Abstract
Objective
For patients with polycystic ovary syndrome (PCOS) to undergo in vitro fertilization (IVF) and embryo transfer (ET), there has been no consensus regarding which protocol is the most optimal for live birth rate in fresh cycles. We sought to evaluate depot gonadotropin-releasing hormone (GnRH) agonist protocol versus GnRH antagonist protocol in IVF outcomes for PCOS patients in a single fertility center.
Methods
In this retrospective cohort, PCOS patients who visited the Second Hospital of Hebei Medical University reproductive center between February 2012 and December 2019 were screened, and 533 PCOS infertility patients were included undergoing their first IVF cycle, with 470 in the depot GnRH agonist group and 63 in the GnRH antagonist group. The primary of this study outcome was the fresh live birth rate (LBR).
Results
PCOS women in the depot GnRH agonist group had a higher LBR (49.79%) than those in the GnRH antagonist group (34.92%, p = 0.027). The multivariable logistic regression also confirmed that women in the depot GnRH agonist group had a higher LBR than those in the GnRH antagonist group (OR = 1.83, 95% CI 1.05~3.18, p = 0.032). After propensity score matching (PSM), the LBR in the depot GnRH agonist group was higher (50.32%) than that of the GnRH antagonist group (35.48%), p = 0.033. The ovarian hyperstimulation syndrome (OHSS) rates were similar between the two groups, with 35 in the depot GnRH group and 6 in the GnRH antagonist group (p = 0.561).
Conclusions
For PCOS patients in fresh embryo transfer cycles, the depot GnRH agonist protocol may lead to a higher LBR than the antagonist protocol with satisfied lower OHSS rates.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among women of reproductive age and is one of the most common causes of female infertility due to anovulation.Citation1 With many PCOS patients needing assisted reproductive technology (ART),Citation2 making safe and efficient ovulation induction protocols for PCOS patients is essential. Many COH protocols have been implemented for PCOS patients receiving IVF.Citation3–5 A meta compared represented that the GnRH antagonist protocol was safer and more cost-effective than the long GnRH agonist protocol for PCOS without compromising the clinical outcomes.Citation6 Two types of GnRH agonist patterns can prevent premature luteinization: a low (0.1 mg) daily dose of GnRH agonist or a single higher dose (3.75 mg) of long-acting GnRH agonist analog. The latter is called the follicular phase depot GnRH agonist.Citation7 The depot GnRH agonist protocol has better clinical outcomes, lower luteinizing hormone (LH), and estradiol/oocyte ratio.Citation8 However, the moderate or severe Ovarian hyperstimulation syndrome (OHSS) rates were higher in the depot GnRH agonist group than in the long GnRH agonist group.Citation9 As GnRH antagonist can block pituitary LH instantly,Citation10 reduce stimulation duration, decrease the usage of gonadotropin, and lower the risk of OHSS,Citation11 therefore GnRH antagonist protocol can improve PCOS patients’ acceptance, but with fewer follicles and oocytes, as well as the incidence of premature LH surge.Citation6
The common strategy for PCOS patients was the GnRH antagonist protocol followed by frozen embryo transfer.Citation12 Meanwhile, a proportion of patients still prefer fresh embryo transfer when the risk of OHSS is under control. However, there remains no consensus on which protocol is the most optimal treatment for PCOS patients to achieve a maximal live birth rate in fresh treatment cycles. In the present study, we sought to evaluate the follicular phase depot GnRH agonist protocol versus the GnRH antagonist protocol in terms of the fresh live birth rate (LBR) and OHSS rate for PCOS patients in a single fertility center.
Methods
Ethics
This was a retrospective cohort at a single center. The Reproductive Ethics Committee approved the study protocol of the Second Hospital of Hebei Medical University (Approval number: 2021-P042).
