Abstract
Tuberculosis, malaria, and HIV are among the most lethal diseases, with AIDS (Acquired Immune Deficiency Syndrome) being a chronic and potentially life-threatening condition caused by the human immunodeficiency virus (HIV). Individually, each of these infections presents a significant health challenge. However, when tuberculosis, malaria, and HIV co-occur, the symptoms can worsen, leading to an increased mortality risk. Mathematical models have been created to study coinfections involving tuberculosis, malaria, and HIV. This systematic literature review explores the importance of coinfection models by examining articles from reputable databases such as Dimensions, ScienceDirect, Scopus, and PubMed. The primary emphasis is on investigating coinfection models related to tuberculosis, malaria, and HIV. The findings demonstrate that each article thoroughly covers various aspects, including model development, mathematical analysis, sensitivity analysis, optimal control strategies, and research discoveries. Based on our comprehensive evaluation, we offer valuable recommendations for future research efforts in this field.
Introduction
Human immunodeficiency virus (HIV) is responsible for causing acquired immunodeficiency syndrome (AIDS).Citation1 The transmission of HIV occurs when infected individuals come into contact with certain bodily fluids, such as blood, semen, breast milk, and vaginal secretions.Citation2 Even though a cure for HIV is not available, the virus can be effectively managed through the use of antiretroviral (ARV) drugs, to control its progression.Citation3 HIV epidemic significantly influences the prevalence of tuberculosis infections andCitation4 the virus compromises the immune system, making individuals who are infected highly susceptible to different infectious diseases.Citation5
Tuberculosis (TB) is transmitted through the air when infected individuals speak, sneeze, or cough, making it an airborne disease. It is caused by Mycobacterium tuberculosis, a bacterium that primarily targets the lungs but can also affect other.Citation6 After successful treatment, individuals who have been declared cured can still be susceptible to reinfection. Failure to consistently take the prescribed medication for the designated duration can lead to the development of Multi-Drug Resistant Tuberculosis (MDR-TB), where the bacteria become resistant to drugs.Citation7 Even individuals who are subjected to treatment can still transmit tuberculosis when the virus remains active in their bodies.Citation8 According to the 2013 global report by the World Health Organization (WHO), approximately one-third of the population is affected by the infection. The 2020 global report stated that around 9.96 million individuals were estimated to have contracted tuberculosis in 2020.Citation9 Furthermore, between 2000 and 2019, the diagnosis and treatment efforts saved the lives of approximately 63 million people.Citation10 Among individuals coinfected with HIV and tuberculosis, tuberculosis is the primary cause of mortality.Citation11 Globally, approximately 10.6 million individuals contracted tuberculosis in 2022, marking an increase from the estimated 10.3 million cases in 2021. There is a possibility of a resurgence in the declining trend observed before the pandemic, anticipated to take place in either 2023 or 2024.Citation12
Malaria is caused by a parasite called Plasmodium.Citation13 Common signs of malaria consist of fever, muscle pain, fatigue, and chills. In severe instances, the illness can result in fatalities. The disease is a highly fatal contagious illness solely transmitted by Anopheles mosquitoes.Citation12 The transmission occurs when female mosquitoes infected with the Plasmodium parasite bite humans, passing on the infection.Citation14 Female mosquitoes require blood meals to produce eggs.Citation15 According to World Health Organization (WHO), the count of malaria-endemic countries reporting fewer than 10,000 cases has risen from 26 nations in 2000 to 47 in 2020. Moreover, the number of countries with fewer than 100 native cases has increased from 6 to 26.Citation16 On a worldwide scale in 2022, an approximate 249 million cases of malaria were reported across 85 countries where malaria is prevalent. The incidence of malaria cases in 2022 stood at 58 per 1000 population at risk.Citation17 Global initiatives are in progress to eliminate malaria, which involves the development of novel vaccines and the implementation of insecticides to prevent mosquito bites.
Based on this data, the imperative to take action becomes evident to mitigate the transmission risk and curb the dissemination of tuberculosis, malaria, and HIV.Citation18 As we progress towards controlling and potentially eliminating malaria, HIV, and TB, mathematical models offer a robust framework for assessing the potential impact of interventions. They help identify areas requiring additional empirical research, prioritize crucial policy and research questions, and, most importantly, necessitate improved communication between modelers and those involved in experiments or fieldwork. This collaboration is essential to fine-tune questions, pinpoint critical data, and ensure that analytical work contributes to enhanced policies and effectively controls all three infections.Citation19 This model divides the population into different compartments based on certain assumptions and characteristics.Citation20 Model can also serve to depict the occurrence of epidemic events involving interactions between two diseases. Using this model enables the estimation of individuals who are infected.Citation21,Citation22 Subsequently, through the application of optimal controls, effective recommendations can be identified to manage and curb the transmission of the disease.
In recent years, progress has been made in the development of coinfection model for tuberculosis, malaria, and HIV. This Systematic Literature Review is dedicated to compiling existing model related to coinfection of these diseases and evaluating the extent of research and its corresponding outcomes. Ultimately, the objective is to provide valuable recommendations for future investigations in this field.
