible nitric oxide synthase; CCL-2,3,4, chemokine (C-C motif) ligand 2,3,4; IL, Interleukin; JAK, Janus kinase; IRF, interferon regulatory factor; M-CSF, macrophage colony-stimulating factor; CD200-Fc, cluster of differentation 200-fragment crystallizable; TGF-β, Transformer growth factor β; RANK, Receptor Activator of NF-KB; RANK-L, Receptor Activator of NF-KB ligand; TRA-8, monomeric monoclonal antibody targeting DR5; DR5, death receptor 5; FAS, a death receptor; TLR-2,4, Toll-like receptor 2,4.
Conclusion
Macrophages play a key role in rheumatic diseases. Their role in the different rheumatic diseases is different according to their M1/M2 macrophage phenotype ().
Pro-inflammatory M1 phenotype expression is prevalently seen in RA, BD, early stage MS, gout, and OA in the presence of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-1, IL-12, and IL-23. On the other hand, M2 macrophages are more predominant in SpA, late stage MS, and SS releasing anti-inflammatory cytokines such as IL-4, IL-10, IL-1ra, and TGF-β and contributing to tissue remodeling and angiogenesis. So, in the future, using specific targeting molecules we can modulate shift versus M1 or M2 phenotype and we can block progression of many rheumatic diseases.
Disclosure
The authors have no conflicts of interest to disclose.
References
- VolkmanAGowansJLThe Origin of Macrophages from Bone Marrow in the RatBr J Exp Pathol196546627014295560
- Burke B; SumnerSMaitlandNLewisCEMacrophages in gene therapy: cellular delivery vehicles and in vivo targetsJ Leukoc Biol9200272341742812223508
- PasslickBFliegerDZiegler-HeitbrockHWIdentification and characterization of a novel monocyte subpopulation in human peripheral bloodBlood1989747252725342478233
- WeberCBelgeKUvon HundelshausenPDifferential chemokine receptor expression and function in human monocyte subpopulationsJ Leukoc Biol20006769970410811011
- RandolphGJSanchez-SchmitzGLiebmanRMSchakelKThe CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue settingJ Exp Med200219651752712186843
- SerbinaNVSalazar MatherTPBironCAKuzielWAPamerEGTNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infectionImmunity72003191597012871639
- BadylakSFGilbertTWImmune Response to Biologic Scaffold MaterialsSemin Immunol200820210911618083531
- MantovaniASozzaniSLocatiMAllavenaPSicaAMacrophage polarization: tumor associated macrophages as a paradigm for polarized M2 mononuclear phagocytesTrends Immunol2002231154955512401408
- BalkwillFCharlesKAMantovaniASmoldering and polarized inflammation in the initiation and promotion of malignant diseaseCancer Cell20057321121715766659
- LebreMCTakPPMacrophage Subsets in Immune-Mediated Inflammatory Disease: Lessons from Rheumatoid Arthritis, Spondyloarthritis, Osteoarthritis, Behçet’s Disease and GoutThe Open Arthritis Journal201031823
- GerlagDMTakPPNovel approaches for the treatment of rheumatoid arthritis: lessons from the evaluation of synovial biomarkers in clinical trialsBest Pract Res Clin Rheumatol200822231132318455687
- Van der HeijdeDMvan’t HofMAvan RielPLJudging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity scoreAnn Rheum Dis199049119169202256738
- MulherinDFitzGeraldOBresnihanBSynovial tissue macrophage populations and articular damage in rheumatoid arthritisArthritis Rheum19963911151248546720
- TakPPBresnihanBThe pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis [review]Arthritis Rheum200043122619263311145019
- HamiltonJATakPPThe Dynamics of Macrophage Lineage Populations in Inflammatory and Autoimmune DiseasesArthritis Rheum20096051210122119404968
- AmbarusCAKrauszSvan EijkMSystematic validation of specific phenotypic markers for in vitro polarized human macrophagesJ Immunol Methods20113751–219620622075274
- LiJHsuHCMountzJDManaging Macrophages in Rheumatoid Arthritis by Reform or RemovalCurr Rheumatol Rep201214544545422855296
- BleesingJPradaASiegelDMThe diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritisArthritis Rheum200756396597117328073
- DennisGJrHolwegCTKummerfeldSKSynovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeuticsArthritis Res Ther2014162R9025167216
- MaruottiNAnneseTCantatoreFPRibattiDMacrophages and angiogenesis in rheumatic diseasesVasc Cell2013511123725043
- ByrneJCNí GabhannJLazzariEGenetics of SLE: functional Relevance for Monocytes/Macrophages in diseaseClin Dev Immunol2012201258235223227085
- KavaiMSzegediGImmune complex clearance by monocytes and macrophages in systemic Lupus erythematosusAutoimmun Rev20076749750217643939
