Abstract
Background
Reporting guidelines have been available for the past 17 years since the inception of the Consolidated Standards of Reporting Trials statement in 1996. These guidelines were developed to improve the quality of reporting of studies in medical literature. Despite the widespread availability of these guidelines, the quality of reporting of medical literature remained suboptimal. In this study, we assess the current adherence practice to reporting guidelines; determine key factors associated with better adherence to these guidelines; and provide recommendations to enhance adherence to reporting guidelines for future studies.
Methods
We undertook a systematic scoping review of systematic reviews of adherence to reporting guidelines across different clinical areas and study designs. We searched four electronic databases (Cumulative Index to Nursing and Allied Health Literature, Web of Science, Embase, and Medline) from January 1996 to September 2012. Studies were included if they addressed adherence to one of the following guidelines: Consolidated Standards of Reporting Trials (CONSORT), Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Quality of Reporting of Meta-analysis (QUOROM), Transparent Reporting of Evaluations with Nonrandomized Designs (TREND), Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). A protocol for this study was devised. A literature search, data extraction, and quality assessment were performed independently by two authors in duplicate. This study reporting follows the PRISMA guidelines.
Results
Our search retrieved 5159 titles, of which 50 were eligible. Overall, 86.0% of studies reported suboptimal levels of adherence to reporting guidelines. Factors associated with better adherence included journal impact factor and endorsement of guidelines, publication date, funding source, multisite studies, pharmacological interventions and larger studies.
Conclusion
Reporting guidelines in the clinical literature are important to improve the standards of reporting of clinical studies; however, adherence to these guidelines remains suboptimal. Action is therefore needed to enhance the adherence to these standards. Strategies to enhance adherence include journal editorial policies endorsing these guidelines.
Background
The medical literature is an integral component of clinical care, education, and research, as it has a serious impact on our understanding of health and disease. There are thousands of medical journals that publish articles related to clinical interventions, prognosis, diagnosis, and risks – among others – with an influence on health and life in general. For example, a quick glance at PubMed shows over 22 million citations for biomedical literature.Citation1 It is therefore a challenge to try to assimilate data presented in the literature and make evidence-based informed decisions. Attempts to summarize these data using systematic reviews are commendable as these reviews aim to provide a summary of the state of knowledge on a specific topic and address the inconsistent findings from single studies. However, these reviews are exponential in number and may report disparate findings. Searching for systematic reviews on depression resulted in 30,038 articles,Citation2 and in cancer resulted in 323,633.Citation3
One way to assimilate and disseminate knowledge that can influence decision-making and provide an understanding of a certain condition is to perform a systematic review of reviews. The past few decades have given rise to a handful of such studies in several clinical areas including lifestyle interventions,Citation4 interventions to improve mental health,Citation5 homeopathy,Citation6 medical education,Citation7 spinal manipulation,Citation8 sleep medicine,Citation9 and cancer,Citation10 among others. Each of these reviews of reviews is focused on a specific clinical question. There is a paucity of systematic reviews that assess the quality of reporting of clinical studies across different clinical areas, and that use different reporting guidelines. The EQUATOR (Enhancing Quality and Transparency in Health Research) network is an international initiative that supports the development and dissemination of such guidelines.Citation11 The EQUATOR website provides guidelines for the minimum information required to report research methods and findings for various kinds of medical research.Citation12
The evidence that is presented in the clinical literature can carry substantial weight in informing professionals and users of health care on multiple aspects of health risks, disease, health care outcomes, and delivery. However, readers of the literature are faced with conflicting results presented in various formats and styles, making interpretations and conclusions challenging even for the most informed readers. For this reason, a consensus on reporting such evidence is needed to establish the quality of such studies. It is also important to ensure that a more uniform method is used by researchers to enable the combination of results from multiple studies and reach more standardized summaries and conclusions; this can minimize heterogeneity, which often complicates meta-analyses in future studies. Furthermore, poorly reported research can cause harm to patients and lead to the use of scarce resources on ineffective treatments.Citation13
To address the concern over the quality of reported studies and ensure transparency in reporting clinical studies, the Consolidated Standards of Reporting Trials (CONSORT)Citation14 statement was produced as a collaborative effort to provide a checklist and flow diagram for authors to have as a guide to prepare reports on randomized controlled trials (RCTs) for publication. The CONSORT Statement was further updated in 2010 based on new evidence and an added focus on specific designs of RCTs.Citation15 The CONSORT is a widely accepted and adopted statement that is well described in many freely accessible publications and websites. In brief, the CONSORT Statement provides a 25-item checklist describing the required criteria for inclusion when reporting RCTs. Such items include the study design, the participants, interventions, outcomes, and sample size among others. It also recommends the inclusion of a flow diagram, accounting for recruitment, randomization, allocation of interventions, and retention in the study.Citation16 Since the introduction of the CONSORT, several extensions and modifications of the original statement have been established to improve the quality of reporting of various study types, including observational studies, systematic reviews, and meta-analysis. Despite the availability of such guidelines for reporting, the quality of reporting of clinical studies has remained suboptimal with several manuscripts in a number of clinical areas missing key items as described in the CONSORT.Citation16–Citation23
Evidence suggests that the use of the CONSORT criteria is associated with improved standards of reporting.Citation24,Citation25 However, it is not clear what the current level of adherence to reporting guidelines is, what factors are associated with improving the reporting of clinical literature, and how the results from different studies on reporting standards can be compiled to provide an overall conclusion on the current state of reporting standards.
