Abstract
Purpose
This study aimed (1) to analyze patients’ perceived shared decision-making (SDM) experiences over 4 weeks between patients participating or not in multidisciplinary tumor conferences (MTCs) and (2) to analyze the association of patients’ active participation in and organizational variables of MTCs with patients’ perceived SDM experience directly after MTC.
Patients and Methods
From the N=317 patients, this observational study included patient surveys, observations, and audio transcripts from MTCs with (N=82) and without (N=145) patient participation in six breast and gynecologic cancer centers. We performed t tests for within- and between-group comparisons and linear regression with “patients’ perceived SDM experiences in MTC” as the dependent variable.
Results
Patients’ perceived SDM experiences increased at 4 weeks after MTC (p<0.001) with lower perceived SDM experiences for participating versus nonparticipating patients (p<0.001). Linear regression showed that the organizational variable “round table seating arrangement” was significantly associated with higher perceived SDM experiences compared with a theater or U-shape arrangement (beta=−0.38, p=0.043; beta=−0.69, p=0.010) directly after MTC.
Conclusion
Results provide first insights into patients’ perceived SDM experiences in MTCs. SDM in MTCs is associated with organizational variables of MTCs. A round table seating arrangement in MTCs with patient participation seems important for patients' perceived SDM experiences. The relatively low perceived SDM experiences of participating patients directly after MTC indicates room for improvement, eg concerning patient-centered communication.
Introduction
Shared decision-making (SDM), an important component of patient-centered health care,Citation1–3 is acknowledged as a key element of high-quality health care internationally and is associated with positive patient outcomes such as knowledge, compliance, and health outcomes.Citation4–7 In oncology, it is particularly important to provide cancer patients with access to health-care services and involve them with their individual preferences in treatment planning over the course of their cancer journey.Citation8 Many treatment decisions in oncology affect patients’ (and their relatives’) quality of life.Citation9,Citation10
For the past two decades, clinical treatment planning in oncology has been oriented toward medical guidelines.Citation11–13 On this basis, health-care providers (HCP) such as medical oncologists, radiation oncologists, surgeons, pathologists, and many more in specialized fields discuss evidence-based recommendations for treatment of patients with cancer in multidisciplinary tumor conferences (MTCs; The frames “multidisciplinary tumor conferences” or “tumor boards” can be seen as synonyms of “multidisciplinary team meetings” which are used in other international studies).Citation14,Citation15 Although important research on the degrees of multidisciplinarity in MTCs is still ongoing,Citation15,Citation16 MTCs are related to increased diagnostic and treatment options and therefore have been implemented in oncology in many countries worldwide.Citation14,Citation17–22
On the basis of the outlined developments of SDM and multidisciplinary health care, some authors have investigated the extent of patient-centeredness of MTCs.Citation23–25 In this context, studies demonstrated that the consideration of patient preferences differed between MTCs and that the discussion often strongly focusses on biomedical information.Citation26–28 As shown by international research, consideration of patient preferences in MTCs can lead to better patient outcomes and better adherence to MTC recommendations.Citation20–22
To involve patients with cancer and their preferences in treatment planning, patient participation in MTCs has been discussed internationally.Citation7,Citation29–32 Some German breast and gynecologic cancer centers invite patients to participate in the discussion of their own case in the MTC. Research on this has shown that this practice is a rare but over the last few years regular healthcare reality, with 5–7% of patients with breast cancer participating in their own case discussion.Citation33–35 There is little evidence on how it is implemented and whether it should be recommended for larger scale implementation.Citation36
Since there are only a few existing studies on patient participation in MTCs, SDM in MTC with patients has hardly been investigated.Citation37–41 First studies indicated that HCP perceives and experiences SDM with patients in MTCs as challenging.Citation37,Citation41,Citation42 In that interview study, the HCP reported that some process steps of SDM can be implemented in limited form and under certain conditions, eg, lack of time, comprehension problems, patient overstrain, in MTCs with patient participation. From the perspective of the HCP experience, patients can potentially ask questions and contribute individual additional information and their preferences. Our own studies showed that MTC participating patients were mostly proposed one treatment option, which in some cases was framed as a final decision and not as a recommendation.Citation40 Furthermore, patients who had a need for psychological support and who were accompanied during their MTC participation asked more questions.Citation39
However, in terms of SDM in MTCs, two main gaps remain in research, which will be addressed in this study. (1) Until now, previous studies have indicated that patients’ active participation in MTCs differs between hospitals and is associated with patients’ need for psychosocial support and whether they were accompanied by a relative.Citation39 However, whether patients’ active participation in MTCs is associated with higher perceived SDM experiences is unknown. (2) Second, in our previous research, we found different implementations of patient participation in MTC due to the variations in the “environment” (in line with the “Conceptual Framework for Patient-Professional Communication: An Application to the Cancer Context”Citation43) of the MTCs, such as seating arrangement, duration, number of cases, technical support, and presence of the core team.Citation36 These first results draw attention to the importance of differences in health care between organizations, because differences in the implementation of patient participation in MTCs and the practical organization of MTCs (eg, seating arrangement) might reflect differences in the organizational behavior and factors of the cancer center. Until now, it has been unclear which environment and implementation are most conducive for patient centeredness and optimizing patients’ SDM experiences in MTCs.
