Abstract
Purpose
This study aimed to evaluate racial disparities in medication use and associated factors among pregnant women receiving prenatal care at Brazilian Unified Health System primary care health units in the northeast region.
Patients and Methods
A total of 1058 pregnant women in the NISAMI Cohort were interviewed between June 2012 and February 2014. Medicines used during pregnancy were classified according to the Anatomical Therapeutic Chemical (ATC) classification system and ANVISA pregnancy risk categories. Prevalence ratios (crude and adjusted) and 95% confidence intervals (CIs) were estimated using Poisson regression with robust error variance. All analyses were stratified by race (Asian, black, brown/mixed, Brazilian indigenous, and white).
Results
Approximately 84% of the pregnant women used at least one medication, with a lower proportion among white women. The most reported medications were antianemic preparations (71.08%; 95% CI 68.27–73.72%), analgesics (21.74%; 95% CI 19.36–24.32%), and drugs for functional gastrointestinal disorders (18.81%; 95% CI 16.57–21.28%). Approximately 29% of women took potentially risky medications during pregnancy, with a higher prevalence among Asian and white women. Factors associated with medication use during pregnancy include a greater number of prenatal consultations, higher education levels, health problems, and smoking. In addition, maternal age above 25 years, smoking status, and two or more previous pregnancies were associated with potentially risky medication use during pregnancy.
Conclusion
A high prevalence of medication use during pregnancy was found; however, this prevalence was lower among white women. Nonetheless, black and brown women used antianemic preparations less frequently. This finding suggests that race is a factor of inequity in prenatal care, demanding public policies to mitigate it.
Introduction
Medication use during pregnancy is common in treating acute and chronic conditions through prescription or self-medication.Citation1 Among the most commonly used medications by pregnant women described in the literature are medicines prescribed to manage pregnancy and nonpregnancy-related conditions, such as ferrous sulfate, folic acid and vitamins, analgesics, antacids, antibiotics, antiemetics, antihistamines, and nonsteroidal anti-inflammatory agents.Citation2–6
A significant increase in medication consumption by pregnant women has been reported in recent years.Citation5,Citation7 The prevalence of at least one medication use during pregnancy reaches proportions greater than 90.0% worldwide.Citation8,Citation9 However, medication use might be related to numerous factors, including healthcare access.Citation10 Furthermore, medicines such as folic acid, insulin, antacids, ondansetron, ranitidine, methyldopa, hydralazine, enoxaparin sodium, nifedipine, and aspirin are more commonly dispensed to pregnant women than to women of childbearing age.Citation11
In addition to the characteristics of healthcare systems, medication use patterns may differ by race because of racism. Racism is a structuring system, historically constituted based on the belief of the inferiority of one race or ethnicity to another. It produces practices, beliefs, behaviors, and prejudices that favor avoidable and unfair inequalities between social groups by obstructing access to goods, resources, services, and opportunities.Citation12,Citation13
Furthermore, institutional racism is a failure by institutions and organizations to provide appropriate and professional services to people because of their race, culture, or ethnicity. Its processes, attitudes, and behaviors cause discrimination due to involuntary prejudice, ignorance, neglect, and racist stereotyping, which disadvantage people of minority groups,Citation14 resulting in differences in the distribution of services, benefits, and opportunities to different racial groups. Institutional racism, including the effective implementation of public policies and the generation of inequalities and inequities, is established in institutional routines.Citation15
Due to racism, there are important differences in socioeconomic status, living conditions, education, and healthcare access between black and brown people compared to white people in Brazil. Historically, black and brown Brazilians have a lower socioeconomic status and poorer health outcomes than white Brazilians.Citation16,Citation17
Therefore, racism is a social determinant of health, as it exposes black and brown people to more vulnerable situations of illness and death,Citation12,Citation13 which does not differ among pregnant women seeking prenatal care. In Brazil, black and brown people have epidemiological and social vulnerabilities that imply difficulty accessing health services and medications.Citation18 Similarly, black and brown Brazilian women have difficulties accessing preventive services and are less likely to undergo prenatal care, gynecological consultations, and prenatal care before the fourth month of pregnancy.Citation18,Citation19
The use of medications during pregnancy is a topic of concern because of their potentially harmful effects on the fetus, as many drugs used during pregnancy can cross the placental barrier and cause pharmacological effects.Citation20 However, the use of medicines during pregnancy may be influenced by social determinants of health, such as race. Therefore, this study provides a pertinent discussion in the context of prenatal care and the use of medications during pregnancy stratified by maternal race.