We therefore undertook a systematic scoping review evaluating systematic reviews addressing the adherence standards to reporting guidelines published since the introduction of the CONSORT Statement in January 1996 to September 2012.
Study aims
In this study, we aimed to examine the extent of adherence to reporting guidelines in published clinical research since the introduction of the CONSORT Statement in 1996. The purpose of this systematic scoping review is to inform researchers, guideline developers, journal editors, and evidence users on the profile of reporting the existing literature and the current state of knowledge in the application of these guidelines. In particular, we will endeavor to address the following questions: (1) what is the current adherence to the reporting standards that include the CONSORT,Citation26 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE),Citation27 Quality of Reporting of Meta-analysis – (QUOROM),Citation28 Transparent Reporting of Evaluations with Nonrandomized Designs (TREND),Citation29 Meta-analysis Of Observational Studies in Epidemiology (MOOSE),Citation30 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines?Citation31 (2) What are the factors that are associated with adherence to reporting standards in medical literature? And (3) what guidance can we provide based on the current state of knowledge on adherence to reporting standards? More specifically the objectives of this review are to:
Report the levels of adherence to the above reporting guidelines in clinical research;
Determine the key factors associated with adherence to good reporting; and
Provide recommendations to enhance adherence to reporting guidelines for future studies.
We preselected the six guidelines above because they are among the oldest and the most popular, spanning through a wide range of study designs and clinical areas, and are therefore likely to be reported in systematic reviews, thus potentially generating a number of reviews to be included in this study.
Methods
We adopted a “systematic” scoping review approach – which is a combination of a scoping review methodology – to ensure the inclusion of broad areas of research and study designs, and a systematic review of reviews methodology.Citation32,Citation33
A scoping review is a relatively new type of study providing an assessment of available evidence from the literature in a broad area of research such as the compliance in the reporting of clinical studies to established guidelines. It also serves to identify gaps in the field and provide recommendations for implementation.Citation32 The methodology of scoping reviews was first described in detail by Arksey and O’MalleyCitation32 in their pivotal paper published in 2005, which provided a foundation for carrying out a scoping review. This framework was further operationalized, and five stages were proposed to be followed when conducting a scoping review, including: (1) the identification of a research question; (2) finding the relevant studies; (3) the selection of studies to be included in the review; (4) data extraction from the included studies; and (5) assembling, summarizing, and reporting the results of the review.Citation34
The methods of conducting a systematic review of systematic reviews follow a similar approach, but include the provision of guidelines and suggestions for clinical practice, education, and research.Citation33 The aim of the methods and search strategy here is to ensure that the systematic review of reviews is comprehensive, thorough, and objective. We will report the results using the PRISMA (formerly QUOROM) reporting guidelines for systematic reviews.Citation35 A protocol was specifically designed for this study outlining the study design, search strategy, and selection criteria.
Data sources and search strategy
Electronic literature databases including Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Embase, and Medline (from January 1996 [date of CONSORT] to September 2012) were searched using a comprehensive search strategy designed with the assistance of a librarian who is experienced in conducting systematic reviews. The reference lists of identified articles were also reviewed for additional studies, and a manual search of key journals like BioMed Central systematic reviews, BioMed Central Research Methodology, and the Cochrane Library was conducted to avoid missing relevant reviews. Such search strategies are well supported for this type of systematic search and retrieval of relevant studies.Citation36,Citation37 The databases were searched for the following key search terms: (Systematic reviews OR reviews OR quality of reporting OR completeness of reporting) AND (CONSORT OR STROBE OR QUOROM OR QUORUM OR PRISMA OR TREND OR MOOSE) OR adherence. For Web of Science, we also performed a forward citation search of the publications pertaining to reporting guidelines, whose acronyms might have other meanings, such as TREND and QUORUM. This helped us to decrease the occurrence of false positives in our search.
Initially, no language limits were set to identify the number of non-English reviews; however, a limit was then set for English language reviews only (which was necessary due to the lack of resources required to translate reviews from other languages). We also set the limits to “human” and “published complete systematic reviews.”
Inclusion criteria
Systematic reviews of clinical studies addressing the quality of reporting of the studies based on at least one of the six preselected reporting guidelines: CONSORT for RCTs; TREND for non-RCTs; STROBE for observational studies; and PRISMA (formerly QUOROM) or MOOSE for systematic reviews of RCTs or observational studies, respectively.
The systematic reviews must be complete (not abstracts only), reported in English, and investigating the quality of reporting in human studies of all age groups using one of the above guidelines.
The quality of reporting guidelines must be the primary focus of the systematic review.
Exclusion criteria
Systematic reviews were excluded if they were published as abstract only; the primary focus of the review was not on the quality of reporting; the quality of reporting was based on the standards of reporting that were different from the ones stated above, or if they were a duplicate publication of existing reviews (commentaries, letters, and editorials).