To close these two research gaps, we aim to analyze and compare patients’ perceived SDM experiences over 4 weeks between MTC-participating and MTC-nonparticipating patients. Furthermore, we analyze the associations between patients’ active participation in MTCs and the MTC environment (organizational variables) with patients’ perceived SDM experiences directly after MTC.
Materials and Methods
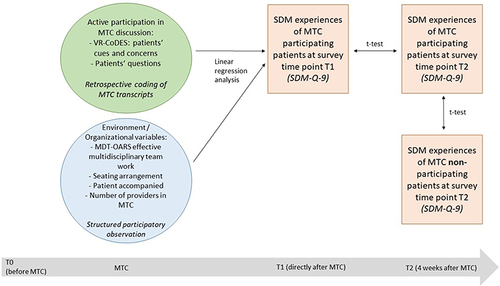
Data collection and data analysis were performed using the approach of mixed-methods research in health services research.Citation44 Here, the triangulation technique was put into practice:Citation45 quantitative patient survey data, qualitative passive participatory observation in MTCs, and qualitative coding of MTC transcripts are combined for each individual patient. The methods section is based on the “Consolidated criteria for reporting qualitative research (COREQ)” a 32-item checklist for interviews and focus groups.Citation46
Study Design and Sample
The presented data are part of a larger multicenter, non-interventional mixed-methods study on patient participation in MTC conducted in breast and gynecologic cancer centers in Germany’s most populous state of North Rhine–Westphalia. The Ethics Committee of the Medical Faculty of the University of Cologne approved this study, and funding was provided by German Cancer Aid. Further details on the PINTU (Patient participation in multidisciplinary tumor conferences in breast cancer care – an exploratory study) study design are reported elsewhere.Citation47
N=6 breast and gynecologic cancer centers participated in the study. In three centers, patients are regularly invited to MTCs and few do participate, and in the other three centers, patients are not regularly invited to MTCs and do not participate. Cancer centers were selected following purposeful sampling criteria,Citation48 varying the size of the center (case volume) and the teaching status (teaching hospital vs nonteaching hospital), because center structures can affect the organization of MTCs.Citation49
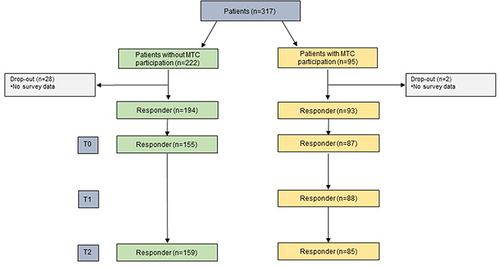
The patient sample consisted of N=317 patients with breast and/or gynecologic cancer (see “Descriptive results”). HCP consecutively recruited patients who were diagnosed with breast or gynecologic cancer (ICD-10 C50–C58 and D05–D07), were at least 18 years old, spoke German, and were cognitively able to complete the questionnaires. Eligible patients provided written informed consent.
Data Collection
Data were collected between November 2018 and February 2020.
Patient Survey
Patients received questionnaires at three points in time. During the recruitment consultation, the first survey (T0) was handed out if the patient had provided written informed consent, with the instruction to fill it out before the MTC (T0). Directly after the MTC, the second survey (T1) was handed out by the research team to patients who participated in the MTC. Four weeks after the MTC, the last survey (T2) was sent as a postal survey to all patients. A maximum of three postal reminders for T1 and T2 according to Dillman’s total design method were sent.Citation50
Active Patient Participation in MTC
We recorded, transcribed verbatim, and pseudonymized the audio data of all case discussions. Transcripts built the basis for coding of emotional expressions and questions during MTCs with patients’ participation using the Verona Coding Definitions of Emotional Sequences (VR-CoDES).Citation51,Citation52
MTC Environment
A group of researchers (sociologist, psychologist, health services researcher) conducted passive participatory observations, two of them simultaneously during each MTC. Field notes were taken using the Multidisciplinary Team-Observational Assessment Rating Scale (MDT-OARS),Citation53 which made it possible to quantify aspects of our qualitative observations into measurable quantitative variables.
Measures
Patient Survey
The questionnaires consisted of validated instruments and self-developed items reported elsewhereCitation47 and were pretested in three cognitive interviews based on established methods.Citation54 In this study, we included data from T0 (patient characteristics), T1 (SDM-Q-9 for linear regression), and T2 (SDM-Q-9 for group differences) (see ). SDM was measured using the German version of the validated SDM-Q-9.Citation55 It consists of nine statements related to decision-making in a medical consultation, eg, that a decision needs to be made, (dis-)advantages of treatment options were explained or that an agreement on further procedures was reached. To measure SDM during the MTC, all nine items were adjusted. Instead of “the doctor” the phrase “the MTC team” was used in agreement with the SDM-Q-9 authors: “Questions about decision-making in the tumor conference: The team in the tumor conference has explicitly told me that…”. All items must be answered on a 6-point scale ranging from 1=completely disagree to 6=completely agree. A relative sum score ranging between 1 and 6 was calculated (9–54 points divided by nine items) for descriptive and multivariate calculations. We based the selection of independent variables on the literature and on an exploratory approach.