Materials and Methods
The method employed for articles to be included in this systematic literature review is the Preferred Reporting Item for Systematic Review and Meta-Analyses (PRISMA).Citation23 PRISMA involves three key stages: identification, screening, and eligibility. In the identification stage, articles pertinent to the research are searched in different databases using specific keywords. During, at the screening stage, all identified articles from multiple databases are consolidated and duplicated entries are removed. The remaining articles are subjected to relevance assessment base on title and abstracts. At this stage, the relevance of the title and abstract in question is adjusted to the keywords used to search for these articles in the previous stage. The article goes to the next stage if the title or abstract contains a combination of these keywords. However, if there is no combination of these keywords, then the article is categorized as irrelevant at this stage. Additionally, the accessibility is checked, and any articles that do not meet the criteria are excluded. Following the screening stage, the articles that pass the initial assessment proceed to the eligibility phase, where a comprehensive evaluation is conducted to ascertain their relevance. At this stage, the relevance of the article in question is adjusted to the research topic of the article to be reviewed, namely mathematical models of tuberculosis, malaria, and HIV/AIDS coinfection in the form of ordinary differential equations. The article goes to the next stage if it is appropriate to the topic. However, if it is inappropriate, the article is categorized as irrelevant at this stage. By following these stages, the selected articles serve as the research material for this systematic literature review, facilitating a thorough exploration of coinfection model for tuberculosis, malaria, and HIV.
This systematic literature review encompasses articles not only focusing on coinfection models involving all three diseases but also combinations of coinfections arising from pairs of distinct diseases. The infectious diseases under investigation in this article include tuberculosis, malaria, and HIV/AIDS. Hence, PRISMA was conducted four times with different keywords. Furthermore, the articles were searched on four databases, namely Dimensions, Scopus, PubMed, and Science Direct. The first PRISMA used keywords: (“Mathematical Model” OR “Mathematical Modelling” OR “Compartmental Model” OR “Transmission Model”) AND (“Coinfection” OR “Co-infection”) AND (“Tuberculosis” OR “TB”) AND (“AIDS” OR “HIV”). The second PRISMA used keywords: (“Mathematical Model” OR “Mathematical Modelling” OR “Compartmental Model” OR “Transmission Model”) AND (“Coinfection” OR “Co-infection”) AND (“Tuberculosis” OR “TB”) AND “Malaria”. Meanwhile, the third used keywords: (“Mathematical Model” OR “Mathematical Modelling” OR “Compartmental Model” OR “Transmission Model”) AND (“Coinfection” OR “Co-infection”) AND “Malaria” AND (“AIDS” OR “HIV”). The last PRISMA used keywords: (“Mathematical Model” OR “Mathematical Modelling” OR “Compartmental Model” OR “Transmission Model”) AND (“Coinfection” OR “Co-infection”) AND (“Tuberculosis” OR “TB”) AND “Malaria” AND (“AIDS” OR “HIV”).
The search limitation for this systematic literature review is as follows:
Research articles published in English language.
Research articles published between 1986 and 2024.
Research articles from Dimensions, ScienceDirect, Scopus, and PubMed databases.
The topic of the research article is the mathematical model of tuberculosis, malaria, and HIV/AIDS coinfection in the form of ordinary differential equations.
Duplication selection employed JabRef, while research topic mapping used VOSviewer. JabRef and VOSviewer were opensource software applications accessible to all users. JabRef enabled users to store their data in a simple text-based file format without being tied to any specific vendor. In contrast, VOSviewer was a software tool designed for creating and visualizing bibliometric networks.
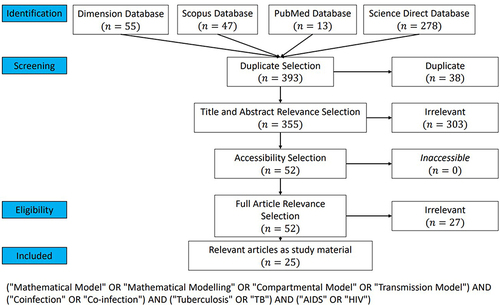
showed the first PRISMA for articles on tuberculosis and HIV/AIDS coinfection models. A total of 55, 47, 13, and 278 articles were obtained from Dimension, Scopus, PubMed, and Science Direct, while 38 articles were duplicated. The screening process yielded 52 articles that proceeded to the eligibility stage. Subsequently, these 25 articles were deemed suitable for inclusion in this systematic literature review.
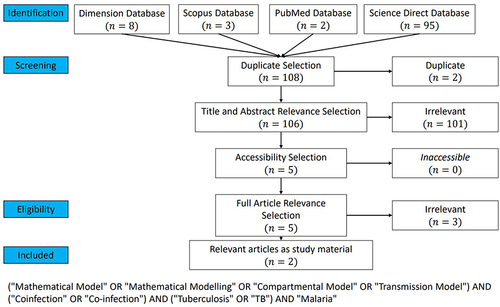
showed the second PRISMA for articles on tuberculosis and malaria coinfection model. A total of 8, 3, 2, and 95 articles were obtained from Dimension, Scopus, PubMed, and Science Direct. After going through all stages of PRISMA, only 2 articles met the criteria for this systematic literature review.