Selection of systematic reviews
Two independent reviewers examined the titles and abstracts of all citations identified in the literature search. Articles were selected for full-text review if the inclusion criteria were met and if both reviewers considered the citation potentially relevant. Disagreement at any stage of study selection was resolved by discussion and consensus between the two reviewers. If agreement could not be reached a third author was recruited to determine eligibility. Initial agreement between the two reviewers was calculated using the kappa statistic.Citation38
Each reviewer independently:
Assessed retrieved titles and abstracts for relevance and duplication;
Screened full text articles deemed eligible for inclusion;
Decided on including or excluding articles;
Extracted relevant data using specifically designed data abstraction forms;
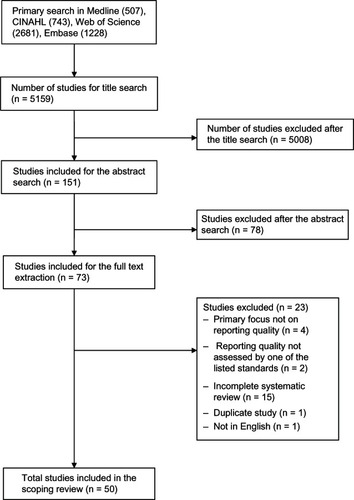
Appraised the quality of the included reviews. A PRISMA flow diagram of included/excluded studies is provided ().Citation35
Quality assessment of systematic reviews
The quality of each systematic review that met the inclusion criteria for the study was assessed using a modified version of the assessment of multiple systematic reviews (AMSTAR, a validated tool to assess the methodological quality of systematic reviews).Citation39–Citation41 Certain items of AMSTAR are not relevant to this type of review and cannot be assessed (eg, item 9, “Were the methods used to combine the findings of studies appropriate?”), as pooling of data may not be feasible in all systematic reviews of methodological quality, and should relate to the study question. In addition, item 10 (“Was the likelihood of publication bias assessed?”) is irrelevant to this review, which is focused on the quality of reporting of published studies. Both of these items were omitted from the quality assessment.
We also used the modified version of the enhanced Overview Quality Assessment Questionnaire (OQAQ) to assess the quality of systematic reviews included in this study.Citation42 In addition to these tools, we assessed the quality of the reviews based on the following criteria: the use of explicit criteria to assess individual study quality using the guidelines checklist; explicit definition of the research question using a flow diagram to explain study selection; and a formal sample size calculation for the assessment of association.
Data abstraction
A spreadsheet was created to record the following items from the selected reviews: authors, year of publication, number of primary studies included in the review, study location, study type, primary outcomes of the study, outcomes measures, and the overall results and conclusions. Two authors independently piloted the data extraction form for this review and modifications were made when necessary before reaching the final data abstraction forms used for this study. Data abstraction disagreements were resolved by discussion and consensus, and a third author extracted the data if an agreement could not be reached. Data collected from each systematic review included the study primary question, primary outcome, number of the studies included in the review, the statement investigated, quality assessment, the factors associated with adherence, the journal of publication, and whether the journal endorsed the statement in question.
Analysis
The level of agreement between raters was estimated using the kappa statistic. The adherence to reporting standards was summarized, and key determinants of adherence were identified in a narrative manner.
Results
Study selection
Our search retrieved 5159 articles from the four electronic databases searched. Following searching through the title, abstract, and full text screening, 50 articles were selected and included for data extraction and quality assessment (). The strength of agreement between two independent raters on abstract screening was substantial (Kappa = 0.65; 95% confidence interval [CI] 0.53, 0.76; P < 0.001), and almost perfect for full text screening (Kappa = 0.94; 95% CI 0.85, 1.00; P < 0.001). Agreement was also substantial for the quality assessment using the modified OQAQ/AMSTAR checklist (Kappa = 0.63; 95% CI 0.42, 0.85; P < 0.001).
Study characteristics
Forty-one studies (82.0%) assessed RCTs using the CONSORT Statement, five (10.0%) studies used the QUOROM checklist, and two studies (4.0%) used the PRISMA tool. The final three systematic reviews (6.0%) consisted of two reviews assessing both RCTs and observational studies using the CONSORT and STROBE guidelines, and the last study used both the QUOROM and PRISMA guidelines. The systematic reviews were published in a wide variety of journals and were led by authors from many different countries (). The median and interquartile range of the number of studies included in each review were 78 and 80.5, respectively.
Table 1 Characteristics of included studies
Adherence to reporting guideline
The adherence of the studies included in the systematic reviews to their respective guidelines, and the author’s conclusions, are shown in . Forty-three (86.0%) of the included studies concluded that the adherence to reporting guidelines was inadequate, poor, medium, or suboptimal, or that some improvement was needed. No combined, quantitative result was generated from the 50 systematic reviews due to differences in the measurement tools used by the individual reviews.
Table 2 Description of the studies’ findings
CONSORT Statement
The adherence of RCTs to the CONSORT Statement was assessed with different versions of the CONSORT checklist. These checklists ranged from eight to 63 items, except for two studies that used the 212 subitem, Nelson–Moberg–Norton Expanded CONSORT instrument and the 201 subitem Nelson–Moberg Expanded CONSORT instrument. The revisions of the CONSORT Statements were usually based on the specific field of the RCT, and the applicability of the items on the CONSORT checklist to that field. For instance, Bian et alCitation43 used a revised 63-item CONSORT checklist designed for Chinese Herbal Medicine clinical trials. In addition to the CONSORT checklist, four studies (Augestad et al,Citation44 Balasubramanian et al,Citation45 Kiehna et al,Citation46 and Moher et al)Citation47 also used the five-point Jadad instrument to assess the quality of the individual RCTs.Citation39
Of the 41 systematic reviews assessing RCTs reporting adherence to the CONSORT Statement, 33 (80%) of them concluded that some improvement was needed, or that the reporting quality was inadequate, poor, medium, or suboptimal (). Furthermore, the authors recommended the use of the CONSORT Statement as a guideline to improve the quality of reporting of RCTs. Eight studies did not report inadequate reporting quality of RCTs. Froud et alCitation48 concluded that cluster randomized trials in oral health had a reasonable quality. Fung et alCitation49 reported that the overall level of reporting was acceptable and the reporting quality has improved since the creation of CONSORT and STROBE statements. Ladd et alCitation25 also concluded that the overall reporting quality had improved since 1994 and the articles published in journals that endorse the CONSORT Statement had the highest levels of adherence to reporting guidelines. Moher et alCitation47 only compared the quality of pediatric complementary and alternative medicine RCTs and reported 40% of the CONSORT checklist items were included in these RCTs. Montgomery et alCitation50 evaluated the RCTs qualitatively and found that there was a varying level of reporting quality in factorial trials of complex interventions in community settings. Plint et alCitation51 compared RCTs from CONSORT-endorsing and nonendorsing journals, and their results suggested some improvement in the quality of reporting when the CONSORT checklist is used. Wangge et alCitation52 suggested that adherence to reporting guidelines for noninferiority trials have improved slightly since the CONSORT Statement has been published. Lastly, Zintzaras et alCitation53 did not comment directly on an overall quality of reporting and concluded that adhering to reporting standards can ensure proper assessment of the results.