The present study delineates the profile of medication use during pregnancy and associated factors, explaining their differences due to race, which may enhance the literature on the subject, especially because few studies in Brazil have demonstrated inequities in medication use during pregnancy, and there is still a lack of information about race, mainly in regions with well-known disparities in healthcare access.Citation4,Citation9
For example, Costa et alCitation4 and Lutz et alCitation9 evaluated the factors associated with medication use during pregnancy in two Brazilian regions and reported a lower use of medicines among black women than among white women (PR 0.96, 95% CI 0.90–1.03; and PR 0.97, 95% CI 0.89–1.06, respectively), although the results were not statistically significant; however, the authors did not stratify their analysis by race group to evaluate potential differences between races and their associated factors; they only used race as an adjustment variable. At the same time, previous studies have explored the use of medicines stratified by the race of pregnant women; however, they limited their analysis to specific drug classes, such as opioids, or low-risk pregnant women.Citation21,Citation22
Moreover, the findings of drug utilization studies can contribute to public health policy decisions and resource allocationCitation23 which, in this case, could focus on diminishing potential racial disparities in the use of medicines during pregnancy.
Thus, this study aimed to evaluate racial disparities in the prevalence of medication use during pregnancy in women who underwent prenatal care at the Brazilian Unified Health System (BUHS) primary care health units in the northeast region, as well as the factors associated with medication use and potentially risky medication use during pregnancy.
From this perspective, the present study aimed to answer the following questions: “Are there differences in medication use during pregnancy according to maternal race?”, and “Considering the maternal race, what factors are associated with medication use during pregnancy by race?”.
Materials and Methods
Study Design and Data Source
This was a cross-sectional study nested within the prospective NISAMI Cohort (Núcleo de Investigação em Saúde Materno Infantil - NISAMI). The NISAMI Cohort resulted from the research project entitled “Maternal Risk Factors for Low Birth Weight, Prematurity and Intrauterine Growth Retardation, in Recôncavo Baiano region”, which was performed by the Center for Research in Maternal and Child Health of the Health Sciences Center, Federal University of Recôncavo da Bahia. The research was conducted in all primary care health units in the urban area of Santo Antônio de Jesus city. Pregnant women who sought prenatal care at the Family Health Unit (FHU) through the Brazilian Unified Health System (BUHS) were enrolled from June 2012 to February 2014. These women were followed prospectively throughout pregnancy until delivery.
Santo Antônio de Jesus is a city located in the state of Bahia, Brazil, and had 90,985 inhabitants in 2010, with 79,299 living in the urban area.Citation24 Public health services are provided in 21 FHUs, one basic health unit, and two hospitals.
Study Population
The study population comprised all pregnant women of any gestational age living in urban areas who attended the FHU for prenatal care. Pregnant women attending rural primary care health units were not eligible because of distance and difficulty accessing them. In addition, pregnant women who had not reported their race were excluded from this study. Thus, all pregnant women residing in urban areas, at any gestational age, and who underwent at least one prenatal consultation were included in this study.