Subjects Enrollment
PCOS patients who visited the human reproductive center of the Second Hospital of Hebei Medical University between February 2012 and December 2019 were screened. The diagnosis of PCOS was established following the Rotterdam consensus criteria.Citation2,Citation13 Individuals meeting at least 2 of the 3 Rotterdam consensus criteria and without phenotypically similar androgen excess disorders such as congenital adrenal hyperplasia (CAH), androgen-secreting tumors, Cushing syndrome, thyroid dysfunction, and hyperprolactinemia, were diagnosed with PCOS.
1. Hyperandrogenism - either clinically by skin manifestations of androgen excess OR hyperandrogenemia (high testosterone in a blood test); 2. Ovulation dysfunction (ie Oligo/Anovulation); 3. Polycystic ovaries on ultrasound.
Inclusion Criteria
Infertile women between 21 and 38 years old
Had either depot GnRH agonist protocol or GnRH antagonist protocol
First IVF or ICSI cycle
Oocytes no more than 20
Exclusion Criteria
Women with endometriosis, hydrosalpinx, uterine malformation, endometrial polyp, history of unilateral oophorectomy or karyotyping.
Women with thyroid disease, diabetes mellitus, hyperprolactinemia, congenital adrenal hyperplasia, or other endocrine or metabolic diseases.
Data were retrieved on patients’ age, BMI (kg/m2), classification of infertility, duration of infertility, and antral follicle counting. All included subjects had been tested for baseline serum levels of follicle-stimulating hormone (FSH) (μIU/mL), luteinizing hormone (LH) (μIU/mL), estradiol (E2) (pg/mL), progesterone (P) (ng/mL), testosterone (T) (ng/mL), and prolactin (PRL) (ng/mL) using commercial kits (Siemens Healthcare Diagnostics) with an automatic chemiluminescence immunoassay analyzer. Exogenously added LH, total GnRH dosage, and numbers of retrieved oocytes were recorded, and the endometrial thickness was measured on the day of triggering with human chorionic gonadotropin (hCG). Patients with PCOS were allocated to either the depot GnRH agonist protocol or the GnRH antagonist protocol, with the decision influenced by physician preferences, discussions between doctors and patients, or the availability of pertinent supplementary medications.
Depot GnRH Agonist Protocol
In the depot, GnRH agonist protocol, patients received 3.75 mg long-acting GnRH agonist on the first to third days of the menstruation for pituitary down-regulation. After 30 days of down-regulation, the endometrium thickness <5 mm, a follicular diameter < 10 mm, and pituitary desensitization was achieved, as indicated by low levels of FSH (≤5 μIU/mL), estradiol (≤20 pg/mL) and LH (≤5 μIU/mL), 150–225 IU of gonadotropin were given. The dosage and duration of gonadotropin were adjusted according to follicle development and serum endocrine. When at least two dominant follicles reached 18 mm, 10,000 IU human chorionic gonadotropin (hCG) (Livzon)/250 μg Ovidrel (Merck Serono) was administered.
GnRH Antagonist Protocol
In the antagonist protocol, patients received gonadotropin on the second day of menstruation, and once daily last for five days. Subsequently, antagonist 0.25 mg cetrorelix acetate (Merck Serono) was given to the modulate serum LH (≥5 μIU/mL) as part of a flexible strategy. The gonadotropin dosage was adjusted according to follicular development, and the antagonist was used until the hCG day.
Oocytes were retrieved 36–38 hours after hCG triggering and were fertilized by either IVF or intracytoplasmic sperm injection (ICSI). There were no differences in semen parameters between both depot and antagonist groups. All embryos were cultured by a standard protocol in the embryo lab. Fresh embryo transfers were performed 3–5 days after oocyte retrieval based on embryo quality and patients’ overall conditions. The transfer was cancelled if the oocyte retrieval was more than twenty or patients were at other high risk for OHSS, or P was over 2 ng/mL on the hCG triggering day. Then luteal-phase support was implemented [90 mg progesterone gel (Merck Serono) plus 20 mg/day dydrogesterone].