Table 3 Studies’ conclusions
PRISMA, QUOROM, and STROBE statements
Three studies examined adherence to the PRISMA guidelines, and all concluded that the adherence of the assessed systematic reviews was poor or moderate. Ma et alCitation74 and Willis et alCitation88 used the 27-item PRISMA checklist to assess the level of adherence. Ma et alCitation74 found that systematic reviews on traditional Chinese medicine published in Chinese journals had low adherence to the PRISMA checklist. Willis et alCitation88 also concluded that adherence to the PRISMA checklist was generally poor for published meta-analyses of diagnostic tests. Weir et alCitation87 used an integrated score consisting of both the PRISMA and QUOROM criteria and found that systematic reviews of empirical computerized provider order-entry research were only of moderate quality.
The assessment of studies’ adherence to the QUOROM guideline was done with the 18-item QUOROM checklist coupled with a ten-item OQAQ checklist in three studies. Bereza et alCitation56 and Junhua et alCitation70 reported that there was a need to improve the quality of reporting of reviews, while Shea et alCitation82 concluded that the quality of Cochrane musculoskeletal systematic reviews was good. Hemels et alCitation68 used only the QUOROM checklist and they concluded that the quality of meta-analyses in studies on major depressive disorder was marginally acceptable. Vigna-Taglianti et alCitation85 used the QUOROM checklist with a specific weighting system for each of the headings and the average score was 29.9/50. No conclusions concerning adherence were made, although the authors did recommend the use of manuals to prepare guidelines for the management of breast and colon cancers. Lastly, as described in the previous paragraph, Weir et alCitation87 used an integrated score containing both PRISMA and QUOROM criteria.
The studies by Fung et alCitation49 and Parsons et alCitation79 assessed the adherence of both RCTs and observational studies to their respective guidelines. Parsons et alCitation79 found there was a general lack of statistical rigor.
Factors associated with adherence to reporting guidelines
Although we included systematic reviews assessing the adherence of research articles to four different guidelines, only systematic reviews related to the CONSORT Statement reported on the factors that were associated with adherence to the guideline (). The exception was Hemel et al,Citation68 who concluded that the overall quality of reporting of meta-analyses using the QUOROM guidelines did not significantly change over time, and that the year of publication was not associated with change in adherence. From the CONSORT-related studies, the following are the factors that were reported to be significantly associated with an increase in adherence to the CONSORT Statement or to the quality of reporting of RCTs, as well as the number of studies reporting these factors: publication in CONSORT-endorsing journals (3); declared funding source (1); high impact factor (3); industrial funding (1); multicenter studies (1); non-Chinese reports (compared to those published in mainland China) (1); number of authors (1); reporting of allocation concealment (1); reporting in a medical journal (1); reporting method of sequence generation (1); sample size (3); trial quality (1); type of intervention (pharmacologic intervention versus nonpharmacologic intervention); and year of publication (before and after CONSORT) (9). These factors are summarized in . Having a positive outcome in RCTs (compared to a neutral or negative outcome) was the only factor reported to be significantly associated with a decrease in adherence to the CONSORT Statement (Spearman correlation = −0.192; 95% CI, −0.351 to −0.011).Citation55 Other factors that reported but did not reach statistical significance for an association with adherence to the CONSORT Statement are also summarized in .
Table 4 Factors associated with reporting quality of articles using the CONSORT guideline
Quality of included studies, measured by the modified OQAQ/AMSTAR checklist
The global score of each of the studies is listed in . The mean global score of the 50 included studies was 16.6 ± 2.4. Twenty-one (42%) out of the 50 studies had a global score of 17 or more. The items with the lowest scores were question 5, “Was information on included and excluded studies provided?” and question 6, “Were the characteristics of included studies provided?” with only 16% and 32% of the studies reporting each of these items correctly, respectively.