- SestakALFürnrohrBGHarleyJBMerrillJTNamjouBThe genetics of systemic Lupus erythematosus and implications for targeted therapyAnn Rheum Dis201170Suppl1i37i4321339217
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell2006124478380116497588
- LafyatisRMarshak-RothsteinAToll-like receptors and innate immune responses in systemic lupus erythematosusArthritis Res Ther20079622218086320
- Marshak-RothsteinAToll-like receptors in systemic autoimmune diseaseNat Rev Immunol200661182383517063184
- LauCMBroughtonCTaborASRNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagementJ Exp Med200520291171117716260486
- LeadbetterEARifkinIRHohlbaumAMChromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptorsNature2002416688160360711948342
- BanchereauJPascualVType I interferon in systemic lupus erythematosus and other autoimmune diseasesImmunity200625338339216979570
- WermelingFChenYPikkarainenTClass A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupusJ Exp Med2007204102259226517893199
- MartinFKearneyJFMarginal-zone B cellsNat Rev Immunol20022532333512033738
- LiYLiHNiDWeigertMAnti-DNA B cells in MRL/lpr mice show altered differentiation and editing patternJ Exp Med2002196121543155212486097
- EnzlerTBonizziGSilvermanGJAlternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like diseaseImmunity200625340341516973390
- KraalGMebiusRNew insights into the cell biology of the marginal zone of the spleenInt Rev Cytol200625017521516861066
- RonnblomLElorantaMLAlmGVThe type I interferon system in systemic lupus erythematosusArthritis Rheum200654240842016447217
- SekiEDe MinicisSOsterreicherCHTLR4 enhances TGF-beta signaling and hepatic fibrosisNat Med200713111324133217952090
- SugiuraHIchikawaTKoaraiAActivation of Toll-like receptor 3 augments myofibroblast differentiationAm J Respir Cell Mol Biol200940665466218988918
- MeneghinAChoiESEvanoffHLTLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiationHistochem Cell Biol2008130597999218633634
- FisherERRodnanGPPathologic observations concerning the cutaneous lesion of progressive systemic sclerosis: an electron microscopic histochemical and immunohistochemical studyArthritis Rheum1960353654513699954
- MunderMEichmannKModolellMAlternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotypeJ Immunol199816011534753549605134
- PopeRMTschoppJThe Role of Interleukin-1 and the Inflammasome in Gout Implications for TherapyArthritis rheum200756103183318817907163
- MartinonFBurnsKTschoppJThe inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-Mol Cell200210241742612191486
- MartinonFTschoppJInflammatory caspases and inflammasomes: master switches of inflammationCell Death Differ2007141102216977329
- van KuijkAWReinders-BlankertPSmeetsTJDijkmansBATakPPDetailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatmentAnn Rheum Dis200665121551155716728461
- BuechlerCRitterMOrsoELangmannTKluckenJSchmitzGRegulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuliJ Leukoc Biol20006719710310648003
- SulahianTHHoggerPWahnerAEHuman monocytes express CD163, which is upregulated by IL-10 and identical to p155Cytokine20001291312132110975989
- BaetenDDemetterPCuvelierCAMacrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathyJ Pathol2002196334335011857499
- BaetenDKruithofEDeRLDiagnostic classification of spondylarthropathy and rheumatoid arthritis by synovial histopathology: a prospective study in 154 consecutive patientsArthritis Rheum20045092931294115457462
- VandoorenBNoordenbosTAmbarusCAbsence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritisArthritis Rheum200960496697519333931
- WargerTHilfNRechtsteinerGInteraction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responsesJ Biol Chem200628132225452255316754684
- FernandesJCMartel-PelletierJPelletierJPThe role of cytokines in osteoarthritis pathophysiologyBiorheology2002391–223724612082286
- Martel-PelletierJPathophysiology of osteoarthritisOsteoarthritis Cartilage200412Suppl AS31S3314698638
- PelletierJPMartel-PelletierJAbramsonSBOsteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targetsArthritis Rheum20014461237124711407681
- BenitoMJVealeDJFitzGeraldOvan den BergWBBresnihanBSynovial tissue inflammation in early and late osteoarthritisAnn Rheum Dis20056491263126715731292
- FarahatMNYanniGPostonRPanayiGSCytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritisAnn Rheum Dis199352128708758311538
- ClarkAGJordanJMVilimVSerum cartilage oligomeric protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis ProjectArthritis Rheum199942112356236410555031