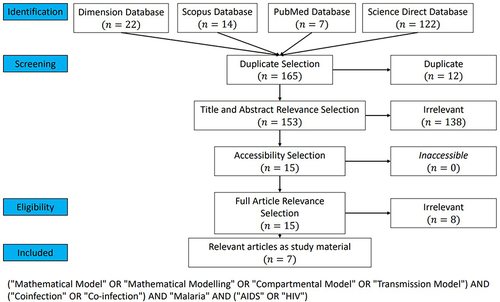
showed the third PRISMA for articles on malaria and HIV/AIDS coinfection model. A total of 22, 14, 7, and 122 articles were obtained from Dimension, Scopus, PubMed, and Science Direct were obtained, while 12 articles are duplicates. The screening result was 15 articles that proceed to the eligibility stage and only 7 articles were included in this systematic literature review.
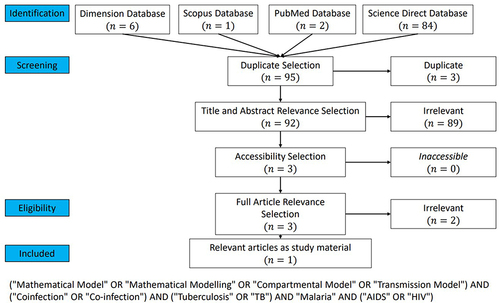
showed the fourth PRISMA for articles on tuberculosis, malaria, and HIV/AIDS coinfection model. A total of 6, 1, 2, and 84 articles were obtained from Dimension, Scopus, PubMed, and Science Direct. After going through all stages of PRISMA, only 1 article met the criteria for this systematic literature review.
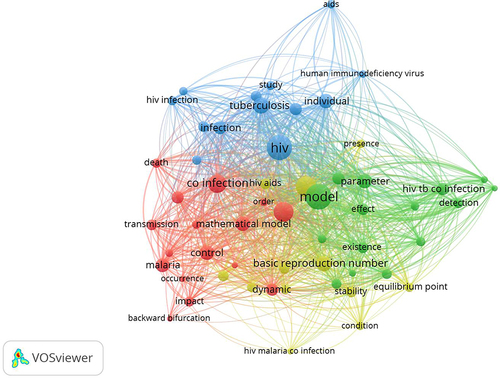
The articles retrieved from the four PRISMA searches were combined, and the keywords were mapped using VOSviewer. presented the resulting mapping, indicating connections between mathematical model, coinfection, HIV/AIDS, malaria, and tuberculosis. The circle shape represents the keywords contained in these articles. The bigger the circle, the more frequently the keyword appears in the articles. The lines connecting the circles illustrate the relationship between these keywords. The circle for the keyword HIV is larger than the circle for the keyword tuberculosis and the circle for the keyword malaria. This is because search results show that HIV co-infection is more common than tuberculosis or malaria. Based on the most occurrences, the keywords that appeared the most were “model” 80 times, “HIV” 73 times, “disease” 49 times, and “co-infection” 40 times. Meanwhile, “tuberculosis” and “malaria” appeared 29 and 22 times.
Results and Discussion
The articles derived from the analysis of the four PRISMA analyses were examined and assessed. The articles were scrutinized, focusing on aspects such as model construction, mathematical analysis, sensitivity analysis, and optimal control. The outcomes were described, and a summary of the findings was provided. In conclusion, recommendations were offered to guide future research in potential areas of development. The objective of this systematic literature review addressed the following inquiries:
1. Which disease coinfection model does the article include?
2. Is the coinfection model mathematically analyzed?
3. What sensitivity analysis method is used in the article?
4. What controls are used in optimal control of coinfection model in the article?
5. How many compartments does the coinfection model consist of, and what are the compartments identified?
6. What are the research results obtained in the article?
Mathematical analysis can encompass various aspects of coinfection models, such as examining their fundamental properties, such as positivity, uniqueness, and invariant regions. This can also involve conducting local and global stability analyses. Additional analyses such as bifurcation may be incorporated. If the article does not discuss one of these analyses, then the article is said to be “No” on mathematical analysis, although all articles explain the formulation of mathematical models.
Sensitivity Analysis is a method for measuring uncertainty in a model. Sensitivity analysis in disease spread models can be used to determine how much influence parameter changes have on changes in basic reproduction numbers () or compartments. Several sensitivity analysis methods can be used to analyze the model: index method for
and Partial Rank Correlation Coefficient (PRCC).
In models of tuberculosis, malaria, and HIV/AIDS coinfection, interventions in the form of various forms of control are often used to reduce the spread of these diseases. Mathematically, this control is used to create an objective function to minimize the spread of the disease. The solution can be found using optimal control theory. Optimal control theory is a subset of control theory focused on determining a control strategy for a dynamic system throughout a specific time frame, aiming to optimize an objective function. These articles were reviewed to find out what type of controls were used in each article.
