 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Matrix metalloproteinases (MMPs) and specific endogenous tissue inhibitors of metalloproteinases (TIMPs) mediate rupture of the fetal membranes in both physiological and pathological conditions. MMPs and TIMPs are subject to regulation by DNA methylation in human malignancies and pre-eclampsia. To determine if membrane type 1 MMP (MT1-MMP), MMP2, and TIMP2 are regulated by DNA methylation in human placentas, we employed an in vitro model where human placental tissues were collected at term gestation and cultured with methylation inhibiting agent 5-AZA-2′-deoxycytidine (AZA) and lipopolysaccharide. The results suggest that DNA methylation is not directly involved in the regulation of MT1-MMP in placental tissue; however, remodeling of chromatin by a pharmacologic agent such as AZA potentiates an infection-related increase in MT1-MMP. MT1-MMP is a powerful activator of MMP2 and this action, coupled with either no change or a decrease in TIMP2 concentrations, favors a gelatinolytic state leading to extracellular matrix degradation, which could predispose fetal membranes to rupture prematurely during inflammation.
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases secreted as latent proenzymes into the extracellular space. Once activated, MMPs are capable of degrading a variety of extracellular matrix (ECM) components.Citation1 Soluble MMPs, particularly MMP2 and MMP9, are major modulators of fetal membrane integrity throughout gestation and are responsible for membrane rupture at the onset of labor.Citation2 Membrane type 1 MMP (MT1-MMP, also known as MMP14) is synthesized as an inactive 63 kDa latent enzyme, which is transported to the cell membrane where it is cleaved into the active 57 kDa form.Citation3 In addition to degrading ECM components, MT1-MMP is a potent physiological activator of soluble MMPs. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of MMPs and regulate their activation by binding in a 1:1 stoichiometry;Citation4 shifting the balance between TIMPs and active MMPs results in excessive proteolytic activity.Citation5
Latent MMP2 (pro-MMP2) is secreted as a 72 kDa proenzyme, and is not readily activated into its active 68 kDa form by general proteolytic cleavage.Citation6–Citation10 Instead, activation is mediated at the cell surface via a series of reactions involving MT1-MMP and TIMP2.Citation4 A pro-MMP2/TIMP2 complex is formed via the C-terminal of both proteins, leaving the inhibitory N-terminal of TIMP2 free to bind MT1-MMP at the cell surface. Once at the cell surface, the pro-MMP2 is activated by a second MT1-MMP, which is free of TIMPs. Alternatively, if TIMP2 is already inhibiting MT1-MMP, this complex may act as a receptor for pro-MMP2.Citation4,Citation5,Citation11
The regulation of MT1-MMP in human placenta is not well understood. MT1-MMP has previously been detected in fetal membranes collected at term gestation from non-laboring cesarean section (CS) deliveries, and from normal vaginal deliveries with the spontaneous onset of labor (spontaneous vaginal delivery [SVD]). Fortunato et al have reported that MT1-MMP mRNA and protein were present in the fetal membranes and that no differences were detected following culture with lipopolysaccharide (LPS), suggesting constitutive expression of this MMP in the fetal membranes.Citation12
There is conflicting information about the role of MMP2 in fetal membrane rupture at term gestation. MMP2 has been reported to be constitutively expressed in fetal membranes throughout gestation.Citation13,Citation14 However, it has more recently been shown to increase with gestational age in amnion, with significantly increased levels at term labor.Citation15 MMP2 has also been implicated in preterm premature rupture of the fetal membranes (pPROM)Citation16 and is increased in the presence of LPS.Citation17 TIMP2 protein increases with advancing gestational age, and decreases with preterm and term labor, pPROM, and in the presence of intra-amniotic infection.Citation18 Furthermore, TIMP2 protein is decreased in cultured fetal membranes exposed to LPS.Citation17,Citation18
Epigenetic regulation refers to modifications to DNA and chromatin that result in heritable changes in gene expression independent of the genetic sequence and include DNA methylation and histone modifications.Citation19 DNA methylation refers to the addition of a methyl group to the cytosine ring in DNA in the context of a CpG dinucleotide, to form methyl cytosine (5-mC). The silencing of TIMP genes via promoter methylation is a hallmark of cancer development, and the MT1-MMP/MMP2/TIMP2 axis is regulated by a combination of DNA methylation and histone modifications in cancer cells.Citation20
In the current study, we hypothesize that MT1-MMP, MMP2, and TIMP2 are regulated by DNA methylation in human term placenta and that aberrant methylation leading to altered gene expression may contribute to fetal membrane rupture.