Table 5 Reporting quality of the 50 included systematic reviews, assessed by the modified AMSTAR/OQAQ (ten items, score out of 20)
Discussion
We undertook a systematic scoping review of systematic reviews to investigate the adherence to reporting guidelines that included the CONSORT, PRISMA, QUOROM, TREND, MOOSE, and STROBE statements. Our systematic review included 50 studies that fulfilled our inclusion criteria, most of which originated from North American and European countries (43/50 studies). Despite the widespread acceptance of the CONSORT Statement and its subsequent extensions, the standards of reporting of clinical studies remained suboptimal. Our study showed that 86.0% of the systematic reviews included in this study concluded that there was a suboptimal quality of reporting across multidisciplinary clinical research topics using different study designs including RCTs and observational studies. The adherence of the assessed studies to reporting standards were not specific to any field of clinical research, but rather spanned across various disciplines including diagnostic procedures, interventions, cancer trials, and alternative medicine, implying the widespread lack of adherence to reporting guidelines in the medical literature. Despite the availability of guidelines and operational definitions of how to use these guidelines to improve reporting and transparency of clinical literature (including providing checklists, flow diagrams, and explicit methods of recruitment and allocationCitation12), the uptake of these guidelines remained low. Several shortcomings of the reporting standards of clinical literature include inadequate reporting of the methods, selective reporting of the results, or misinterpretation of the results.Citation91 Studies have shown that the use of these guidelines was associated with better reporting of studies of acupuncture trials,Citation92 and only minimal improvement in the adherence to reporting guidelines of studies that investigated diagnostic accuracy.Citation93 It is possible that the lack of adherence may relate to the narrow focus of these guidelines on specific clinical areas or study designs, and therefore further guidelines need to be developed. Such new guidelines can be developed based on sets of tools and criteria, as proposed previously.Citation94 The poor adherence to reporting guidelines seen in the clinical literature is also seen in other settings including the failure to follow the National Institute of Health guidelines for reporting sex and ethnicity in clinical trials.Citation95 Efforts to address the gap between the standards set by the guidelines and the actual standards of the published literature are therefore needed.
The most striking observation from our study was the lack of consistency in methods of recording the adherence to the reporting guidelines, and therefore it was not possible to combine the results to provide a summary statistic. This highlights the need for a consensus statement on the reporting of methodological quality of studies addressing the adherence to CONSORT and other statements.
Despite the suboptimal adherence to reporting guidelines in most of the studies reviewed, we observed that RCTs have a better adherence to reporting standards than non-RCTs. In addition, studies published in journals endorsing the CONSORT Statement have higher adherence to reporting standards. Not surprisingly, studies published after the introduction of CONSORT showed a better reporting quality and adherence to reporting guidelines. These findings are encouraging and provide a platform to disseminate knowledge generated by this study to multiple disciplines in health research to stress the need for improvement in adherence to reporting guidelines.
The strengths of our study are that we conducted a rigorous systematic review and included studies investigating the quality of reporting across various clinical areas of research, thus adding a scoping review methodology to a systematic review. We have also extracted relevant data and attempted to provide a summary statistic; however, the diversity of the findings did not allow for the computation of results.
Our study results are limited by the lack of reviews addressing adherence standards to other guidelines (MOOSE, TREND, QUOROM), the inability to combine the overall study findings, and the unavailability of tools designed to assess the quality of systematic reviews investigating methodological quality. Furthermore, the design, conduct, analysis, and reporting of the results of the reviews including definitions of outcomes (and predictor variables) varied substantially within and between the guidelines. This is mainly due to the lack of an established framework or standard for the conduct and reporting of reviews assessing the adherence to guidelines.
The study findings are nonetheless important for educators, authors, editors, sponsors, health consumers, and research ethics boards.
Summary and recommendations
Factors that are associated with reporting standards can be grouped into four categories:
Study design: Better reporting standards were seen in studies with large sample sizes; RCT design; transparency in reporting randomization, adverse events, and secondary outcomes; and studies of drug interventions.
Timing of publication: Studies that were published more recently were associated with better quality of reporting.
Study sponsor: Studies with an industrial sponsor were also associated with a better quality of reporting.
Journal: Journals with a high impact factor and those endorsing the CONSORT Statement and its extensions tended to publish studies with better adherence to reporting standards.
Recommendations for educators
Educators are at the forefront of teaching research methodology and applications in clinical settings, and therefore they play an important role in improving the reporting standards of clinical literature. Educators need to emphasize the importance of reporting standards and incorporate the guidelines in research training. They also need to provide ongoing training through workshops at professional meetings, and highlight the factors shown to improve the quality of reporting to foster improved reporting standards of the clinical literature.
Recommendations for authors
Authors should use the reporting standards appropriate to the study design as a guide to planning and reporting studies, and provide a flow diagram and checklist that will not only improve the reporting standard and adherence to guidelines, but will also help with transparency and reproducibility of the study. The use of the guidelines will also help to minimize reporting bias. For resources on using reporting standards, see the EQUATOR Network website.Citation12
Recommendations for editors
Studies published in journals endorsing the CONSORT and its extensions were described as having better reporting quality and increased adherence to guidelines. Therefore, editors must endorse the reporting standards as part of their journal editorial policy.
Furthermore, inclusion of the respective guideline checklist must also be part of the editorial policy. Editors need to consider assessing the adherence to reporting guidelines as a requirement for peer review, and they should revise the peer review process to incorporate these assessments.
Recommendations for sponsors
Sponsors can ensure that the quality of the study methodology and transparency are meeting these standards by requesting adherence to the respective reporting guidelines appropriate for the study design.
Recommendations for research ethics boards
Institutional Review Boards or Research Ethics Boards have a substantial responsibility to ensure ethical and sound methodological quality of clinical studies. Therefore, we recommend that Institutional Review Boards/Research Ethics Boards require that protocols be submitted for ethical approval to clearly state what reporting standards the study will be using based on the study design, and that reporting guidelines checklist are part of the application for ethics approval.
Recommendation for health consumers
In accordance with the general principles of evidence-based health care practice,Citation96 we encourage consumers or health care users to be actively involved in their health care by discussing their care options with their providers. Understanding information presented in published studies can be an important ingredient in these discussions. We suggest that health care users consider the evaluation of the quality of the information presented in the literature by looking for a guideline statement and a checklist to ensure the study reporting followed a certain standard that is appropriate for the particular study design.