Outcomes
The primary outcome was live birth after the first fresh-cycle embryo transfer. A blood hCG test was taken 14 days post-embryo transfer. The positive hCG result was confirmed as a biochemical marker of pregnancy.Citation14 Clinical intrauterine pregnancy was defined by the observation of fetal cardiac activity by sonograph 30 days after the embryo transfer, which included ectopic pregnancy.Citation14 Both the chemical pregnancy and the clinical pregnancy were secondary outcomes. LBR was defined as the number of delivery cycles that resulted in at least one live-born baby/number of embryo transfer cycles.Citation15 The outcomes also included miscarriage and a pregnancy loss before 28 weeks.Citation15 Severe OHSS was characterized by one of the following criteria: ascites, hydrothorax, and dyspnea.Citation16 The use of abdominal puncture to release ascites was recorded.
Statistical Analysis
Continuous variables with normal distribution were presented as the mean, followed by standard deviation. The non-normal distribution of continuous variables was presented as the median, followed by the interquartile range (IQR). Student’s t-test and Wilcoxon rank-sum test were used for normally and non-normally distributed quantitative data for inter-group comparisons. Categorical variables at baseline were analyzed using the Chi-square or Fisher’s exact test for inter-group baseline comparisons.
Multivariate logistic regression analysis and Cox regression were performed to identify the associations between dependent and independent variables. The results are expressed as adjusted odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (95% CIs).
Assuming a live birth rate of 30% in the GnRH Antagonist group and 50% in the Depot GnRH Agonist group, with an α (Type I error) of 0.05 and a β (Type II error) of 0.2, and with a group ratio of 1:5, the necessary sample size for the GnRH Antagonist group was 57, while for the Depot GnRH Agonist group, it was 285.
To obtain comparable groups, propensity score matching was performed to match patients of the GnRH antagonist group with the depot GnRH agonist group. The propensity score model was constructed using the multivariable logistic regression model, which included age, spouse age, BMI, infertility type, duration of infertility, natural cycle, baseline FSH level, baseline E2 level, baseline P level, baseline PRL level, baseline LH level, baseline T level, antral follicle count (AFC) level, and intimal thickness.
For the primary endpoint, logistic regression was used to assess the association between live births and the two treatment regimens. The model was adjusted by female age, infertility type, baseline PRL, retrieved oocytes, and treatment regimens. Propensity score-matched data were analyzed for sensitivity analysis for the entire cohort. The caliper width is equivalent to 0.2 of the pooled standard deviation of the logit of the propensity score. Patients in the GnRH antagonist group were matched 1:5 to patients in the Depot GnRH agonist group.
All hypothesis tests were two-sided, and values of <0.05 were considered statistically significant.
Stata SE 13 (Serial number 401306302851), R software version 3.6.1 (http://cran.r-project.org/), easy-R (www.empowerstats.com), and prism (https://www.graphpad.com/scientific-software/prism/) were applied for the data analysis.