Materials and methods
Tissue collection and explant system
The present study was designed in accordance with the principles set out in the Declaration of Helsinki. Ethical approval for the study was obtained from Northern X Regional Ethics Committee (NTX/10/07/062), and tissues were collected following written informed consent. Villous placental tissues were collected from women at term with uncomplicated, singleton pregnancies (38–40 weeks gestation) following elective CS (mean maternal age 35 years, mean gestational age 38 weeks). Tissue culture protocol including doses and length of treatments were based on a previously published in vitro tissue explant system,Citation21–Citation23 with modifications.
Samples of villous tissue were taken randomly across the placenta from midsections of cotyledons (halfway between the maternal and fetal sides). Large vessels were removed using blunt dissection and leaving only villous tissue, which was further dissected into 20 mg pieces. Villous explants were plated separately (six pieces per well) and were equilibrated for 24 hours in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 containing L-glutamate (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific) and 1% antibiotic solution (final concentrations 100 U/mL penicillin and 100 μg streptomycin; Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 and 8% O2.
After equilibration, tissues were washed and media were replaced with DMEM/F12 supplemented with 0.1% bovine gamma globulin (Sigma-Aldrich Co, St Louis, MO, USA) containing 5 μM 5-AZA-2′-deoxycytidine (AZA; Sigma-Aldrich Co) or dimethyl sulfoxide (DMSO, a vehicle control; Sigma-Aldrich Co). Following 48 hours culture, tissues were extensively washed in sterile phosphate-buffered saline (PBS), and tissues were further incubated in the presence or absence of 5 μg/mL LPS and Escherichia coli 055.B5 (Sigma-Aldrich Co). Tissues were cultured with LPS to determine if an inflammatory response induced changes in TIMP1 expression and/or DNA methylation. Culture was terminated at 24 hours and 48 hours post-LPS treatment; tissues were snap-frozen, and conditioned media were reserved. Tissues and media samples were stored at −80°C and −20°C, respectively.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was isolated from tissues using Trizol (Thermo Fisher Scientific) according to the manufacturer’s instructions. RNA concentrations were quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Following DNase treatment, reverse transcription and cDNA synthesis were performed using a Transcriptor First Strand cDNA Synthesis Kit (Hoffman-La Roche Ltd, Basel, Switzerland) according to the manufacturer’s instructions using 1 μg of total RNA for each preparation. The resulting cDNA was stored at −20°C until required.
MT1-MMP, MMP2, and TIMP2 mRNA expression was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) using the LightCycler® 480 and LightCycler 480 SYBR® Green Master Mix (Hoffman-La Roche Ltd). Gene-specific primers used are detailed in . RPLPO and RPL13a were used as endogenous controls to normalize gene expression, and the average transcript quantity in treated tissue explants was calculated using the delta-delta CT method.Citation24
Table 1 gene-specific primers used for qRT-PcR
Western blotting
Western blotting was performed on whole cell lysates using rabbit monoclonal anti-MT1-MMP antibody (1:1,000 dilution, product number ab51074, Abcam, Cambridge, UK). Proteins were separated by weight by sodium dodecyl sulfate polyacrylamide gel electrophoresis using 4%–12% Bis-Tris gels (Thermo Fisher Scientific), and were transferred onto polyvinylidene fluoride membrane. Following pre-incubation with a blocking solution, membranes were incubated in primary antibody overnight. The membranes were then extensively washed, incubated in horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000 dilution, product number A5045; Sigma Aldrich Co), and were visualized using Pierce Super-Signal® West Dura Extended Duration Substrate (Thermo Fisher Scientific). Relative protein levels were obtained using densitometric quantification within the linear range (Quantity One® software; Bio-Rad Laboratories Inc., Hercules, CA, USA) and were normalized to β-actin.