Lastly, one element that all parties need to take into consideration is the importance of conducting large studies. Large studies have been shown to have a better quality of reporting.Citation81,Citation84,Citation97 Large studies are also less prone to problems of bias and have better precision.
Conclusion
Reporting guidelines help to improve the quality and transparency of clinical studies and allow for systematic reviews and meta-analyses to provide evidence worthy of changing practice, improving knowledge, and better management of health and disease. The current reporting standards and adherence to guidelines are poor and are in need of major improvement. Steps need to be taken by all involved in the conducting and reporting of clinical research in order to achieve better standards of reporting, thus minimizing bias and providing reproducible studies that can be combined to reach conclusive evidence.
Acknowledgments
Special thanks to Neera Bhatnagar for the detailed discussion on literature search strategies. This study was funded in part by funds from the CANNeCTIN program.
ZS designed and wrote the first draft of the protocol and current manuscript; LM, DK, VBD, RD, SZ, VF, BD, and MB contributed to the protocol design and writing of the manuscript; LT conceived of the idea for the study and contributed to the design and writing of the manuscript. All authors read and approved the final draft.
Disclosure
The authors report no conflicts of interest in this work.
References
- US National Library of Medicine National Institutes of HealthPubMed.gov [homepage on the Internet]Bethesda, MDNational Center for Biotechnology Information, US National Library of Medicine Available from: http://www.ncbi.nlm.nih.gov/pubmedAccessed February 1, 2013
- US National Library of Medicine National Institutes of HealthPubMed.gov [webpage on the Internet]Bethesda, MDNational Center for Biotechnology Information, US National Library of Medicine2013 Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=depression+systematic+reviewAccessed February 1, 2013
- US National Library of Medicine National Institutes of HealthPubMed.gov [webpage on the Internet]Bethesda, MDNational Center for Biotechnology Information, US National Library of Medicine2013 Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=cancer+systematic+reviewAccessed February 1, 2013
- GreavesCSheppardKAbrahamCSystematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventionsBMC Public Health201111111921333011
- TennantRGoensCBarlowJDayCStewart-BrownSA systematic review of reviews of interventions to promote mental health and prevent mental health problems in children and young peopleJournal of Public Mental Health2007612532
- ErnstEA systematic review of systematic reviews of homeopathyBr J Clin Pharmacol200254657758212492603
- BloomBSEffects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviewsInt J Technol Assess Health CareSummer200521338038516110718
- ErnstECanterPHA systematic review of systematic reviews of spinal manipulationJ R Soc Med200699419219616574972
- De NietGJTiemensBGKloosMWHutschemaekersGJReview of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomniaInt J Evid Based Healthc20097423324221631864
- BrouwersMCGarciaKMakarskiJDarazLThe landscape of knowledge translation interventions in cancer control: What do we know and where to next? A review of systematic reviewsImplementation science: IS2011613022185329
- RichardsDThe EQUATOR network and websiteEvid Based Dent20078411718158552
- EQUATOR NetworkEnhancing the QUAlity and Transparency Of health Research [homepage on the Internet]Oxford, UKEQUATOR Network2011 Available from: http://www.equator-network.org/home/Accessed May 13, 2012
- SimeraIAltmanDGMoherDSchulzKFHoeyJGuidelines for reporting health research: the EQUATOR network’s survey of guideline authorsPLoS Med200856e13918578566
- CONSORTCONSORT Transparent Reporting of Trials [homepage on the Internet] Available from: http://www.consort-statement.org/Accessed May 13, 2012
- SchulzKAltmanDMoherDGroup The CONSORT GroupCONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trialsBMC Medicine2010811820334633
- ShikataSNakayamaTYamagishiHQuality of surgical randomized controlled trials for acute cholecystitis: assessment based on CONSORT and additional check itemsJ Hepatobiliary Pancreat Surg200815329730318535768
- SütNSenocakMUysalOKöksalanHAssessing the quality of randomized controlled trials from two leading cancer journals using the CONSORT statementHematol Oncol Stem Cell Ther200811384320063527
- UetaniKNakayamaTIkaiHYonemotoNMoherDQuality of reports on randomized controlled trials conducted in Japan: evaluation of adherence to the CONSORT statementIntern Med200948530731319252352
- ZiogasDCZintzarasEAnalysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statementAnn Epidemiol200919749450019523596
- AlvarezFMeyerNGourraudPAPaulCCONSORT adoption and quality of reporting of randomized controlled trials: a systematic analysis in two dermatology journalsBr J Dermatol200916151159116519681881
- AreiaMSoaresMDinis-RibeiroMQuality reporting of endoscopic diagnostic studies in gastrointestinal journals: where do we stand on the use of the STARD and CONSORT statements?Endoscopy201042213814720140830
- DasíFNavarro-GarcíaMMJiménez-HerediaMEvaluation of the quality of publications on randomized clinical trials using the Consolidated Standards of Reporting Trials (CONSORT) statement guidelines in a Spanish tertiary hospitalJ Clin Pharmacol20125271106111421593281
- HeJDuLLiuGQuality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviewsTrials20111212221569452
- SmithBALeeHJLeeJHQuality of reporting randomized controlled trials (RCTs) in the nursing literature: application of the consolidated standards of reporting trials (CONSORT)Nurs Outlook2008561313718237622