Results
Baseline of All the Participants
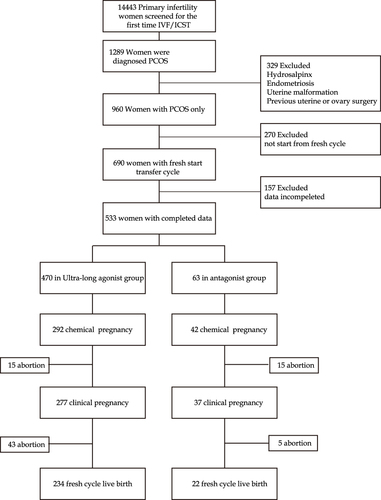
The flow chart of the study is shown in . Between February 2012 and December 2019, a total of 14,443 eligible primary infertility patients underwent IVF/ICSI for their first cycle. Of them, 1289 patients were diagnosed with PCOS. Three hundred and twenty-nine patients were excluded from this analysis because of hydrosalpinx, malformation, endometriosis, or previous uterine/ovary surgery. Another 270 patients were excluded due to a lack of a fresh embryo transplant (ET) cycle, and 157 patients were excluded due to incomplete data. The final analysis included 533 eligible women, with 470 in the depot GnRH agonist group and 63 in the GnRH antagonist group. Baseline characteristics were comparable between the groups, including age, spouse age, infertility type, BMI, duration of infertility, and the cycle of pregnancy. The baseline E2 level in the depot GnRH agonist group was 64.88 ± 63.80 (median 43) pg/mL, while it was 45.21 ± 34.70 (median 41) pg/mL in the GnRH antagonist group (p = 0.024). The baseline P level in the depot GnRH agonist group was 1.43 ± 3.38 (median 0.71) pg/mL, while it was 0.82 ± 1.11 (median 0.60) pg/mL in the GnRH antagonist (p = 0.007). There were no statistical differences in age, spouse age, stimulate cycle, BMI, baseline serum FSH, baseline serum LH, baseline serum PRL, baseline serum T, AFC, and endometrium thickness (EMT). All details of the study parameters are shown in .
Table 1 Baseline Characteristics for All 533 Subjects
ART Outcomes for All 533 Subjects
The total gonadotropin (Gn) dose was higher in the depot GnRH agonist group (median 2475) than that in the GnRH antagonist group (median 1800) because of protocol differences (p < 0.001).
There was no statistically significant difference in the number of oocytes retrieved (p = 0.849), clinical pregnancy rate (p = 0.975), and OHSS rate (p = 0.561) between the two groups. For the fresh cycle, 234 of 470 (49.79%) women in the depot GnRH agonist group had a live birth, while 22 of the 63 (34.92%) women in the GnRH antagonist group had a fresh live birth (p = 0.027) ().
Table 2 ART Outcomes for All 533 Subjects
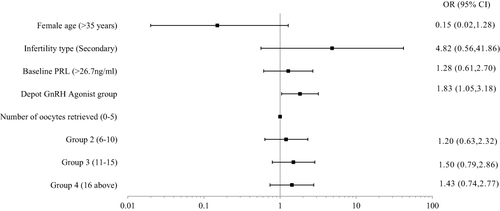
Multivariable logistic regression showed that women in the depot GnRH agonist group had a higher LBR than those in the GnRH antagonist group (OR = 1.83, p = 0.032, 95% CI 1.05 ~ 3.18) ( and ).
Table 3 Single Factor Analysis and Logistic Regression for Fresh Cycle for All 533 Subjects
Figure 2 Multivariable logistic regression showed the depot GnRH agonist group had a higher LBR than those in the GnRH antagonist group.
The biochemical pregnancy rate was 62.39% in the Depot GnRH Agonist group and 66.67% in the GnRH Antagonist group, whereas the clinical pregnancy rate stood at 58.94% for the Depot GnRH Agonist group and 58.73% for the GnRH Antagonist group (as indicated in Table S1).
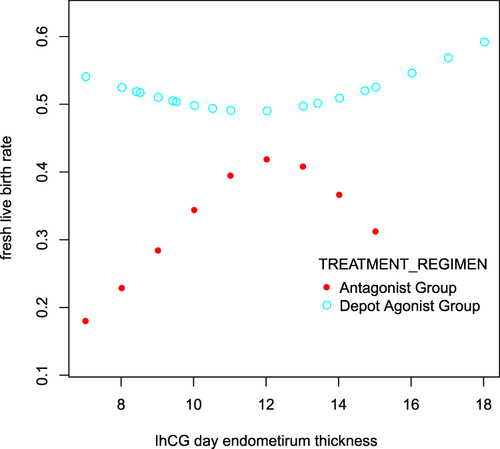
Restricted cubic spline analyses generated a curve () showing the relationship between endometrial thickness (EMT) and the probability of LBR, adjusted age, infertility type, duration of infertility, natural cycle, BMI, baseline FSH, E2, P, PRL, LH, T, AFC, LH, the total dose of Gn, and the number of oocytes retrieved. The turning point of endometrial thickness in the depot GnRH agonist group was 11 mm, and the live birth rate of fresh cycle attempts increased after this point. In contrast, women in the GnRH antagonist group had a peak live birth rate of 40% when the endometrial thickness was approximately 12 mm.