Active Patient Participation in MTC
Active participation was operationalized through the number of questions patients and relatives asked during the MTCCitation56 and the number of unpleasant feelings (cues) and concerns verbally indicated from patients and relatives. Cues and concerns were coded with the help of VR-CoDES.Citation51,Citation52 While previous data were often collected from dyadic conversations, in this study, we coded the cues and concerns from patients and relatives with a whole HCP team.
MTC Environment
Structural and process variables were operationalized using the established MDT-OARS.Citation53 On this observational assessment tool, the characteristics of an effective MDT and dimensions of patient-centered care can be rated on a 4-point scale ranging from 1=very poor to 4=very good or for some items between 0=“no” and 1=“yes.” The main categories are Team (2–22 points), Infrastructure (2–8 points), Organization and Logistics (6–22 points), and Clinical Decision-Making (1–7 points). Furthermore, we expanded the MDT-OARS to add a fifth dimension of “Communication” (2–7 points) as MTCs with patient participation were observed. The detailed observational assessment tool is shown in the “Appendix 1” (own illustration with adaptations). MDT-OARS makes it possible to quantify aspects of our qualitative observations (eg, attendance of different health-care providers, leadership, patient notes and documentation, team-working) into measurable quantitative variables. Finally, for each MTC, we protocolled the structural variables including seating arrangement, accompaniment of the patient, and the presence of caregivers. Since these variables are clustered information for each MTC, we merged the information to the individual patient data level. See for all measures and analyses used.
Data Analysis
Patient Survey
Regarding survey data, we used descriptive statistics for sample description, t test for between-group comparisons regarding SDM experiences 4 weeks after MTC (T2 with and without MTC participation), and within-group comparisons of SDM experiences for participating patients between T1 and T2. All SDM scores were normally distributed. After testing the preconditions (eg, multicollinearity), we performed a linear regression model with the dependent variable “SDM experiences in MTC” (from T1) and independent variables of active patient participation using the coded cues, concerns, and questions using the VR-CoDES, as well as the MTC environment variables using participatory observation data from the MDT-OARS. IBM SPSS version 27 was used for all analyses. Missing data were handled using pairwise deletion.
Active Patient Participation in MTC
Regarding VR-CoDES and the number of questions raised by patients, four researchers coded the cues, concerns, and questions of patients and relatives independently in two teams. For validation, the identified codes were discussed and consented in regular research meetings within the team. Interrater reliability concerning VR-CoDES was based on Cohen’s Kappa calculation for a random sample of 10 case discussions. The agreement was substantial (Cohen’s Kappa=0.74).
MTC Environment
Two researchers simultaneously and independently documented and quantified the structural and process variables. When necessary (eg, for MDT-OARS ratings of the MTC), the two quantifications were first summarized by calculating the mean and then merged to the individual patient data level. Interrater reliability concerning MDT-OARS ratings was based on Cohen’s Kappa calculation for a random sample of 10 case discussions. The agreement was substantial (Cohen’s Kappa=0.83).
Results
Descriptive Analysis
Patient Survey
The sample for descriptive analysis consisted of 317 patients (average age=60 years), of whom 95 participated in the MTC (see for details). shows the relevant patient characteristics for both groups. Regarding the regression analysis for patients who participated in the MTC, complete data (observations and surveys) were available for 82 of 95 patients.
Table 1 Characteristics of Survey Sample at T0 (n=242, n=82 with Participation, n=155 Without Participation)
Active Patient Participation in MTC
In the 82 case discussions with patients, 230 cues and concerns indicated an active patient participation, with a median of 2 cues and concerns per patient (range=0–24). Patients and their relatives asked 607 questions, with a median of 6 questions asked per patient (range=0–26).
MTC Environment
MTCs with patient participation took place in theater style (6%), in a U-shape (21%), or at a round table (73%). Nearly half of the patients were accompanied by a relative. The average case discussion with patient participation was 7:26 min (range, 1:25–20:54 min). The MDT-OARS score was high overall, with a mean of 54 points and a median of 56 points out of 66 possible points (range, 39–64 points).
Within- and Between-Subgroup Differences
Patients’ perceived SDM experiences increased from 3.1 immediately after MTC (T1) to 3.5 at 4 weeks after MTC (T2; p < 0.001; d=0.32) for MTC-participating patients (see ) on a relative sum scale ranging from 1 to 6. At 4 weeks after MTC, MTC-participating patients showed lower perceived SDM experiences than nonparticipating patients did (T2; 3.5 versus 4.5; p<0.001; d=0.79) (see ) on a relative sum scale ranging from 1 to 6.