In this systematic literature review, a total of 35 articles were examined from the four PRISMA analyses. The analysis aimed to gain insights into the coinfection scenarios involving tuberculosis-malaria, tuberculosis-HIV, malaria-HIV, as well as tuberculosis-malaria-HIV. Detailed information from all the articles was extracted, including author names, publication years, details of mathematical analysis, sensitivity methods, and the controls used in model, as shown in . The specifics regarding the compartments employed in model are available in . Detailed descriptions of the model parameters can be seen in the reference article. The research results of these articles will be briefly explained in this systematic literature review.Citation24
Table 1 Detail of the Articles
Table 2 Compartments of the Models
The only article about a coinfection model of tuberculosis and malaria based on the second PRISMA. Model formulation of malaria and tuberculosis coinfection can be seen in model (1). In model (1), the entire human population is categorized into various subpopulations: susceptible individuals denoted as , individuals currently exposed to malaria alone represented as
, individuals exclusively infected with malaria noted as
, individuals who have recovered from both TB and malaria referred to as
, individuals exposed to TB marked as
, individuals infected with TB indicated as
, individuals infected with TB who are undergoing treatment as
, and individuals concurrently infected with both TB and malaria denoted as
. The author carries out a fundamental analysis of the model to prove that the model is well posed by establishing the positivity of the solution, invariance region, and stability of the equilibrium point locally and globally. The author also proves that model (1) experiences backward bifurcation. To obtain the best strategy for dealing with tuberculosis and malaria coinfection, the author used optimal control with five types of control and then carried out numerical simulations for several optimal control combinations.
with and
Based on the results obtained from the third PRISMA, six articles discuss malaria and HIV coinfection.Citation27 Of the six articles, the article has detailed and coherent discussion content. The author divides the model into sub-models and then looks for the balance point and stability of each balance point, both local stability and global stability. Model formulation of malaria and HIV coinfection can be seen in model (2). In model (2), the human population is categorized into six distinct compartments, each mutually exclusive: susceptible , productive HIV-only infected individuals
, non-productive HIV-only infected individuals
, AIDS patients
, non-productive individuals infected with malaria only
, and individuals dually infected with both HIV and malaria
. Similarly, the vector population is divided into three exclusive compartments: susceptible mosquitoes
, exposed mosquitoes
, and infected mosquitoes
. Model (2) also experiences backward bifurcation. The author also added 5 optimal controls and carried out numerical simulations to get the best control.
with and
.
Based on the results obtained from the first PRISMA, sixteen articles discuss tuberculosis and HIV coinfection.Citation52 Of the sixteen articles, research on a comprehensive model of tuberculosis and HIV coinfection, starting from basic analysis to model simulations. Model formulation of tuberculosis and HIV coinfection can be seen in model (3). The overall population is divided into seven distinct classes: Susceptible individuals represented as , those infected with tuberculosis as
, individuals who have both HIV and TB infections as
, individuals with TB who are undergoing treatment as
, individuals with both HIV and TB infections who are receiving treatment as
, individuals affected by AIDS as
due to the progression of HIV-infected individuals who have chosen not to undergo treatment, and lastly, individuals who have recovered from tuberculosis infection, denoted as
. The author carries out a fundamental analysis of the model to prove that the model is well posed by establishing the positivity of the solution, invariance property, existence of the solution, and stability of the equilibrium point both locally and globally.
According to the fourth PRISMA, only one article constructs a model from tuberculosis, malaria, and HIV coinfection.Citation58 Model formulation of malaria and tuberculosis coinfection can be seen in model (4). The entire population is categorized into five distinct classes, which include the susceptible , individuals with treatable malaria infections
, those with mTB infections
, the HIV-infected population
, and the class of people living with AIDS
. Model (4) considers one-way infection from malaria or mTB infection to malaria-HIV coinfection and mTB-HIV coinfection. In addition, this model also does not consider coinfection between malaria and mTB. The author analyzes local stability, performs several numerical simulation cases, and concludes that malaria and mTB infection significantly increase HIV/AIDS infection. From this information, this model still has many things that can be developed.
provides an overview of the included articles, showing 7, 25, 2, and 1 article on malaria-HIV coinfection model, tuberculosis-HIV coinfection model, tuberculosis-malaria and tuberculosis-malaria-HIV coinfection model.Citation33,Citation40,Citation45 The authors did not incorporate mathematical analysis into their articles.Citation33 One of this research featured highly intricate model that posed challenges for mathematical analysis.Citation26–30,Citation37,Citation39,Citation52 Furthermore, articles performed a sensitivity analysis on model, employing an indexing method to assess the impact on basic reproduction number. Basic Reproduction Number is a threshold value that can reference whether a disease will spread or be eliminated from a population. Sensitivity analysis of a parameter is carried out to see how the parameter influences changes in
.Citation57 Other Article use PRCC method to analyze the sensitivity of the parameter. Obtaining trustworthy data poses a common challenge in mathematical biology. Although specific parameter values are available in other articles, some parameters had to be estimated. Consequently, it is crucial for the estimation of
to exhibit relatively low sensitivity to these parameter values.Citation26 Example of sensitivity analysis result, if HIV infection predominantly influences
, a 10% reduction in the transmission rate is approximately mirrored by a 10% decline in
. However, a 10% reduction in the death rate results in a 7% rise in R0 for Malawi and an 8% increase for sub-Saharan Africa.Citation30 Other article result, increasing infection rate for malaria by 10%, increases the reproduction number by 5%. Thus, increasing natural death rate δ by 10% decreases the basic reproduction number by 10%.Citation33 Other articles employed sensitivity analysis with an indexing method applied to different variables. In general transmission model, sensitivity analysis employs an index for
. The more modest the sensitivity of a parameter to its
, the more accurate the estimated value of that parameter. For some cases, the results of sensitivity analysis are used as a reference for selecting parameters to be controlled using optimal control theory. This can prove the influence of these parameters on the spread of disease in the population.