Figure 3 Relationship between the endometrial thickness (EMT) and the probability of LBR. Restricted cubic spline curve of the relationship between EMT and the probability of LBR, adjusted age; infertility type; duration of infertility; natural cycle; BMI; baseline FSH, E2, P, PRL, LH, T, and AFC; whether LH was added; total dose of Gn; and the number of oocytes retrieved.
ART Outcomes for 372 PSM Subjects
After PSM, the baseline characteristics between the two groups were comparable in female age, spouse age, duration of infertility, natural cycle, BMI, FSH, E2, P, PRL, LH, T, AFC, and endometrium thickness on hCG day (). The LBR between the two treatment groups differed. Of the 156 of 310 (50.32%) patients in the depot GnRH agonist group had a live birth, while 22 of 62 (35.48%) women in the GnRH antagonist group had a live birth (p = 0.033). The number of oocytes retrieved and the clinical pregnancy rate between the two groups were comparable (). The depot GnRH agonist group has a higher LBT than the GnRH antagonist group (OR = 1.84, 95% CI 1.05%~3.24%, p = 0.034) ().
Table 4 Baseline Characteristics for 372 PSM Subjects
Table 5 ART Outcomes for 372 PSM Subjects
Table 6 Live Birth Rate of Fresh Cycle for PSM Subjects
OHSS
Both depot GnRH agonist and GnRH antagonist groups used hCG for ovulation triggering. Because we froze all the embryos when more than 20 oocytes were retrieved, no significant differences were observed in severe OHSS rates between the two protocols (35 in the depot GnRH agonist group, 6 in the GnRH antagonist group, p = 0.561).
Discussion
The present study is a retrospective cohort that evaluated the LBR of GnRH antagonist and depot GnRH agonist protocols in PCOS patients for their first ART cycle. The LBR was higher using the depot GnRH agonist protocol than the GnRH antagonist protocol for fresh embryo transfer. The main reasons for the higher LBR of the depot GnRH agonist protocol with fresh transfer cycles are as follows.
Firstly, it may be possible due to the progesterone level elevation. PCOS patients with a high number of oocytes retrieved tended to have premature luteinizationCitation17 and indicating an increased progesterone concentration (≥1.3 ng/mL) on the day of hCG administration so that the implantation window would shift forward.Citation18 The fact that LBR is better in the depot GnRH agonist protocol may be associated with a reduction in P level on the day of hCG administration for pituitary down-regulation before gonadotrophin stimulation. Secondly, another possible factor is that a high level of LH can lead to follicular atresia. The depot GnRH agonist protocol can decrease LH levels and reduce follicular atresia, so it may increase the number of oocytes retrieved.Citation19 The lower LBR with the GnRH antagonist may be due to the LH surge not being suppressed entirely and the fact that these surges occurred before the antagonist started.Citation20 Thirdly, the beneficial effect of the depot GnRH agonist protocol for a fresh cycle might be due to endometrial receptivity.Citation21 A study showed that GnRH agonist protocol has a beneficial impact on endometrial receptivity by regulates IL-6 and IL-11 expression.Citation22 Other studies have shown that GnRH agonist protocol may improve endometrial receptivity by protecting the expression of HOXA10, MEIS1, and LIF receptivity markers.Citation23
Many studies have shown that the OHSS rate was significantly lower when the antagonist was used.Citation24–26 The GnRH antagonist protocol can reduce OHSS because GnRH antagonists decrease VEGF mRNA expression.Citation27 As the risk of OHSS is due to the effect of hCG,Citation28 using GnRH agonist protocols to trigger ovulation and reducing hCG usage can reduce OHSS in the GnRH antagonist protocol.Citation29,Citation30 For our center, there were only a few mild OHSS cases in the two groups. Those patients with a high potential risk of OHSS occurrence on embryo transfer day will not have fresh cycle implementation. The criteria for evaluating potential risks of OHSS occurrence included ovary size, ascitic fluid, and symptoms of abdominal distension, which have been implemented at our site for the past 20 years.