Gelatin zymography
MMP2 activity was measured in conditioned media from placental explant cultures using gelatin zymography. Samples were separated by electrophoresis (125 V for 90 minutes) on Novex® 10% gelatin zymogram gels (Thermo Fisher Scientific). Gels were incubated in denaturing buffer for 30 minutes, and then in developing buffer overnight at 37°C. Gels were stained in a solution containing 0.3% coomassie blue, after which areas on the gel that had been degraded appeared as clear bands against a dark background. Molecular weights were determined using a standard of conditioned media from HT-1080 human fibrosarcoma cells, which constitutively express MMP2 and MMP9. Gels were scanned with a GS-800 calibrated densitometer (Bio-Rad Laboratories Inc.) and were analyzed using Quantity One software (Bio-Rad Laboratories Inc.).
Enzyme-linked immunosorbent assay
Total TIMP2 protein in conditioned media from tissue explant experiments was measured using a human TIMP2 DuoSet® enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Optical density was read at 450 nm and was corrected with 540 nm on a Synergy™ 2 multimode plate reader (BioTek, Winooski, VT, USA). Levels of secreted TIMP2 were normalized with respect to the total protein content as measured by microplate bicinchoninic assay protein assay kit (Thermo Fisher Scientific).
DNA extraction and methylation analysis of MT1-MMP promoter
Genomic DNA was extracted from tissues using the QiaAMP DNA extraction kit (Qiagen NV, Venlo, the Netherlands) as per manufacturer’s instructions and quantified using a NanoDrop ND-1000 spectrophotometer.
Methylation analysis using the Sequenom™ EpiTyper® MassARRAY platform was undertaken by the Australian Genome Research Facility (AGRF). Primer sets to cover two regions (A and B) of the major CpG island in the MT1-MMP promoter region were designed in-house by AGRF. Region A covered 30 CpG sites and region B covered 38 CpG sites. Primers used for Sequenom analysis are detailed in . Methylation analysis was carried out on bisulfite-converted DNA using 200 ng for each preparation. Methylation levels for each CpG unit were expressed as a percentage, which was calculated from the ratio of mass signals between methylated and nonmethylated DNA in each sample.
Table 2 sequenom™ primers used for MT1-MMP promoter methylation analysis
Chromatin accessibility assay
Chromatin assembly of treated villous explant tissues was carried out using the EpiQuik chromatin accessibility assay kit (Epigentek, Farmingdale, NY, USA). Chromatin was isolated from tissues and treated with a nuclease (Nse) mix. DNA was then isolated and amplified using quantitative PCR and gene-specific primers for MT1-MMP (). Control primers were provided to determine the successful digestion of the chromatin. The fold enrichment (FE) was calculated by the ratio of amplification efficiency of the Nse-treated DNA sample over that of the control sample not treated with nuclease (No-Nse):
Changes in chromatin structure in treated tissues were identified by the degree of Ct shift between digested and undigested samples. DNA in heterochromatin was inaccessible to the nucleases, resulting in insignificant Ct shifts between digested and undigested (control) samples, whereas DNA in euchromatin was accessible to nucleases, resulting in a large Ct shift.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Comparisons between tissues collected from CS and SVD pregnancies were carried out using the Mann–Whitney U test. One-way analysis of variance was used for explant experiments and if significantly different (α=0.05), post hoc multiple comparisons using Dunn’s test were performed for comparison to time-matched controls. Values are presented as mean±standard error of the mean (SEM).