Table 2 Results of t Test with Perceived SDM Experiences in Patients Participating in the MTC Over the Course of 4 Weeks After MTC (n=82)
Table 3 Results of t Test with Perceived SDM Experiences Between MTC-Participating and MTC-Nonparticipating Patient Groups 4 Weeks After MTC
Regression Analysis
The results of the linear regression analysis showed that patients had significantly lower perceived SDM experiences when the MTC took place in a theater or U-shape seating arrangement as compared with a round table arrangement (beta=−0.38, p=0.043; beta=−0.69, p=0.010, respectively). Patients showed higher perceived SDM experiences when more HCP participated in the MTC, but no significant association was found. In addition, although higher numbers of expressed emotions and questions were associated with higher perceived SDM experiences, no significant association was observed. shows the model with all variables.
Table 4 Linear Regression Model with Patients’ Perceived SDM Experiences at T1 as the Dependent Variable (n=82)
Discussion
This study aimed to analyze patients’ perceived SDM experiences over 4 weeks after MTC between participating and nonparticipating patients and to analyze the associations between patients’ active participation in MTCs and environmental (organizational) variables of MTCs with patients’ perceived SDM experiences. Results of a between-group t test showed that perceived SDM experiences 4 weeks after MTC were significantly higher in patients who did not participate in the MTC. A within-group t test showed that perceived SDM experiences of MTC-participating patients significantly increased from immediately after the MTC to 4 weeks after the MTC. Both results seem contra-intuitive. Linear regression analysis with the dependent variable “Perceived SDM experiences in MTC” including independent variables of active patient participation and MTC environment (organizational variables) demonstrated significant associations between the seating arrangement of the MTC with patients’ perceived SDM experiences. Thus, this study continues important research on patient participation and SDM in medical consultations,Citation57,Citation58 especially by focusing on patients who raise questionsCitation56,Citation59 and who express emotions during consultations.Citation59–61
These results must be discussed in terms of current international research on SDM and MTCs. In our previous study, we found that in the reality of the clinical setting, SDM in MTCs with patients is challenging.Citation37 The present study now adds important knowledge on and from the patients’ perspective in terms of patient-reported experience measures.Citation62,Citation63 We found that participation during MTCs is not associated with higher perceived SDM experiences. Instead, the MTC environment is partly associated with perceived SDM experiences. Furthermore, compared with participating patients, nonparticipating patients showed higher perceived SDM experiences 4 weeks after MTC related to the decision that was discussed in MTC. It is important to keep in mind that all patients have a consultation with HCP before and after the MTC, where SDM might have taken place, and MTC might just be one part of the treatment process. Differences could be explained by different patient expectations of MTC participation and whether their expectations were fulfilled. Furthermore, the need for participation might also be a factor in the differing perceived SDM experiences. If one patient has a high need for MTC participation, he/she might be disappointed after such participation.
Differences in the MTC environment (eg, seating arrangement) might reflect differences in the organizational behavior of the cancer center. Seating arrangements can be the expression of a certain patient-centered behavior in and between the centers. Statistically, because the seating arrangement varies among organizations (cancer centers), it is partly a proxy variable for cancer centers, and this could be merged with individual patient data with the help of mixed-methods triangulation. By interpreting the results of the seating arrangement, we can argue that there is preliminary evidence that some seating arrangements foster greater SDM in MTCs,Citation64 possibly related to different organizational behaviors. However, larger studies and multilevel analyses are needed to confirm this. This finding makes it possible to carefully understand the interactions between the organizational meso-level and patient micro-level, understand differences between the organizations, and understand more about structures and processes in these organizations (cancer centers). The perspective of organizational behavior helped to describe how these organizations (cancer centers) act regarding MTCs and also to understand processes of oncological healthcare better (here the perceived SDM experiences), which might be connected with a certain organizational behavior.Citation65,Citation66 These structural and process variables were made measurable and taken into account with the integration of VR-CoDES and MDT-OARS. Here, our study is in line with and extends findings from important research on patient-centered communication in (dyadic) medical encounters.Citation58,Citation67
Although the seating arrangement is a prerequisite for higher perceived SDM experiences, no causal effect can be postulated. Involving patients at a round table might result in communication with (better) eye contact and give patients the feeling to be taken seriously. More raised cues, concerns, and questions are associated with higher perceived SDM experiences, which is in line with the research on VR-CoDES. However, this association was not significant in the linear regression model, which is not surprising, given our previous research showed that participation in the MTC is not feasible for all patients from the HCP perspective because of clinical reality (eg time pressure).Citation41 Furthermore, based on the results from our interview study with HCP, patients’ emotional situations can be only partly met during MTCs.Citation42 Combined with these results, there is still a great barrier to organizing MTCs and communicating in a patient-centered manner to fulfill SDM. This stands in line with previous research on barriers on SDM implementation.Citation68
Finally, the unexpected between-group results of higher perceived SDM experiences for nonparticipating patients indicate the need to account for the difference between (1) a solely guideline-based recommendation in MTC (standardization) and (2) actual decision-making with patients that considers their preferences, which takes place after the MTC (individualization). Before, during, and after MTCs, HCP faces the complex task of transferring evidence-based recommendations to individual patients in the form of personalized medicine and customized psychosocial, social, and cultural characteristics.Citation69 From our findings, this seems to be important, as we still have no knowledge on patients’ expectations concerning SDM in MTCs. These expectations contain preferences for participation in treatment decisions but also general expectations on the structure and processes on MTCs.