Optimal control plays a crucial role in determining the most appropriate and efficient interventions for model. Several articles employ these methods, incorporating different types of prevention and intervention controls.Citation28,Citation31,Citation34,Citation38,Citation39,Citation45,Citation49,Citation58 Certain articles determine the best strategy to reduce infection by altering parameter values.Citation26,Citation33,Citation40 However, some articles did not incorporate any control measures in their analysis.
illustrates that model with theCitation36 lowest andCitation37 highest number of compartments consist of 4 and 21 compartments, respectively. Despite the complexity of their model, the authors conducted mathematical and sensitivity analyses, and utilized optimal control to obtain results.
The presented articles have yielded recommendations for mitigating the transmission of tuberculosis, malaria, and HIV.Citation24 The integration of Long-Lasting Insecticide-Treated Nets (LLITN), malaria treatment, tuberculosis treatment, Indoor Residual Spraying (IRS), and tuberculosis prevention proves to be effective in reducing the spread of tuberculosis, malaria, as well as their coinfection.
The results obtained for the malaria-HIV coinfection model differ. According toCitation30 article, the most effective method to diminish malaria-HIV coinfection involves the combination of malaria prevention measures and antiretroviral (ARV) treatment. However,Citation31 article obtained the result that treating malaria and HIV individually proved to be more effective in reducing infection compared to administering combined treatment.Citation28 The escalation of HIV/AIDS prevalence due to coinfection with malaria, highlights the significance of treatment in mitigating this interplay, particularly for individuals already affected by AIDS.Citation26 The mortality rate rises with coinfection and doubles when the infectivity escalates by 30%.Citation27 The most cost-effective control to inhibit the spread of HIV-Malaria coinfection is prevention. Furthermore,Citation29 significant reductions or potential eradication of HIV prevalence can be achieved by ensuring high bed-net coverage, a high rate of malaria treatment to effectively minimize the incidence of malaria-HIV coinfection.
The outcomes derived from coinfection model of tuberculosis and HIV exhibit variations.Citation40 The infections exert a significant impact on the population due to the presence of a hidden population with tuberculosis infection.Citation33 Tuberculosis and HIV are intricately interconnected with each other, while AIDS is influential on tuberculosis infection. Likewise,Citation34 the presence of tuberculosis infection can expedite the progression of HIV, potentially leading to more rapid development of AIDS.
In terms of optimal control,Citation36 article implemented effective isolation measures to restrict the contact and transmission of infections within the population, to eliminate tuberculosis-HIV coinfection. Furthermore,Citation37 the integration of case findings and prevention treatment failure of tuberculosis proved to be effective in reducing the spread of tuberculosis and HIV.Citation38 Screening plays a crucial role in managing and containing the transmission of HIV and tuberculosis.Citation39 Individuals with weakened immune systems are more vulnerable to contracting HIV and tuberculosis. The overall burden arising from coinfection can be minimized by employing well-selected strengths and initiating antiretroviral therapy (ARV).Citation43 Additionally, maintaining a higher early treatment rate compared to the late treatment rate throughout the entire treatment program is essential.Citation45 Early detection of HIV and tuberculosis cases and the prompt initiation of treatment can effectively decrease the rate of infection, slow down the progression of HIV infected individuals toward AIDS, and reduce the occurrence of coinfection.Citation46
The combination of prevention and treatment gives good results from economic and epidemiological perspectives. In addition,Citation49 vaccination leads to rapid recovery in individuals.Citation48 Optimal detection and integrated therapy, administered at the appropriate time, also yield superior clinical outcomes.Citation50 The optimal result can be obtained by combining all the detection or one of tuberculosis or HIV only for a longer period.
To decrease the prevalence of infection and fatalities caused by the disease, it is necessary to implement all control measures collectively and at an optimal level. However,Citation51 this method carries the potential risk of inducing immune reconstitution inflammatory syndrome (IRIS) in infected individuals.Citation52 Enhancing awareness through education results in a decline in the cumulative occurrence of new cases of coinfection within a population.Citation53 The most effective method to minimize infection, maximize the rate of recovery, and control the disease progression is through a combination of vaccination and treatment.
Based on the method employed to search for articles, only one addresses coinfection model involving tuberculosis, malaria, and HIV. However,Citation58 this model refrains from employing vectors as compartments, and the management of malaria and tuberculosis can potentially decelerate the progression of HIV.