Results
Effect of AZA and LPS treatments on MT1-MMP mRNA and protein
MT1-MMP mRNA was significantly increased in placental explants pretreated with AZA and then stimulated with LPS for 24 hours or 48 hours compared to controls. Significant increase was also observed in explants following LPS stimulation alone for 48 hours (). MT1-MMP protein was significantly increased in placental explants pre-treated with AZA and then stimulated with LPS for 24 hours and 48 hours, and treated with LPS alone for 24 hours ().
Figure 1 MT1-MMP mRNA and protein expression in villous explants.
Notes: (A) qRT-PcR was used to detect MT1-MMP mRNA expression in tissues treated with/without 5 μg/mL LPS with/without prior 5 μM AZA treatment. MT1-MMP mRNA was significantly increased in explants pre-treated with AZA and then cultured with LPS for 24 hours and 48 hours. LPS treatment alone increased MT1-MMP mRNA in explants after 48 hours. (B) Western blotting was used to detect MT1-MMP protein in tissues treated with/without 5 μg/mL LPS with/without prior 5 μM AZA treatment. MT1-MMP protein was significantly increased in placental explants pre-treated with AZA and then stimulated with LPS for 24 hours and 48 hours and in MT1-MMP protein treated with LPS alone for 24 hours. In (A), data are presented as mean fold change in MT1-MMP transcript normalized to RPLPO and RPL13a compared to time-matched controls (±SEM; n=8 in each group), *P<0.05. In (B), data are presented as mean fold change in MT1-MMP protein normalized to β-actin optical density, compared to time-matched controls (±SEM; n=8 in each group); *P<0.05.
Abbreviations: qRT-PcR, quantitative real-time polymerase chain reaction; MT1-MMP, membrane type 1 matrix metalloproteinase; LPS, lipopolysaccharide; AZA, 5-aza-2′-deoxycytidine; RPLPO, large ribosomal protein; RPL13, ribosomal protein type 13; SEM, standard error of the mean; h, hours; n, number in each group.
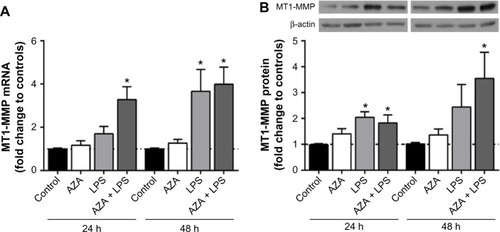
Effect of AZA and LPS treatments on MMP2 mRNA and protein secretion
MMP2 mRNA expression was not altered by AZA or LPS treatment alone or in combination in any tissue (data not shown). MMP2 activity, however, was significantly increased in the culture media from placental explants pre-treated with AZA and subsequently cultured with LPS for 48 hours ().
Figure 2 Secreted MMP2 activity from villous explants.
Notes: Gelatin zymography was used to assess MMP2 activity in conditioned media from tissue explants treated with/without 5 μg/mL LPS with/without prior 5 μM AZA treatment. Pro-MMP2 activity is shown by white bars; activated MMP2 activity is shown by gray bars. a representative zymogram is shown above the graph. levels of the secreted MMPs were normalized with respect to the total protein content and expressed as a percentage of time-matched controls (mean ± SEM; n=8 in each group); *P<0.05.
Abbreviations: MMPs, matrix metalloproteinases; MMP2, MMP type 2; pro-MMP2, latent MMP2; LPS, lipopolysaccharide; AZA, 5-aza-2′-deoxycytidine; SEM, standard error of the mean; h, hours; n, number in each group.
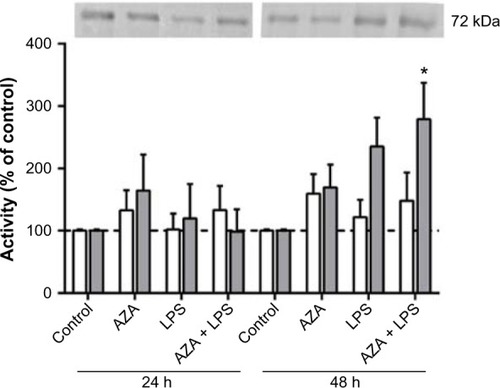
Effect of AZA and LPS treatments on TIMP2 mRNA and protein secretion
There were no significant differences in TIMP2 mRNA expression in relation to any treatment in any tissue (data not shown). TIMP2 protein production was significantly decreased in the culture media from placental explants pre-treated with AZA and subsequently cultured with LPS for 48 hours ().