- LaddBOMcCradyBSManuelJKCampbellWImproving the quality of reporting alcohol outcome studies: effects of the CONSORT statementAddict Behav201035766066620207490
- MoherDHopewellSSchulzKFCONSORTCONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trialsInt J Surg2012101285522036893
- NoahNThe STROBE initiative: STrengthening the Reporting of OBservational studies in Epidemiology (STROBE)Epidemiol Infect2008136786518482461
- MoherDCookDJEastwoodSOlkinIRennieDStroupDFImproving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analysesLancet199935491931896190010584742
- TreasureEThe TREND statementEvid Based Dent200454889115608704
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) groupJAMA2000283152008201210789670
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaborationPLoS Med200967e100010019621070
- ArkseyHO’MalleyLScoping studies: towards a methodological frameworkInt J Soc Res Methodol2005811932
- SmithVDevaneDBegleyCMClarkeMMethodology in conducting a systematic review of systematic reviews of healthcare interventionsBMC Med Res Methodol20111111521291558
- BrienSELorenzettiDLLewisSKennedyJGhaliWAOverview of a formal scoping review on health system report cardsImplement Sci201052220205791
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaborationBMJ2009339b270019622552
- MontoriVMWilczynskiNLMorganDHaynesRBOptimal search strategies for retrieving systematic reviews from Medline: analytical surveyBMJ200533074826815619601
- WilczynskiNLHaynesRBEMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviewsJ Clin Epidemiol2007601293317161751
- CohenJA coefficient of agreement for nominal scalesEducational and Psychological Measurement196020137
- SheaBJHamelCWellsGAAMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviewsJ Clin Epidemiol200962101013102019230606
- SheaBJBouterLMPetersonJExternal validation of a measurement tool to assess systematic reviews (AMSTAR)PLoS One2007212e135018159233
- SheaBJGrimshawJMWellsGADevelopment of AMSTAR: a measurement tool to assess the methodological quality of systematic reviewsBMC Med Res Methodol200771017302989
- OxmanADGuyattGHSingerJAgreement among reviewers of review articlesJ Clin Epidemiol199144191981824710
- BianZXMoherDDagenaisSImproving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting quality. Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine200643233242
- AugestadKMBerntsenGLassenKBellikaJGWoottonRLindsetmoROStandards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision supportJ Am Med Inform Assoc2012191132121803926
- BalasubramanianSPWienerMAlshameeriZTiruvoipatiRElbourneDReedMWStandards of reporting of randomized controlled trials in general surgery: can we do better?Annals of surgery2006244566366717060756
- KiehnaENStarkeRMPouratianNDumontASStandards for reporting randomized controlled trials in neurosurgeryJ Neurosurg2011114228028521054137
- MoherDSoekenKSampsonMBen-PoratLBermanBAssessing the quality of reports of systematic reviews in pediatric complementary and alternative medicineBMC Pediatr20022311914146
- FroudREldridgeSDiaz OrdazKMarinhoVCDonnerAQuality of cluster randomized controlled trials in oral health: a systematic review of reports published between 2005 and 2009Community Dent Oral Epidemiol201240Suppl 131422369703
- FungAEPalankiRBakriSJDepperschmidtEGibsonAApplying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studiesOphthalmology2009116228629619091408
- MontgomeryAAAstinMPPetersTJReporting of factorial trials of complex interventions in community settings: a systematic reviewTrials20111217921771302
- PlintACMoherDMorrisonADoes the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic reviewThe Medical journal of Australia2006185526326716948622
- WanggeGKlungelOHRoesKCde BoerAHoesAWKnolMJRoom for improvement in conducting and reporting non-inferiority randomized controlled trials on drugs: a systematic reviewPLoS One2010510e1355021048948
- ZintzarasEKitsiosGDPapathanasiouAARandomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysisClin Ther201032222123720206780
- Al-NamankanyAAAshleyPMolesDRParekhSAssessment of the quality of reporting of randomized clinical trials in paediatric dentistry journalsInt J Paediatr Dent200919531832419320912
- BathFJOwenVEBathPMQuality of full and final publications reporting acute stroke trials: a systematic reviewStroke19982910220322109756604
- BerezaBGMachadoMEinarsonTRAssessing the reporting and scientific quality of meta-analyses of randomized controlled trials of treatments for anxiety disordersAnn Pharmacother200842101402140918728102
- BousquetPJCalderonMADemolyPThe Consolidated Standards of Reporting Trials (CONSORT) Statement applied to allergen-specific immunotherapy with inhalant allergens: a Global Allergy and Asthma European Network (GA(2)LEN) articleThe Journal of Allergy and Clinical Immunology20111271495656e41e1121112079
- CapiliBAnastasiJKGeigerJNAdverse event reporting in acupuncture clinical trials focusing on painClin J Pain2010261434820026952
- CavadasVBrancoFCarvalhoFLOsorioLGomesMJSilva-RamosMThe quality of reporting of randomized controlled trials in pelvic organ prolapseInt Urogynecol J20112291117112521484364
- ChowersMYGottesmanBSLeiboviciLPielmeierUAndreassenSPaulMReporting of adverse events in randomized controlled trials of highly active antiretroviral therapy: systematic reviewAntimicrob Chemother2009642239250
- CookDALevinsonAJGarsideSMethod and reporting quality in health professions education research: a systematic reviewMed Educ201145322723821299598
- de VriesTWvan RoonENLow quality of reporting adverse drug reactions in paediatric randomised controlled trialsArch Dis Child201095121023102620551194