Limitations and Strengths
As a limitation of SDM research, it must be noted that we did not obtain data on consultations occurring after the MTC or consultations from nonparticipating patients with HCP (no longitudinal decision-making processes). Moreover, we do not know which expectations concerning SDM in MTCs participating and nonparticipating patients have, and we were unable to control for this in our regression model. Although one could argue that “active participation” is not fully operationalized with patient cues, concerns, and questions, the longstanding development of and research on VR-CoDES show the importance of our operationalization. We did not include patient characteristics in the regression model; our sample consisted of women with a solid tumor, and our results and recommendations are therefore related only to this patient group. This study has a multilevel data structure of patients clustered in centers, but the numbers of centers and patients were not sufficient for multilevel modeling. Instead, organizational-level variables were merged with the individual patient data level, which could lead to an overestimation of these variables in the regression model (disaggregation effect). Regarding sample size, 82 patients seem to be an objectively low case number for multivariate statistics, resulting in a small power.
Nevertheless, patient participation in MTCs is rare. Thus, one strength of this study is that, to our knowledge, it includes the largest sample of patients participating in MTC. Furthermore, this is one of the first studies analyzing MTC patient participation and SDM. We were able to perform a mixed-methods analysis with an individual data level matching of survey data, observation data, and retrospective coding of MTC transcripts of the same patients. The drop-out in survey data is low. Within each of these methodologic fields, we used common statistical analysis; extended common forms of analysis, as VR-CoDES was used not for dyadic but for multipersonal analysis in MTCs with patients; and extended the statistical analysis, including the adaption of MDT-OARS to MTCs with patient participation. The results represent an example of the differences between health-care organizations that can affect patient outcomes.
Implications
Our results provide the first insights into patients’ SDM experiences in MTCs. MTC environment variables were associated with the SDM experiences of patients in MTCs. Nevertheless, we still do not have a clear picture of SDM in MTCs with patients. Therefore, in terms of research implications, we must consider other study designs: on one hand, it is important to gain a deeper understanding of patients’ experiences in MTCs, and a qualitative interview study seems helpful. On other hand, there are questions regarding the influencing factors and context that can be answered only with a Phase III intervention study,Citation70 which does not seem possible at the moment. In general, future studies should also investigate whether patient participation in MTCs occurs in other oncologic entities in Germany or other countries with other patient groups such as male patients with cancer or those with nonsolid tumors. Concerning the important research on patient centeredness in MTCs with patient participation, two main topics seem particularly important for this and future projects. First, knowledge on patients’ expectations concerning SDM in MTCs from MTC-participating patients must be increased. It might be possible that SDM expectations are associated with the patients’ decision to (non)participate in the MTC. Second, additional analysis is needed on specific communication in MTCs, especially regarding elements of patient-centered communication in MTCs (eg, the type of questions HCP asks). From the perspective of organizational health services research, future research and analysis should take the clustered data structure into account when analyzing MTC outcomes. Furthermore, knowledge on the possible associations between organizational-level characteristics and MTC outcomes seems important.
SDM in MTCs is challenging. As practical implications it is important to provide patients with information on the purpose and processes of MTCs in order to regulate their experiences and promote patient-centered communication in MTCs to HCP. Furthermore, this work highlighted that a round table with possible eye contact might help start a discussion between patients, caregivers, and HCP. If patients do express cues, concerns, or ask questions, they might experience greater SDM. To support patients in potentially stressful situations in the MTC, HCP knowledge about patient-centered communication is strongly recommended. Finally, if patients do not wish to participate in an MTC, there might be alternative ways to include patient preferences in the MTC discussion, such as the standardized participation of a breast care nurse or a comprehensive documentation of alternative options during MTC for the discussion with the patients after MTC.
Conclusion
This study has helped to fill the research gap on SDM experiences from patients who participated in the discussion of their own case in an MTC. Active participation in the MTC discussion does not automatically mean that patients with breast and gynecologic cancer experience greater SDM. Instead, characteristics of organizing the MTC (eg, seating arrangement) are associated with patients’ perceived SDM experiences in MTC. However, until now, providing recommendations for organizations regarding patient-centered MTCs and their implementation has been difficult. Future studies and practical recommendations should take this into account.
Data Sharing Statement
Data and all other materials for this study are kept at the Center for Health Communication and Health Services Research, University of Bonn, University Hospital Bonn, Germany. The datasets generated and analyzed during the current study are not publicly available due to terms of written informed consent to which the participants agreed but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of the Medical Faculty of the University of Cologne, Germany. Written informed consent was obtained from all individual participants included in the study.
Trial Registration
German Clinical Trials Register (DRKS), DRKS00012552, registered prospectively on 16.06.2017, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00012552.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
IS has been members of the executive board of the International Shared Decision Making Society, which has a mission to foster SDM implementation. LA declares grants from German Cancer Aid, during the conduct of the study. The authors report no other conflicts of interest in this work.
Acknowledgments
We would like to thank all of the patients and the breast and gynecological cancer centers for participating in this study.