Figure 3 Secreted TIMP2 protein from villous explants.
Notes: ELISA was used to assess TIMP2 protein in conditioned media from tissue explants treated with/without 5 μg/mL LPS with/without prior 5 μM AZA treatment. levels of secreted TIMP2 were normalized with respect to the total protein content (mean ± SEM; n=8 in each group); *P<0.05.
Abbreviations: TIMP2, tissue inhibitor of metalloproteinase type 2; ELISA, enzyme-linked immunosorbent assay; LPS, lipopolysaccharide; AZA, 5-aza-2′-deoxycytidine; SEM, standard error of the mean; h, hours; n, number in each group.
Methylation analysis of MT1-MMP promoter region
MT1-MMP promoter was generally hypomethylated across all tissue explants (ranging 4%–6% on average). In addition, no changes in methylation were observed in cultured tissue explants in response to any treatment ().
Figure 4 MT1-MMP promoter methylation in villous explants.
Notes: Sequenom™ EpiTyper® MassaRRaY was used to measure methylated cytosine residues (5-mC) within the MT1-MMP promoter in tissues treated with 5 μg/mL LPS with/without prior 5 μM AZA treatment. % of 5-Methylcytosine (%5-mC) was calculated from the ratio of mass signals between methylated and nonmethylated DNA in each sample. Data are presented as mean %5-mC across all CpGs ± SEM (n=8).
Abbreviations: MT1-MMP, membrane type 1 matrix metalloproteinase; LPS, lipopolysaccharide; AZA, 5-aza-2′-deoxycytidine; SEM, standard error of the mean; h, hours; n, number in each group.
Chromatin accessibility of treated tissue explants
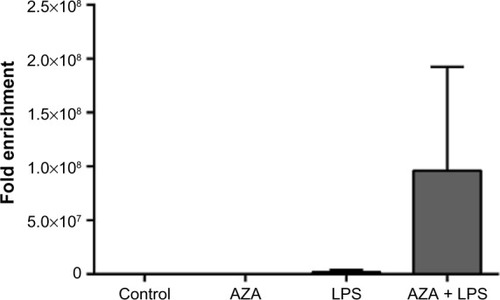
Changes in chromatin structure were most evident in villous tissue explants treated with AZA and subsequently exposed to LPS, as shown by the large Ct shift between digested and undigested samples (). Due to sample constraints, chromatin accessibility was only assessed in two villous sample sets, resulting in large variation. While no significant differences were noted, a relative increase was seen in AZA + LPS treatment groups.
Figure 5 Chromatin accessibility assay of treated villous explants.
Notes: Chromatin structures of treated villous explants were determined by specific nucleases. DNA in heterochromatin was inaccessible to the nucleases, resulting in insignificant ct shifts between digested and undigested samples, whereas DNA in euchromatin was accessible to nucleases, resulting in a large ct shift shown as fold enrichment. Treatment with combined AZA and LPS resulted in large fold enrichment, signifying the DNA is more open than in the control, AZA alone, or LPS alone treatments. Data are presented as the ratio of amplification efficiency of the Nse-treated DNA sample over that of the No-Nse (control) sample (mean fold enrichment) ± SEM (n=2).
Abbreviations: LPS, lipopolysaccharide; AZA, 5-aza-2′-deoxycytidine; Nse, nuclease; SEM, standard error of the mean; n, number in each group; No-Nse, control samples not treated with nuclease.
Discussion
The present study investigated the response of MT1-MMP, MMP2, and TIMP2 to methylation inhibiting (AZA) and immunomodulating (LPS) treatments in term human gestational tissue explants. We report an increase in MT1-MMP mRNA and protein in response to AZA treatment followed by LPS stimulation. In addition, we examined promoter-specific methylation of the MT1-MMP gene.