- EthgenMBoutronLStegPGRoyCRavaudPQuality of reporting internal and external validity data from randomized controlled trials evaluating stents for percutaneous coronary interventionBMC Med Res Methodol200992419358717
- EyawoOLeeCWRachlisBMillsEJReporting of noninferiority and equivalence randomized trials for major prostaglandins: a systematic survey of the ophthalmology literatureTrials200896919055743
- FarrokhyarFChuRWhitlockRThabaneLA systematic review of the quality of publications reporting coronary artery bypass grafting trialsCan J Surg200750426627717897515
- GagnierJJDeMeloJBoonHRochonPBombardierCQuality of reporting of randomized controlled trials of herbal medicine interventionsAm J Med20061199800e801e81116945616
- HalpernSHDaraniRDouglasMJWightWYeeJCompliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trialsInt J Obstet Anesth200413420721415477048
- HemelsMEVicenteCSadriHMassonMJEinarsonTRQuality assessment of meta-analyses of RCTs of pharmacotherapy in major depressive disorderCurr Med Res Opin200420447748415119985
- HerdanARothRGrassDImprovement of quality of reporting in randomised controlled trials to prevent hypotension after spinal anaesthesia for caesarean sectionGynecol Surg20118212112721654900
- JunhuaZHongcaiSXiumeiGMethodology and reporting quality of systematic review/meta–analysis of traditional Chinese medicineJ Evid Based Complementary Altern Med2007138797805
- KoberTTrelleSEngertAReporting of randomized controlled trials in Hodgkin lymphoma in biomedical journalsJ Natl Cancer Inst200698962062516670387
- LiJYZhangYFSmithGSQuality of reporting of randomized clinical trials in tai chi interventions-a systematic reviewEvidence-Based Complementary and Alternative Medicine2011201138324519351709
- LuLZengJChenYQuality of reporting in randomized controlled trials conducted in China on the treatment of cancer painExpert Rev Anticancer Ther201111687187721707284
- MaBGuoJQiGEpidemiology, quality and reporting characteristics of systematic reviews of traditional Chinese medicine interventions published in Chinese journalsPLoS One201165e2018521633698
- MarshmanZFaridFThe quality of reporting of randomised controlled trials in dental public healthCommunity dental health201027425325621473363
- Moberg-MogrenENelsonDLEvaluating the quality of reporting occupational therapy randomized controlled trials by expanding the CONSORT criteriaAm J Occup Ther200660222623516596926
- MontaneEVallanoAVidalXAguileraCLaporteJRReporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic reviewBMC Clin Pharmol2010102
- Norton-MabusJCNelsonDLReporting of randomized controlled trials in occupational therapy and speech therapy: evaluation using an expansion of the CONSORT statementOTJR (Thorofare N J)20082826471
- ParsonsNRHiskensRPriceCLAchtenJCostaMLA systematic survey of the quality of research reporting in general orthopaedic journalsJ Bone Joint Surg Br20119391154115921911523
- PiggottMMcGeeHFeuerDHas CONSORT improved the reporting of randomized controlled trials in the palliative care literature? A systematic reviewPalliat Med2004181323814982205
- RiosLPOdueyungboAMoitriMORahmanMOThabaneLQuality of reporting of randomized controlled trials in general endocrinology literatureJ Clin Endocrinol Metab200893103810381618583463
- SheaBBouterLMGrimshawJMScope for improvement in the quality of reporting of systematic reviews. From the Cochrane Musculoskeletal GroupJ Rheumatol200633191516267878
- StrechDSoltmannBWeikertBBauerMPfennigAQuality of reporting of randomized controlled trials of pharmacologic treatment of bipolar disorders: a systematic reviewJ Clin Psychiatry20117291214122121294992
- ThabaneLChuRCuddyKDouketisJWhat is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trialsInt J Obes (Lond)200731101554155917452988
- Vigna-TagliantiFVineisPLiberatiAFaggianoFQuality of systematic reviews used in guidelines for oncology practiceAnn Oncol200617469170116461333
- WalleserSHillSRBeroLACharacteristics and quality of reporting of cluster randomized trials in children: reporting needs improvementJ Clin Epidemiol201164121331134021775103
- WeirCRStaggersNLaukertTReviewing the impact of computerized provider order entry on clinical outcomes: The quality of systematic reviewsInt J Med Inform201281421923122342868
- WillisBHQuigleyMThe assessment of the quality of reporting of meta-analyses in diagnostic research: a systematic reviewBMC Med Res Methodol20111116322151233
- ZhongYZWeiJiangHongliFan Taoet al. Quality of reporting of two-group parallel randomized controlled clinical trials of multi-herb formulae: A survey of reports indexed in the Science Citation Index ExpandedEur J Integr Med201134E303E310
- ZiogasDCZ EAnalysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statementAnn Epidemiol200919749450019523596
- SimeraIMoherDHirstAHoeyJSchulzKFAltmanDGTransparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR NetworkBMC Medicine201082420420659
- PradySLRichmondSJMortonVMMacphersonHA systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trialsPLoS One200832e157718270568
- SmidtNRutjesAWvan der WindtDAThe quality of diagnostic accuracy studies since the STARD statement: has it improved?Neurology200667579279716966539
- MoherDSchulzKFSimeraIAltmanDGGuidance for developers of health research reporting guidelinesPLoS medicine2201072e100021720169112
- GellerSAdamsMCarnesMAdherence to federal guidelines for reporting of sex and race/ethnicity in clinical trialsJ Womens Health (Larchmt)200615101123113117199453
- GuyattGHHaynesRBJaeschkeRZUsers’ Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users’ Guides to patient care. Evidence-Based Medicine Working GroupJAMA2000284101290129610979117
- AleissaSParsonsDGrantJHarderJHowardJDeep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factorsCan J Surg201154426326921658334