Some coinfection models consider the effects of prevention and intervention in various forms. Prevention is in the form of Net insecticides and mass spraying to reduce mosquito populations. There is also in the form of education and use of condoms or in any form without specifying the prevention. Meanwhile, intervention can take the form of treatment for malaria and tuberculosis or various therapies for HIV/AIDS. Based on these articles, prevention and intervention significantly influence the dynamics of disease spread. Prevention and intervention in various forms can be used as optimal control in reducing the spread of disease in a population.
Mathematical models of coinfection between tuberculosis, malaria, and HIV/AIDS can be used to help in deciding intervention policies that must be implemented to suppress the spread of these diseases.Citation11 One of the important mathematical models in the spread of infectious diseases is the use of country-specific dynamic models to estimate the incidence and mortality of TB in that period 2020–2022. Estimates for 2020–2022 were generated utilizing a dynamic model tailored to each country, considering the impact of disruptions to tuberculosis diagnosis and treatment caused by the Covid-19 pandemic. Determining the burden of TB during the Covid-19 pandemic and its aftermath poses challenges, and the current approach involves the use of country- and region-specific dynamic models, particularly for many low- and middle-income countries. These models also used by WHO.Citation52
Article use data relevant to Kogi state of Nigeria to study the coinfection of tuberculosis and HIV. Analytical results supported by numerical simulation prove that educational awareness campaigns and treatment can reduce the burden of tuberculosis, HIV, and the coinfection of tuberculosis and HIV.Citation40 Article use population and health statistic from the Ministry of Interior, the Ministry of Public Health, Thailand and the World Health Organization to estimate the parameter value of the model. The extended duration of latent TB infection means that newly infected cases do not exhibit clinical symptoms immediately. Consequently, these cases remain unnoticed for a considerable period. To account for this delay, the development of a time-delay differential equation model becomes essential.Citation26
Global parameter estimates were derived from data collected in sub-Saharan Africa. The complete biological interactions between the malaria parasite and HIV are not yet fully understood. However, it is plausible that coinfection might result in a magnitude increase in the parasite or viral load. Future research endeavors should involve parameter fitting to data. Exploring coinfection at a cellular level would be needed as well.
Conclusion
In conclusion, a comprehensive search was conducted for articles using four different databases and specific sets of keywords. The selection process involved removing duplicated articles, assessing titles and abstracts, checking accessibility, and evaluating the relevance of entire article. Furthermore, the search yielded a total of 761 articles with the specified keywords. After completing the selection process, 35 articles were identified for analysis in systematic literature review. The articles covered different aspects, including model construction, mathematical analysis, sensitivity analysis, optimal control, and research findings. Valuable research and findings were also presented, contributing to the field of tuberculosis, malaria, and HIV coinfection model. Infectious diseases are a global health problem, especially deadly diseases such as tuberculosis, malaria and HIV/AIDS. This can become a more crucial health problem when coinfection occurs between these diseases. Mathematical models of tuberculosis, malaria, and HIV/AIDS coinfection can find the most effective intervention policies in reducing the spread of disease. Several studies on tuberculosis, malaria, and HIV/AIDS coinfections have been carried out in several countries using mathematical models and parameter estimation using data resulting from collaboration with local departments. For example, in Nigeria, Thailand, and Africa. This research produced several optimal solutions to reduce the spread of disease, including educational awareness campaign and treatment. Mathematical models of disease spread can produce recommendations for the government to create policies to reduce the spread of disease. However, there was still potential for further development of coinfection model by considering factors such as lifestyle, hospitalization, traditional treatment, and bacterial or virus evolution. Future research can also carry out parameter fitting estimation methods or use time delay models. Even the research object can be modified, namely from distribution at the human level to distribution at the cellular level.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
This research was supported and funded by Universitas Padjadjaran Research Grant within the Padjadjaran Postgraduate Excellent Scholarship with contract number 1549/UN6.3.1/PT.00/2023. The author would also like to thank the Directorate of Research and Community Service (DRPM) of Universitas Padjadjaran for the Article Processing Charge (APC) support.
References
- UNAIDS. Global HIV & AIDS statistics. SA Pharm J. 2010;77:57.
- World Health Organization. Global Health Sector Strategy on HIV 2016-2021; 2016.
- UNAIDS. Tuberculosis and AIDS: point of View; 1997.
- World Health Organization. World Health Organization Strategic Framework to Decrease the Burden of TB/HIV Stop TB Department and Department of HIV/AIDS; 2002.
- Zaman K. Tuberculosis: a global health problem. J Heal Popul Nutr. 2010;28:111–113.
- Fouad RM, Laerte Rda SJJ, Carolina GFA, et al. Tuberculosis treatment. J Bras Pneumol. 2017;43(6):472–486. doi:10.1590/s1806-37562016000000388
- Pontali E, D’Ambrosio L, Centis R, et al. Multidrug-resistant tuberculosis and beyond: an updated analysis of the current evidence on bedaquiline. Eur Respir J. 2017;2017:49.
- Floyd K, Anderson L, Baddeley A, et al. Global tuberculosis report; 2018:1–277.
- World Health Organization. Tuberculosis reports; 2020; 188.