Additionally, we freeze all embryos if the number of retrieved follicles is greater than 20. A systematic review showed that, in fresh cycles, LBR reaches a plateau or even declines when more than 15–20 oocytes are retrieved, and therefore, freeze-all embryos can eliminate severe OHSS.Citation31 A multicenter RCT demonstrated that frozen embryo transfer could effectively reduce the risk of OHSS.Citation32
Our restricted cubic spline figure indicates that the depot GnRH agonist protocol generated better LBR than the GnRH antagonist protocol at any EMT, which explains why the depot GnRH agonist protocol was more advantageous in fresh embryo transfer cycles. In the GnRH antagonist group, the EMT cut-off value was 12 mm. When the EMT was less than 12 mm, LBR increased with EMT, indicating that EMT acts as a good indicator for fresh live birth rate outcomes. When the EMT was thicker than 12 mm, LBR decreased coupled with EMT increase, suggesting that the GnRH antagonist protocol had a gradually negative effect on LBR as EMT increased. The restricted cubic spline figure shows that, in the GnRH agonist group, the EMT cut-off value was 11 mm. Before the cut-off value, LBR decreased slightly as EMT increased. After the cut-off values, LBR increased as EMT increased.
Our results demonstrated that with fresh transfer cycles, the live birth rate was significantly lower in the GnRH antagonist group than in the depot GnRH agonist group. Still, close attention to the endometrial thickness on hCG day might allow the pregnancy rate of fresh transfer cycles to reach a similar level between the GnRH antagonist and depot GnRH agonist protocols. We should try to improve endometrial receptivity to increase the pregnancy rate of fresh embryo transfer with the GnRH antagonist protocol in PCOS patients because the GnRH antagonist protocol has a lower OHSS rate.
For PCOS patients to ensure the pregnancy rate and decrease the incidences of moderate and severe OHSS, it is better to undergo frozen-thawed embryo transfer after GnRH antagonist protocol-induced ovulation.
For PCOS patients whose oocyte retrieving was under 20 and willing to have fresh embryo transfers, the depot GnRH agonist protocols can be applied directly. In contrast, the GnRH antagonist protocols need to be implemented for those whose EMT is around 12 mm.
As a retrospective study, this work has several limitations. Our analysis depends on previously recorded data, and not all PCOS patients were analyzed based on the inclusion and exclusion criteria. Therefore, some patients could not be collected, which may have led to bias and confounding. To overcome this issue, we used multiple statistical strategies (multivariable adjustment, propensity score matching) to adjust for the differences in the baseline that may influence the outcomes. More studies are needed to evaluate the safety issues surrounding OHSS and birth defects. This study is based on fresh cycles, we need further study about frozen cycles.
Conclusion
In our center, the depot GnRH agonist protocol provides a higher LBR than the antagonist protocol in fresh cycles among those PCOS patients whose retrieved oocytes were no more than 20, together with a satisfying low OHSS rate.
Ethics Approval and Consent to Participate
As a retrospective analysis, this study cannot get registration before patients recruitment. But we affirm that this research study has been conducted in strict adherence to the principles delineated in the Declaration of Helsinki. The study analyzed the data of fresh embryo transfer (ET) cycles performed at the reproductive center of the Second Hospital of Hebei Medical University between February 2012 and December 20191. As a retrospective research, informed consents were not obtained from patients prior to this study. The ethical committee approved this work of the Second Hospital of Hebei Medical University (Approval number: 2021-P042). All subjects signed their informed consents before treatments. All methods were performed by the relevant guidelines and regulations.