A previous study reported that MT1-MMP mRNA was not increased by LPS treatment in amnion-chorion fetal membrane samples.Citation12 We observed an increase in MT1-MMP transcription and protein treatment with LPS alone for 24 hours and 48 hours, respectively. When AZA and LPS treatments were combined, we observed a significant increase in MT1-MMP mRNA at 24 hours and 48 hours, and in MT1-MMP protein at 48 hours.
TIMP2 protein was decreased in tissues treated with AZA and subsequently stimulated with LPS. Furthermore, although not significant, there is an apparent decrease in TIMP2 protein secretion in tissues treated with LPS only at both time points. This observation is supported by previous studies, which reported a decrease in TIMP2 protein secretion by gestational membranes treated with LPS.Citation25,Citation26 Furthermore, decreased TIMP2 expression has previously been correlated to promoter hypermethylation.Citation27
It was interesting to note that although AZA treatment had no effect basally, when combined with LPS, the two treatments appeared to act synergistically to increase MT1-MMP mRNA and protein production. AZA is a methylation-inhibiting agent that is incorporated into the DNA as a cytidine analog and prevents methylation by irreversibly binding to DNA methyltransferases, leading to passive hypomethylation over successive rounds of cell division.Citation28 Interrogation of the MT1-MMP promoter revealed hypomethylation in all term gestational tissues. Furthermore, no change in MT1-MMP promoter-specific methylation was observed in explant tissues in response to culture treatments. Thus, as MT1-MMP was hypomethylated in the placenta, any AZA-induced changes in methylation were negligible.
It is becoming increasingly apparent that AZA has more far-reaching effects than hypomethylation alone; evidence suggests that AZA is capable of inducing reorganization of the chromatin structure.Citation29,Citation30 In this way, AZA may be enhancing the availability of transcriptional regulatory elements in the MT1-MMP promoter, or directly interacting with the transcription factors themselves. Preliminary analysis of the chromatin structure of treated explants was assessed using a commercially available kit, which determines the extent of chromatin accessibility to exogenous nucleases. We observed that, for this study, villous explants treated with AZA and LPS had a more open chromatin structure compared to controls, AZA treatments, or LPS treatments alone. This result was represented by a larger shift in qRT-PCR Ct values, calculated as fold enrichment between digested and undigested samples.
A major limitation of this experiment was the low sample number, due to sample constraints. Although significant differences were not noted, the apparent increase observed between treatment groups would be clarified by an increase in sample number. AZA has been shown to reactivate gene expression independent of DNA methylation.Citation31,Citation32 AZA can act as an inhibitor of histone methylation, which may account for the reexpression of unmethylated genes.Citation33 Both hypomethylation of CpG islands and low levels of histone H3 lysine-27 trimethylation are features of transcriptionally active MT1-MMP in cancer cell lines.Citation34
Co-treatment of human colorectal cancer cell lines with combined AZA and pro-inflammatory cytokines IL-1, IL-6, or TNF-α induces MMP2 and MMP9 mRNA to a greater extent than cytokine treatment alone. This cooperative regulation is due to functional interactions between AZA and transcription factors c-Jun and C/EBP.Citation35 MT1-MMP contains a number of regulatory binding sites in its promoter, including several for C/EBP-β; thus, the direct interaction between AZA and transcription factors is a potential mechanism of MT1-MMP mRNA regulation in the placenta and fetal membranes in the presence of inflammation.Citation36 Although we did not observe increased MMP2 mRNA with combined AZA and LPS treatments, MT1-MMP induction resulted in a significant increase in MMP2 activity in placenta explants after 48 hours.