- Godfrey-Faussett P, Maher D, Mukadi YD, et al. How human immunodeficiency virus voluntary testing can contribute to tuberculosis control. IAPAC Mon. 2003;9(3):54–60.
- World Health Organization. Global tuberculosis report 2023; 2023.
- White NJN, Pukrittayakamee S, Hien TTT, Faiz MA, Mokuolu OAO, Dondorp AAM. Malaria. Lancet. 2014;383(9918):723–735. doi:10.1016/S0140-6736(13)60024-0
- M.a P, Burrows JN, Manyando C, et al. Malaria. Nat Rev Dis Prim. 2017;2017:3.
- League GP, Degner EC, Pitcher SA, et al. The impact of mating and sugar feeding on blood-feeding physiology and behavior in the arbovirus vector mosquito Aedes aegypti. PLoS Negl Trop Dis. 2021;15(9):1–29. doi:10.1371/journal.pntd.0009815
- World Health Organization. World Malaria Report 2021; 2021.
- World Health Organization. World Malaria Report 2023; 2023.
- Chu CS, White NJ. The prevention and treatment of plasmodium vivax malaria. PLoS Med. 2021;18(4):1–21. doi:10.1371/journal.pmed.1003561
- Childs LM, Abuelezam NN, Dye C, et al. Modelling challenges in context: lessons from malaria, HIV, and tuberculosis. Epidemics. 2015;10:102–107. doi:10.1016/j.epidem.2015.02.002
- Pangestu DS, Tresna ST, Inayaturohmat F, et al. Covid-19 transmission model with discrete time approach. Commun Math Biol Neurosci. 2022;2022:1–12.
- Inayaturohmat F, Anggriani N, Supriatna AK. A mathematical model of tuberculosis and COVID-19 coinfection with the effect of isolation and treatment. Front Appl Math Stat. 2022;2022:8.
- Inayaturohmat F, Anggriani N, Supriatna AK. Optimal control and sensitivity analysis of Covid-19 transmission model with the presence of waning immunity in west java, Indonesia. commun. Math Biol Neurosci. 2022;2022:1–13.
- Purwani S, Inayaturohmat F, Tresna ST. Covid-19 EPIDEMIC MODEL: STUDY OF NUMERICAL METHODS AND SOLVING OPTIMAL CONTROL PROBLEM THROUGH FORWARD-BACKWARD SWEEP METHOD. Commun Math Biol Neurosci. 2022;2022:1–19.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;2009:6.
- Alzahrani AK, Khan MA. The Co-dynamics of malaria and tuberculosis with optimal control strategies. Filomat. 2022;36(6):1789–1818. doi:10.2298/FIL2206789A
- Afolabi MA, Adewale SO. Sensitivity analysis on mathematical modeling of transmission dynamics of tuberculosis–malaria co-infections. Int J Math Trend Tech. 2021;67(12):21–40. doi:10.14445/22315373/IJMTT-V67I12P503
- Barley K, Murillo D. A mathematical model of HIV and malaria co-infection in Sub-Saharan Africa. J AIDS Clin Res. 2012;S8:3. doi:10.4172/2155-6113
- Seidu B, Makinde OD, Seini IY. Mathematical analysis of the effects of HIV-malaria co-infection on workplace productivity. Acta Bio. 2015;63(2):151–182. doi:10.1007/s10441-015-9255-y
- Nyabadza F, Bekele BT, Rúa MA, et al. The Implications of HIV treatment on the HIV-malaria coinfection dynamics: a modeling perspective. Biomed Res Int. 2015;2015:1–14. doi:10.1155/2015/659651
- Mohammed-Awel J, Numfor E. Optimal insecticide-treated bed-net coverage and malaria treatment in a malaria-HIV co-infection model. J Biol Dyn. 2017;11(sup1):160–191. doi:10.1080/17513758.2016.1192228
- Windarto F, Hanif L. Application of optimal control strategies to HIV-malaria co-infection dynamics. J Phys Conf Ser. 2018;974:1–14.
- Saha AK, Niger AM, Podder CN. Impact of treatment on HIV-malaria coinfection based on mathematical modeling. GANIT J Bangladesh. Math Soc. 2019;39:45–62. doi:10.3329/ganit.v39i0.44165
- Oladapo AO, Olayiwola MO, Adedokun KA, et al. Mathematical analysis of sensitive parameters on the dynamical transmission of HIV-malaria co-infection. Jambura Journal of Biomathematics. 2023;4(1):37–45. doi:10.34312/jjbm.v4i1.18972
- Massad E, Burattini MN, Coutinho FAB, et al. Modeling the interaction between aids and tuberculosis. Math Comput Model. 1993;17(9):7–21. doi:10.1016/0895-7177(93)90013-O
- Naresh R, Sharma D, Tripathi A. Modelling the effect of tuberculosis on the spread of HIV infection in a population with density-dependent birth and death rate. Math Comput Model. 2009;50(7–8):1154–1166. doi:10.1016/j.mcm.2009.05.033
- Bhunu CP, Garira W, Mukandavire Z. Modeling HIV/AIDS and Tuberculosis Coinfection. Bulletin of Mathematic Biology. 2009;71(7):1745–1780. doi:10.1007/s11538-009-9423-9
- Gakkhar S, Chavda N. A dynamical model for HIV-TB co-infection. Appl Math Comput. 2012;218:9261–9270.