Disclosure
The authors declare no conflicts of interest in this work.
Data Sharing Statement
The datasets presented in this article are not readily available because of Chinese regulations and conditions for informed consent. Requests to access the dataset should be directed to Jiajia Zhai, [email protected].
Additional information
Funding
References
- Zehravi M, Maqbool M, Ara I. Polycystic ovary syndrome and infertility: an update. Int J Adolesc Med Health. 2021;34(2):1–9. doi:10.1515/ijamh-2021-0073
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–680. doi:10.1016/S2213-8587(22)00163-2
- Orvieto R, Meltcer S, Homburg R, Nahum R, Rabinson J, Ashkenazi J. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization? Fertil Steril. 2009;91(4 Suppl):1466–1468. doi:10.1016/j.fertnstert.2008.07.1711
- Wang N, Zhu Q, Ma M, et al. Comparison of a progestin-primed ovarian stimulation protocol with a flexible GnRH antagonist protocol in patients with polycystic ovary syndrome who are participating in an IVF programme: study protocol for a randomised controlled trial. BMJ open. 2020;10(12):e038153. doi:10.1136/bmjopen-2020-038153
- Wang D, Chu T, Yu T, Zhai J. Is early-follicular long-acting GnRH agonist protocol an alternative for patients with polycystic ovary syndrome undergoing in vitro fertilization? Reprod Biol Endocrinol. 2022;20(1):137. doi:10.1186/s12958-022-01007-z
- Kadoura S, Alhalabi M, Nattouf AH. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis. Sci Rep. 2022;12(1):4456. doi:10.1038/s41598-022-08400-z
- Zhang Y, Zhao W, Han Y, et al. The follicular-phase depot GnRH agonist protocol results in a higher live birth rate without discernible differences in luteal function and child health versus the daily mid-luteal GnRH agonist protocol: a single-centre, retrospective, propensity score matched cohort study. Reprod Biol Endocrinol. 2022;20(1):140.
- Song J, Wu W, Jiang L, Duan C, Xu J. Effects of different exposure days to gonadotropin-releasing hormone agonist (GnRH-A) on live birth rates in the depot GnRH-A protocol: a retrospective analysis of 7007 Cycles. Med Sci Monit. 2021;27:e929854. doi:10.12659/MSM.929854
- Xia M, Zheng J. Comparison of clinical outcomes between the depot gonadotrophin-releasing hormone agonist protocol and gonadotrophin-releasing hormone antagonist protocol in normal ovarian responders. BMC Pregnancy Childbirth. 2021;21(1):372. doi:10.1186/s12884-021-03849-8
- Li R, Gong F, Chen H, Wang Q, Qiao J. Correlation of LH level and steroid concentrations in GnRH antagonist protocol: a sub-analysis of Ganirelix Phase III study of China. J Gynecol Obstet Hum. 2022;51(5):102363. doi:10.1016/j.jogoh.2022.102363
- Yang R, Guan Y, Perrot V, Ma J, Li R. Comparison of the long-acting GnRH agonist follicular protocol with the GnRH antagonist protocol in women undergoing in vitro fertilization: a systematic review and meta-analysis. Adv Ther. 2021;38(5):2027–2037. doi:10.1007/s12325-020-01612-7
- Costello MF, Garad RM, Hart R, et al. A review of second- and third-line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med Sci. 2019;7(7). doi:10.3390/medsci7070075
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi:10.1016/j.fertnstert.2016.05.003
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi:10.1016/j.fertnstert.2017.06.005
- Duffy JMN, Bhattacharya S, Bhattacharya S, et al. Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Fertil Steril. 2021;115(1):201–212. doi:10.1016/j.fertnstert.2020.11.013