AZA could also be increasing the expression of toll-like receptors (TLRs) in these tissues, leading to enhanced LPS stimulation of MT1-MMP. TLRs are innate immune receptors that are the principle sensors of bacterial pathogens, and are highly expressed by gestational tissues.Citation37 TLR type 4 (TLR4) mediates LPS-induced immune responses, and is methylated in human intestinal epithelial cell lines and mice embryonic stem cells.Citation38,Citation39 Treatment with AZA restores TLR4 expression in these cells, thereby enhancing LPS responsiveness. In one study, TLR4 reactivation was augmented by combined AZA and TSA (a histone deacetylase inhibitor) treatments, suggesting cooperative control of TLR4 by DNA methylation and histone modifications.Citation38
pPROM is associated with increased MMP2 and MT1-MMP concentrations, and decreased TIMP2 protein in fetal membranes and amniotic fluid.Citation13,Citation16,Citation18 In the current study, we report significantly increased MT1-MMP mRNA and protein and MMP2 activity with an associated decrease in TIMP2 protein in placental explants treated with combined AZA and LPS treatments. MT1-MMP and TIMP2 are major mediators of MMP2 activity, and while high concentrations of MT1-MMP facilitate the activation of MMP2, low TIMP2 concentration would result in less inhibition of the activated enzymes.Citation40 Increased MMP levels in the presence of unchanged or decreased TIMP2 would shift the balance in favour of gelatinase activity, resulting in ECM degradation.
Conclusion
Our results imply that promoter methylation is not directly involved in the regulation of MT1-MMP in human placenta. Rather, alterations in chromatin structure instigated by pharmacologic agents such as AZA could expose regulatory regions in the MT1-MMP promoter, allowing for the binding of transcription factors. Furthermore, recent evidence demonstrates the direct interaction between AZA and transcriptional factors in the presence of inflammatory mediators leads to increased MMP transcription. Together, this evidence supports a mechanistic link between epigenetic modifications and inflammation in the control of MMP expression. Enhanced MT1-MMP in gestational tissues alongside an associated increase in activation of MMP2 would alter the balance of MMPs and TIMPs, favouring a proteolytic state and thereby making the fetal membranes vulnerable to ECM degradation and subsequent membrane rupture.
Acknowledgments
We thank staff and patients at Auckland City Hospital for donation and collection of tissue samples. We also thank Dr Devaki De Silva for technical input.
Disclosure
The authors report no conflicts of interest in this work.
References
- ReichRThompsonEWIwamotoYEffects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cellsCancer Res19884812330733122836052
- WeissAGoldmanSShalevEThe matrix metalloproteinases (MMPS) in the decidua and fetal membranesFront Biosci20071264965917127325
- NagaseHWoessnerJFJrMatrix metalloproteinasesJ Biol Chem199927431214912149410419448
- VisseRNagaseHMatrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistryCirc Res200392882783912730128
- NagaseHVisseRMurphyGStructure and function of matrix metalloproteinases and TIMPsCardiovasc Res200669356257316405877
- WoessnerJFJrMatrix metalloproteinases and their inhibitors in connective tissue remodelingFASEB J199158214521541850705
- MurphyGCockettMIWardRVDochertyAJMatrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP)Biochem J1991277Pt 12772791649600
- MurphyGWardRHembryRMReynoldsJJKuhnKTryggvasonKCharacterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenaseBiochem J198925824634722539808
- HippsDSHembryRMDochertyAJReynoldsJJMurphyGPurification and characterization of human 72-kDa gelatinase (type IV collagenase). Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblastsBiol Chem Hoppe Seyler199137242872961647782
- WoessnerJFJrThe family of matrix metalloproteinasesAnn N Y Acad Sci199473211217978784
- NelsonARFingletonBRothenbergMLMatrisianLMMatrix metalloproteinases: biologic activity and clinical implicationsJ Clin Oncol20001851135114910694567
- FortunatoSJMenonRLombardiSJExpression of a progelatinase activator (MT1-MMP) in human fetal membranesAm J Reprod Immunol19983953163229602249