- SowersMJannauschMSteinEJamadarDHochbergMLachanceLC-reactive protein as a biomarker of emergent osteoarthritisOsteoarthritis Cartilage200210859560112479380
- HaywoodLMcWilliamsDFPearsonCIInflammation and angiogenesis in osteoarthritisArthritis Rheum20034882173217712905470
- DanksLSabokbarAGundleRAthanasouNASynovial macrophage-osteoclast differentiation in inflammatory arthritisAnn Rheum Dis2002611091692112228163
- van der KraanPMVittersELvan de PutteLBvan den BergWBDevelopment of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee jointsAm J Pathol19891356100110142556924
- van LentPLBlomABvan derKPCrucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formationArthritis Rheum200450110311114730606
- BlomABvan LentPLHolthuysenAESynovial lining macrophages mediate osteophyte formation during experimental osteoarthritisOsteoarthritis Cartilage200412862763515262242
- BlomABBrockbankSMvan LentPLInvolvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1Arthritis Rheum200960250151219180479
- BlomABvan LentPLLibregtsSCrucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3Arthritis Rheum200756114715717195217
- SakaneTTakenoMSuzukiNInabaGBehcet’s diseaseN Engl J Med1999341171284129110528040
- CaneteJDCelisRNoordenbosTDistinct synovial immunopathology in Behcet disease and psoriatic arthritisArthritis Res Ther2009111R1719196489
- AlpsoyEKodeljaVGoerdtSOrfanosCEZouboulisCSerum of patients with Behcet’s disease induces classical (proinflammatory) activation of human macrophages in vitroDermatology2003206322523212673080
- BabanBLiuJYAbdelsayedRMozaffariMSReciprocal relation between GADD153 and Del-1 in regulation of salivary gland inflammation in Sjogren syndromeExp Mol Pathol201395328829724060278
- RoescherNLoddeBMVostersJLTemporal changes in salivary glands of non-obese diabetic mice as a model for Sjogren’s syndromeOral Dis20121819610621914088
- van der VeenBSChenMMullerREffects of p38 mitogen-activated protein kinase inhibition on anti-neutrophil cytoplasmic autoantibody pathogenicity in vitro and in vivoAnn Rheum Dis201170235636521062851
- JiangZJiangJXZhangGXMacrophages: a double-edged sword in experimental autoimmune encephalomyelitisImmunol Lett20141601172224698730
- MironVEBoydAZhaoJWM2 microglia/macrophages drive oligodendrocyte differentiation during CNS remyelinationNat Neurosci20131691211121823872599
- LiuCLiYYuJTargeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudilPLoS One201382e5484123418431
- SavageCOThe evolving pathogenesis of systemic vasculitisClin Med200225458464
- ParameswaranNPatialSTumor necrosis factor-alpha signaling in macrophagesCrit Rev Eukaryot Gene Expr20102028710321133840
- CatrinaAITrollmoCaf KlintEEvidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended reportArthritis Rheum2005521617215641091
- TaylorPCPetersAMPaleologEReduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritisArthritis Rheum2000431384710643698
- CornishALCampbellIKMcKenzieBSChatfieldSWicksIPG-CSF and GM-CSF as therapeutic targets in rheumatoid arthritisNat Rev Rheumatol200951055455919798030
- EhrensteinMREvansJGSinghACompromised function of regulatory t cells in rheumatoid arthritis and reversal by anti-tnfalpha therapyJ Exp Med2004200327728515280421
- GoedkoopAYKraanMCPicavetDIDeactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapyArthritis Res Ther200464R326R33415225368
- NakaharaHSongJSugimotoMAnti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritisArthritis Rheum20034861521152912794819
- SzekaneczZKochAEChemokines and angiogenesisCurr Opin Rheumatol200113320220811333349
- SzekaneczZKochAEMechanism of disease: angiogenesis in inflammatory diseasesNat Clin Pract Rheumatol200731163564317968334
- SinghJABegSLopez-OlivoMATocilizumab for rheumatoid arthritis: a Cochrane systematic reviewJ Rheumatol2011381102020952462
- CampbellIKRichMJBischofRJHamiltonJAThe colony-stimulating factors and collagen-induced arthritis: Exacerbation of disease by m-csf and g-csf and requirement for endogenous m-csfJ Leukoc Biol200068114415010914502
- CookADBraineELCampbellIKRichMJHamiltonJABlockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (gm-csf): Requirement for gm-csf in the effector phase of diseaseArthritis Res20013529329811549370
- AndoWHashimotoJNampeiAImatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (CIA)J Bone Miner Metab200624427428216816921
- HamiltonJAColony-stimulating factors in inflammation and autoimmunityNat Rev Immunol20088753354418551128