- Agusto FB, Adekunle AI. Optimal control of a two-strain tuberculosis-HIV/AIDS co-infection model. BioSystems. 2014;119:20–44. doi:10.1016/j.biosystems.2014.03.006
- Kaur N, Ghosh M, Bhatia SS. The role of screening and treatment in the transmission dynamics of HIV/AIDS and tuberculosis co-infection: a mathematical study. J Biol Phys. 2014;40(2):139–166. doi:10.1007/s10867-014-9342-3
- Adewale SO, Olopade IA, Adeniran GA, et al. Mathematical modelling and sensitivity analysis of HIV-TB co-infection. J Advance in Mathematics. 2015;1111.
- Bunwong K, Sae-Jie W, Boonsri N. A modeling approach for assessing the spread of tuberculosis and human immunodeficiency virus co-infections in Thailand. Kasetsart J Nat Sci. 2015;49:990–1000.
- Kaur N, Ghosh M, Bhatia SS. HIV-TB co-infection: a simple mathematical model. Journal of Advanced Research in Dynamical and Control Systems. 2015;7:66–81.
- Nthiiri JK, Lawi GO, Manyonge A. Mathematical modelling of tuberculosis as an opportunistic respiratory Co-Infection in HIV/AIDS in the presence of protection. Applied Mathematical Sci. 2015;9(5215):–. doi:10.12988/ams.2015.54365
- Mallela A, Lenhart S, Vaidya NK. HIV–TB co-infection treatment: modeling and optimal control theory perspectives. J Comput Appl Math. 2016;307:143–161. doi:10.1016/j.cam.2016.02.051
- Olopade IA, Adewale SO, Mohammed IT, Ajao SO, Oyedemi OT. Mathematical analysis of the role of detection rate in the dynamical spread of HIV-TB co-infection. J Adv Math. 2016;11:10.
- Bolarin G, Omatola U, Aiyesimi Y. Semi-analytic solution of HIV and TB co-infection model. J Appl Sci Environ. Manage. 2017;26:203–208.
- Awoke TD, Kassa SM. Optimal control strategy for TB-HIV/AIDS Co-infection model in the presence of behaviour modification. Processes. 2018;6(5):1–25. doi:10.3390/pr6050048
- Muthuri GG, Malonza DM. Mathematical modeling of TB - HIV co infection, case study of tigania west sub county, Kenya. J Adv Math Com Sci. 2018;27(5):1–18. doi:10.9734/JAMCS/2018/41850
- Tahir M, Shah SIA, Zaman G. Prevention strategy for superinfection mathematical model tuberculosis and HIV associated with AIDS. Cogent Math. 2019;6(1):1637166. doi:10.1080/25742558.2019.1637166
- Tanvi AR, Aggarwal R. Stability analysis of a delayed HIV-TB co-infection model in resource limitation settings. Chaos, Solitons and Fractals. 2020;140(140):110138. doi:10.1016/j.chaos.2020.110138
- Tanvi AR, Aggarwal R. Dynamics of HIV-TB co-infection with detection as optimal intervention strategy. Int J Non Linear Mech. 2020b;120:103388. doi:10.1016/j.ijnonlinmec.2019.103388
- Tanvi AR. Estimating the impact of antiretroviral therapy on HIV-TB co-infection: optimal strategy prediction. Int J of Biomathematics. 2020c;14:1.
- Omale AJ, Bolaji B. Mathematical model for transmission dynamics of HIV and tuberculosis co-infection in Kogi State, Nigeria. J Math Comput Sci. 2021;11:5580–5613.
- Biswas MHA, Samad SA, Parvin T, et al. Optimal control strategy to reduce the infection of pandemic HIV associated with tuberculosis. Commun Biomath Sci. 2022;5(1):20–39. doi:10.5614/cbms.2022.5.1.2
- Pitchaimani M, Devi AS. Threshold dynamics of an HIV-TB co-infection model with multiple time delays. Tamkang Journal of Mathematics. 2022;53:201–228.
- Adeyomo S, Sangotola A, Korosteleva O. Modeling transmission dynamics of tuberculosis–HIV co-infection in South Africa. Epidemiologia. 2023;4(4):408–419. doi:10.3390/epidemiologia4040036
- Teklu SW, Abebaw YF, Terefe BB, Mamo DK. HIV/AIDS and TB co-infection deterministic model bifurcation and optimal control analysis. Informatics in Medicine Unlocked. 2023;41:101328. doi:10.1016/j.imu.2023.101328
- Torres M, Tubay J, Reyes ADL. Quantitative assessment of a dual epidemic caused by tuberculosis and HIV in the Philippines. Bulletin of Mathematical Biology. 2023;85(7):56. doi:10.1007/s11538-023-01156-1
- Singh A, Jain M, Sharma GC. Stability and numerical analysis of malaria- mTB- HIV/AIDS co-infection. Int J Eng Trans a Basics. 2013;26:729–742.