- AthaydeNRomeroRGomezRMatrix metalloproteinases-9 in preterm and term human parturitionJ Matern Fetal Med19998521321910475503
- XuPAlfaidyNChallisJRExpression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term laborJ Clin Endocrinol Metab20028731353136111889208
- OtaAYonemotoHSomeyaAItohSKinoshitaKNagaokaIChanges in matrix metalloproteinase 2 activities in amniochorions during premature rupture of membranesJ Soc Gynecol Investig2006138592597
- ChelbiSTMondonFJammesHExpressional and epigenetic alterations of placental serine protease inhibitors: SERPINA3 is a potential marker of preeclampsiaHypertension2007491768317088445
- FortunatoSJMenonRLombardiSJMMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane ruptureJ Perinat Med199927536236810642956
- MaymonERomeroRPacoraPA role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infectionJ Perinat Med200129430831611565199
- ProbstAVDunleavyEAlmouzniGEpigenetic inheritance during the cell cycleNat Rev Mol Cell Biol200910319220619234478
- ChernovAVSounniNERemacleAGStronginAYEpigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cellsJ Biol Chem200928419127271273419286653
- MitchellMDUnique suppression of prostaglandin H synthase-2 expression by inhibition of histone deacetylation, specifically in human amnion but not adjacent choriodeciduaMol Biol Cell200617154955316251350
- SatoTAMitchellMDMolecular inhibition of histone deacetylation results in major enhancement of the production of IL-1beta in response to LPSAm J Physiol Endocrinol Metab20062903E490E49316234266
- SimpsonKLKeelanJAMitchellMDLabor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodeciduaJ Clin Endocrinol Metab19988312433243379851773
- LivakKJSchmittgenTDAnalysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) MethodMethods200125440240811846609
- FortunatoSJMenonRLombardiSJAmniochorion gelatinase-gelatinase inhibitor imbalance in vitro: a possible infectious pathway to ruptureObstet Gynecol200095224024410674587
- SkinnerSJCamposGALigginsGCCollagen content of human amniotic membranes: effect of gestation length and premature ruptureObstet Gynecol19815744874897243099
- PulukuriSMPatibandlaSPatelJEstesNRaoJSEpigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumorsOncogene200726365229523717325663
- EggerGLiangGAparicioAJonesPAEpigenetics in human disease and prospects for epigenetic therapyNature2004429699045746315164071
- TerrySYVallisKARelationship between chromatin structure and sensitivity to molecularly targeted auger electron radiation therapyInt J Radiat Oncol Biol Phys20128341298130522336201
- NguyenCTWeisenbergerDJVelicescuMHistone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2′-deoxycytidineCancer Res200262226456646112438235
- ZhengZLiLLiuX5-AZA-2′-deoxycytidine reactivates gene expression via degradation of pRb pocket proteinsFASEB J201226144945921990374
- SchmelzKSattlerNWagnerMLübbertMDörkenBTammIInduction of gene expression by 5-aza-2′-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanismsLeukemia200519110311115510208
- ZhuWGDaiZDingHIncreased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cellsOncogene200120537787779611753657
- ChernovAVSounniNERemacleAGStronginAYEpigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cellsJ Biol Chem200928419127271273419286653
- CouillardJEstèvePOPradhanSSt-PierreY5-AZA-2′-deoxycytidine and interleukin-1 cooperate to regulate matrix metalloproteinase-3 gene expressionInt J Cancer201112992083209221170958
- Hernandez-BarrantesSBernardoMTothMFridmanRRegulation of membrane type-matrix metalloproteinasesSemin Cancer Biol200212213113812027585
- AdamsKMLucasJKapurRPStevensAMLPS induces translocation of TLR4 in amniotic epitheliumPlacenta2007285–647748117055575
- ZampetakiAXiaoQZengLHuYXuQTLR4 expression in mouse embryonic stem cells and in stem cell-derived vascular cells is regulated by epigenetic modificationsBiochem Biophys Res Commun20063471899916814255
- TakahashiKSugiYHosonoAKaminogawaSEpigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasisJ Immunol2009183106522652919846881
- BernardoMMFridmanRTIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMPBiochem J2003374Pt 373974512755684