Additional information
Funding
References
- Stewart M. Patient-Centered Medicine: Transforming the Clinical Method. Thousand Oaks Calif. u.a.: SAGE Publ; 1997.
- Scholl I, Zill JM, Härter M, Dirmaier J. An integrative model of patient-centeredness - a systematic review and concept analysis. PLoS One. 2014;9(9):e107828. doi:10.1371/journal.pone.0107828
- Epstein RM, Franks P, Fiscella K, et al. Measuring patient-centered communication in patient-physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528. doi:10.1016/j.socscimed.2005.02.001
- Finset A. Research on person-centred clinical care. J Eval Clin Pract. 2011;17(2):384–386. doi:10.1111/j.1365-2753.2010.01608.x
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423–1433.
- Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600–607. doi:10.1111/j.1745-7599.2008.00360.x
- Vahdat S, Hamzehgardeshi L, Hessam S, Hamzehgardeshi Z. Patient involvement in health care decision making: a review. Iran Red Crescent Med J. 2014;16(1):e12454. doi:10.5812/ircmj.12454
- Rabinowitz B. Interdisciplinary breast cancer care: declaring and improving the standard. Oncology. 2004;18(10):1263–1268.
- Koch L, Bertram H, Eberle A, et al. Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psycho-oncology. 2014;23(5):547–554. doi:10.1002/pon.3452
- Izci F, Sarsanov D, Erdogan Zİ, et al. Impact of Personality Traits, Anxiety, Depression and Hopelessness Levels on Quality of Life in the Patients with Breast Cancer. Eur J Breast Health. 2018;14(2):105–111. doi:10.5152/ejbh.2018.3724
- Esmo. Guidelines. Available from: Available from: https://www.esmo.org/guidelines. Accessed July 19, 2021.
- ASCO. Guidelines, Tools, & Resources. Available from: https://www.asco.org/research-guidelines/quality-guidelines/guidelines. Accessed July 19, 2021.
- Leitlinienprogramm Onkologie [Guideline program oncology]. Leitlinien. Available from: https://www.leitlinienprogramm-onkologie.de/leitlinien/. Accessed July 19, 2021.
- Soukup T, Lamb BW, Arora S, Darzi A, Sevdalis N, Green JS. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49–61. doi:10.2147/JMDH.S117945
- Soukup T, Murtagh G, Lamb BW, Green JSA, Sevdalis N. Degrees of Multidisciplinarity Underpinning Care Planning for Patients with Cancer in Weekly Multidisciplinary Team Meetings: conversation Analysis. J Multidiscip Healthc. 2021;14:411–424. doi:10.2147/JMDH.S270394
- Horlait M, Baes S, Dhaene S, van Belle S, Leys M. How multidisciplinary are multidisciplinary team meetings in cancer care? An observational study in oncology departments in Flanders, Belgium. J Multidiscip Healthc. 2019;12:159–167. doi:10.2147/jmdh.s196660
- Taylor C, Munro AJ, Glynne-Jones R, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi:10.1136/bmj.c951
- Hoinville L, Taylor C, Zasada M, Warner R, Pottle E, Green J. Improving the effectiveness of cancer multidisciplinary team meetings: analysis of a national survey of MDT members’ opinions about streamlining patient discussions. BMJ Open Qual. 2019;8(2):e000631. doi:10.1136/bmjoq-2019-000631
- Houssami N, Sainsbury R. Breast cancer: multidisciplinary care and clinical outcomes. Eur J Cancer. 2006;42(15):2480–2491. doi:10.1016/j.ejca.2006.05.023
- Lucarini A, Garbarino GM, Orlandi P, et al. From “Cure” to “Care”: the Role of the MultiDisciplinary Team on Colorectal Cancer Patients’ Satisfaction and Oncological Outcomes. J Multidiscip Healthc. 2022;15:1415–1426. doi:10.2147/JMDH.S362550
- Schuler M, Schildmann J, Trautmann F, et al. Cancer patients’ control preferences in decision making and associations with patient-reported outcomes: a prospective study in an outpatient cancer center. Support Care Cancer. 2017;25(9):2753–2760. doi:10.1007/s00520-017-3686-8
- Street RL, Elwyn G, Epstein RM. Patient preferences and healthcare outcomes: an ecological perspective. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):167–180. doi:10.1586/erp.12.3
- Lamb BW, Taylor C, Lamb JN, et al. Facilitators and barriers to teamworking and patient centeredness in multidisciplinary cancer teams: findings of a national study. Ann Surg Oncol. 2013;20(5):1408–1416. doi:10.1245/s10434-012-2676-9
- Taylor C, Finnegan-John J, Green JSA. ”No decision about me without me” in the context of cancer multidisciplinary team meetings: a qualitative interview study. BMC Health Serv Res. 2014;14:488. doi:10.1186/s12913-014-0488-2
- Soukup T, Sevdalis N, Green JSA, Lamb BW, Chapman C, Skolarus TA. Making Tumor Boards More Patient-Centered: let’s Start With the Name. JCO Oncol Pract. 2021;OP2000588. doi:10.1200/OP.20.00588
- Hahlweg P, Hoffmann J, Harter M, Frosch DL, Elwyn G, Scholl I. In Absentia: an Exploratory Study of How Patients Are Considered in Multidisciplinary Cancer Team Meetings. PLoS One. 2015;10(10):e0139921. doi:10.1371/journal.pone.0139921