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International committee for monitoring assisted reproductive technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Tandulwadkar S, Gupta S, Singh A, Mishra S, Singhania S. Medroxyprogesterone acetate versus gonadotropin-releasing hormone antagonist for the prevention of premature luteinizing hormone surge in hyper-responder women undergoing controlled ovarian stimulation for IVF/ICSI Cycles. JBRA Assist Reprod. 2022. doi:10.5935/1518-0557.20220006
- Drakopoulos P, Racca A, Errázuriz J, et al. The role of progesterone elevation in IVF. Reprod Biol. 2019;19(1):1–5. doi:10.1016/j.repbio.2019.02.003
- Li G, Wu Y, Niu W, et al. Analysis of the number of euploid embryos in preimplantation genetic testing cycles with early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol. Front Endocrinol. 2020;11:424. doi:10.3389/fendo.2020.00424
- Zhang D, Zhang D, Sun Z, Deng C, Yu Q, Zhen J. The effect of a transient premature luteinizing hormone surge without elevated serum progesterone on in vitro fertilization outcomes in a gonadotropin-releasing hormone antagonist flexible protocol. Gynecol Endocrinol. 2020;36(6):550–553. doi:10.1080/09513590.2019.1683730
- Song J, Duan C, Cai W, et al. Comparison of GnRH-A prolonged protocol and short GnRH-A long protocol in patients with thin endometrium for assisted reproduction: a retrospective cohort study. Drug Des Devel Ther. 2020;14:3673–3682. doi:10.2147/DDDT.S270519
- Li L, Liu L, Kou Z, Huo M, An J, Zhang X. GnRH agonist treatment regulates IL-6 and IL-11 expression in endometrial stromal cells for patients with HRT regiment in frozen embryo transfer cycles. Reprod Biol. 2022;22(2):100608. doi:10.1016/j.repbio.2022.100608
- Xu B, Geerts D, Hu S, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod. 2020;35(6):1306–1318. doi:10.1093/humrep/deaa086
- Lambalk C, Banga F, Huirne J, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–579. doi:10.1093/humupd/dmx017
- Al-Inany H, Youssef M, Ayeleke R, Brown J, Lam W, F B. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750. doi:10.1002/14651858.CD001750.pub4
- Kuroda K, Nagai S, Ikemoto Y, et al. Incidences and risk factors of moderate-to-severe ovarian hyperstimulation syndrome and severe hemoperitoneum in 1,435,108 oocyte retrievals. Reprod Biomed Online. 2021;42(1):125–132. doi:10.1016/j.rbmo.2020.09.001
- Wang B, Wang J, Liu Y, et al. sRAGE downregulates the VEGF expression in OHSS ovarian granulosa cells. Gynecol Endocrinol. 2021;37(9):836–840. doi:10.1080/09513590.2021.1942453
- Hortu I, Karadadas E, Ozceltik G, et al. Oxytocin and cabergoline alleviate ovarian hyperstimulation syndrome (OHSS) by suppressing vascular endothelial growth factor (VEGF) in an experimental model. Arch Gynecol Obstet. 2021;303(4):1099–1108. doi:10.1007/s00404-020-05855-1
- Deepika K, Suvarna R, Sumi M, et al. HCG trigger versus GnRH agonist trigger in PCOS patients undergoing IVF cycles: frozen embryo transfer outcomes. JBRA Assist Reprod. 2021;25(1):48–58. doi:10.5935/1518-0557.20200028
- Shen X, Yang Q, Li L, Lu W. Clinical pregnancy and incidence of ovarian hyperstimulation syndrome in high ovarian responders receiving different doses of hCG supplementation in a GnRH-Agonist trigger protocol. Evid Based Complement Alternat Med. 2021;2021:2180933. doi:10.1155/2021/2180933
- Mizrachi Y, Horowitz E, Farhi J, Raziel A, AJHru W. Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update. 2020;26(1):118–135. doi:10.1093/humupd/dmz037
- Shi Y, Sun Y, Hao C, et al. Transfer of Fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi:10.1056/NEJMoa1705334