- Geerts PAF, van der Weijden T, Savelberg W, et al. The Next Step Toward Patient-Centeredness in Multidisciplinary Cancer Team Meetings: an Interview Study with Professionals. J Multidiscip Healthc. 2021;14:1311–1324. doi:10.2147/JMDH.S286044
- Wihl J, Rosell L, Frederiksen K, Kinhult S, Lindell G, Nilbert M. Contributions to Multidisciplinary Team Meetings in Cancer Care: predictors of Complete Case Information and Comprehensive Case Discussions. J Multidiscip Healthc. 2021;14:2445–2452. doi:10.2147/JMDH.S309162
- Choy ET, Chiu A, Butow P, Young J, Spillane A. A pilot study to evaluate the impact of involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Breast. 2007;16(2):178–189. doi:10.1016/j.breast.2006.10.002
- Butow P, Harrison JD, Choy ET, Young JM, Spillane A, Evans A. Health professional and consumer views on involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Cancer. 2007;110(9):1937–1944. doi:10.1002/cncr.23007
- Harrison JD, Choy ET, Spillane A, Butow P, Young JM, Evans A. Australian breast cancer specialists’ involvement in multidisciplinary treatment planning meetings. The Breast. 2008;17(4):335–340. doi:10.1016/j.breast.2008.03.001
- Massoubre J, Lapeyre M, Pastourel R, et al. Will the presence of the patient at multidisciplinary meetings influence the decision in head and neck oncology management? Acta Otolaryngol. 2018;138(2):185–189. doi:10.1080/00016489.2017.1384059
- Ansmann L, Kowalski C, Pfaff H, Wuerstlein R, Wirtz MA, Ernstmann N. Patient participation in multidisciplinary tumor conferences. Breast. 2014;23(6):865–869. doi:10.1016/j.breast.2014.09.004
- Heuser C, Diekmann A, Kowalski C, et al. Health literacy and patient participation in multidisciplinary tumor conferences in breast cancer care: a multilevel modeling approach. BMC Cancer. 2019;19(1):330. doi:10.1186/s12885-019-5546-z
- Diekmann A, Heuser C, Ernstmann N, et al. How do breast cancer patients experience multidisciplinary tumor conferences? - A description from the patient perspective. Breast. 2019;44:66–72. doi:10.1016/j.breast.2018.12.012
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714–6724. doi:10.1002/cam4.4213
- Bohmeier B, Schellenberger B, Diekmann A, Ernstmann N, Ansmann L, Heuser C. Opportunities and limitations of shared decision making in multidisciplinary tumor conferences with patient participation - A qualitative interview study with providers. Patient Educ Couns. 2020;104(4):792–799. doi:10.1016/j.pec.2020.09.007
- Schellenberger B, Diekmann A, Heuser C, Gambashidze N, Ernstmann N, Ansmann L. Decision-Making in Multidisciplinary Tumor Boards in Breast Cancer Care - An Observational Study. J Multidiscip Healthc. 2021;14:1275–1284. doi:10.2147/JMDH.S300061
- Schellenberger B, Heuser C, Diekmann A, et al. Questions and emotional expressions from patients and companions while participating in multidisciplinary tumor conferences in breast and gynecological cancer centers. Patient Educ Couns. 2021. doi:10.1016/j.pec.2021.12.010
- Schellenberger B, Heuser C, Diekmann A, et al. How shared is decision-making in multidisciplinary tumour conferences with patient participation? An observational study. Health Expect. 2022;25(6):3297–3306. doi:10.1111/hex.13638
- Heuser C, Diekmann A, Schellenberger B, et al. Patient Participation in Multidisciplinary Tumor Conferences from the Providers’ Perspective: is It Feasible in Routine Cancer Care? J Multidiscip Healthc. 2020;13:1729–1739. doi:10.2147/JMDH.S283166
- Diekmann A, Heuser C, Schellenberger B, et al. Patient participation in multidisciplinary tumor conferences: providers’ perceptions of patients’ need satisfaction and emotional experiences. Psycho-oncology. 2020;29(8):1263–1271. doi:10.1002/pon.5413
- Feldman-Stewart D, Brundage MD, Tishelman C. A conceptual framework for patient-professional communication: an application to the cancer context. Psycho-oncology. 2005;14(10):801. doi:10.1002/pon.950
- O’Cathain A, Murphy E, Nicholl J. The quality of mixed methods studies in health services research. J Health Serv Res Policy. 2008;13(2):92–98. doi:10.1258/jhsrp.2007.007074
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ. 2010;341:c4587. doi:10.1136/bmj.c4587
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi:10.1093/intqhc/mzm042
- Heuser C, Diekmann A, Ernstmann N, Ansmann L. Patient participation in multidisciplinary tumour conferences in breast cancer care (PINTU): a mixed-methods study protocol. BMJ Open. 2019;9(4):e024621. doi:10.1136/bmjopen-2018-024621
- Palinkas LA, Aarons GA, Horwitz S, Chamberlain P, Hurlburt M, Landsverk J. Mixed method designs in implementation research. Adm Policy Ment Health. 2011;38(1):44–53. doi:10.1007/s10488-010-0314-z
- Radecki SE, Nyquist JG, Gates JD, Abrahamson S, Henson DE. Educational Characteristics of Tumor Conferences in Teaching and Non‐teaching Hospitals. J Cancer Educ. 2009;9(4):204–216.
- Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York; 1978.
- Del Piccolo L, de Haes H, Heaven C. Development of the Verona coding definitions of emotional sequences to code health providers’ responses (VR-CoDES-P) to patient cues and concerns. Patient Educ Couns. 2011;82(2):149–155. doi:10.1016/j.pec.2010.02.024
- Zimmermann C, Del Piccolo L, Bensing J, et al. Coding patient emotional cues and concerns in medical consultations: the Verona coding definitions of emotional sequences (VR-CoDES). Patient Educ Couns. 2011;82(2):141–148. doi:10.1016/j.pec.2010.03.017
- Taylor C, Atkins L, Richardson A, Tarrant R, Ramirez A-J. Measuring the quality of MDT working: an observational approach. BMC Cancer. 2012;12:202. doi:10.1186/1471-2407-12-202
- Prüfer P, Rexroth M. Two-Phases-Pretesting: [Zwei-Phasen-Pretesting]. ZUMA-Arbeitsbericht. 2000;8:548.
- Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80(1):94–99. doi:10.1016/j.pec.2009.09.034
- Stivers T, Enfield NJ. A coding scheme for question–response sequences in conversation. J Pragmat. 2010;42(10):2620–2626. doi:10.1016/j.pragma.2010.04.002
- Street RL, Gordon HS, Ward MM, Krupat E, Kravitz RL. Patient participation in medical consultations: why some patients are more involved than others. Med Care. 2005;43(10):960–969. doi:10.1097/01.mlr.0000178172.40344.70
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312. doi:10.1016/j.pec.2005.06.010
- Amundsen A, Nordøy T, Lingen KE, Sørlie T, Bergvik S. Is patient behavior during consultation associated with shared decision-making? A study of patients’ questions, cues and concerns in relation to observed shared decision-making in a cancer outpatient clinic. Patient Educ Couns. 2018;101(3):399–405. doi:10.1016/j.pec.2017.10.001
- Butow PN, Brown RF, Cogar S, Tattersall MHN, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psycho-oncology. 2002;11(1):47–58. doi:10.1002/pon.556
- Finset A, Heyn L, Ruland C. Patterns in clinicians’ responses to patient emotion in cancer care. Patient Educ Couns. 2013;93(1):80–85. doi:10.1016/j.pec.2013.04.023
- Bull C, Byrnes J, Hettiarachchi R, Downes M. A systematic review of the validity and reliability of patient-reported experience measures. Health Serv Res. 2019;54(5):1023–1035. doi:10.1111/1475-6773.13187
- Koster EB, Baars EW, Delnoij DMJ. Patient-reported quality of care in anthroposophic and integrative medicine: a scoping review. Patient Educ Couns. 2020;103(2):276–285. doi:10.1016/j.pec.2019.09.010
- Soukup T, Gandamihardja TAK, McInerney S, Green JSA, Sevdalis N. Do multidisciplinary cancer care teams suffer decision-making fatigue: an observational, longitudinal team improvement study. BMJ Open. 2019;9(5):e027303. doi:10.1136/bmjopen-2018-027303
- Ansmann L, Baumann W, Gostomzyk J, et al. DNVF-Memorandum III – Methoden für die Versorgungsforschung, Teil 4 – Konzept und Methoden der organisationsbezogenen Versorgungsforschung. Kapitel 1 – Definition und Konzept der organisationsbezogenen Versorgungsforschung. [DNVF-Memorandum III - Methods For Health Services Research, Part 4 - Concept and Methods For Organizational Health Services Research. Chapter 1 - Definition and Concept of Organizational Health Services Research]. Gesundheitswesen. 2019;81(3):e64–e71. doi:10.1055/a-0862-0527
- Kočo L, Siebers CCN, Schlooz M, et al. Mapping Current Organizational Structure and Improvement Points of Breast Cancer Multidisciplinary Team Meetings - An Interview Study. J Multidiscip Healthc. 2022;15:2421–2430. doi:10.2147/JMDH.S380293
- Street RL, Millay B. Analyzing patient participation in medical encounters. Health Commun. 2001;13(1):61–73. doi:10.1207/S15327027HC1301_06
- Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi:10.1016/j.pec.2013.10.031
- Ansmann L, Pfaff H. Providers and Patients Caught Between Standardization and Individualization: individualized Standardization as a Solution Comment on “(Re) Making the Procrustean Bed? Standardization and Customization as Competing Logics in Healthcare”. Int J Health Policy Manag. 2018;7(4):349–352. doi:10.15171/ijhpm.2017.95
- Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. doi:10.1136/bmj.n2061