Data Collection
Trained interviewers collected data longitudinally during prenatal consultations. A Fieldwork Guidance Manual was prepared to describe in detail all activities related to data collection. Daily visits to prenatal services were carried out from June 2012 to February 2014, where pregnant women were interviewed using a standardized questionnaire with 116 questions grouped into seven axes: sociodemographic characteristics, nutritional information, gynecological-obstetric information, oral health information, blood and biochemical tests, medication use information (before and during pregnancy), and anthropometry. In addition to the self-reported interviews, information related to the obstetric history was obtained from the prenatal services’ clinical records.Citation25
The supervisors’ team reviewed all the completed questionnaires. Data validation was performed by comparing the information obtained from the questionnaires with that recorded on the prenatal cards. Whenever necessary, the interviewers returned to the patients’ homes to confirm the data from the interviews. Quality control of the interviews was performed to identify any inaccuracies, systematic errors, or frauds. Approximately 20% of the pregnant women were revisited by field supervisors who reapplied selected parts of the questionnaires. The data were compared with the original interviews. Questionnaires with inaccuracies that could not be corrected were excluded.Citation26
Outcomes
The Outcomes of interest were medication use and potentially risky medication use through prescription and/or over-the-counter at any time during pregnancy. During the interview, the women were asked,
Have you used any medication during this pregnancy for high blood pressure, diabetes (high blood sugar), pain or colic, nausea or vomiting, cough, vaginal discharge, infection, respiratory problems, and/or other reasons?
If pregnant women answered yes, they provided all their medication names and dosages.
Medications were coded up to the third level (pharmacological subgroup) according to the Anatomical Therapeutic Chemical Classification System (ATC) of the World Health Organization.Citation27 Additionally, medications were classified according to the Food and Drug Administration (FDA) risk classification system adopted by the Agência Nacional de Vigilância Sanitária (ANVISA) to identify potential risks to the fetus due to the use of medications during pregnancy.Citation28 Although the FDA has recommended discontinuation of this classification since 2015, it is still used by ANVISA.
According to this classification, medications were divided into five risk categories: category A, drugs without risk to the fetus; category B, drugs for which animal studies have not demonstrated a fetal risk, but there are no human studies; category C, drugs for which animal studies have shown adverse effects on the fetus, but there are no adequate human studies; category D, drugs for which the experience of use during pregnancy showed an association with malformations, whose risk-benefit should be evaluated; and category X, drugs associated with fetal abnormalities in animal and human studies and/or whose risk-benefit contraindicates their use in pregnancy.Citation28 Potentially risky medications during pregnancy were those classified as C, D, or X due to adverse effects on the fetus.
Exposure
Information regarding exposure was self-reported maternal race, recorded during the interview, and categorized into five groups: Asian, black, Brazilian indigenous, brown, and white.
Covariates
Covariates were selected after a literature review and included socioeconomic and demographic, maternal, and health service utilization variables: maternal age (≤ 24; 25 to 29; 30 to 35; ≥ 36 years); education (≤ 8; 9 to 11; ≥ 11 years); marital status (with a partner; without a partner); employment status (with employment; without employment); family income – according to the 2013 minimum wage (one minimum wage in 2013: R$ 678.00/US$ 301.33) (MW) (≤ 1 MW; > 1 MW); smoking during pregnancy (yes; no); number of previous pregnancies (< 2; ≥ 2); onset of prenatal care (during the 1st trimester; after the 1st trimester); number of prenatal consultations (≤ 3; > 3); history of miscarriage (yes; no); and health problems (yes; no) – anemia, asthma, tuberculosis, pneumonia, diabetes, hypertension, kidney disease, urinary tract infection, and/or bleeding (Table S1).
Statistical Analysis
Descriptive analyses were performed using central tendency and dispersion measures for quantitative variables and frequency distributions for qualitative variables. Percentages and 95% confidence intervals (95% CI) were used for figures. The normality of the data was verified using the Shapiro‒Wilk test. Pearson’s chi-square and Fisher’s exact tests were used to examine the differences between strata. The analyses were stratified by race.
Prevalence ratios (PRs) and their 95% CIs were estimated using Poisson regression with robust error variance using each maternal race as an independent variable and medication use and potentially risky medication use as the dependent variables, categorized as binary variables (yes; no). Multivariate Poisson regression analysis included all variables associated with outcomes at a level lower than 20% in the bivariate analysis and those considered of interest according to the scientific literature. For all tests and the permanence of the variable in the final model, a significance level of 5% was assumed. The final model selection was carried out using backward elimination, consecutively eliminating those variables with lower statistical significance until only those with a significance level of 5% remained.
Analyses were conducted using R software, version 4.1.2Citation29 and the sandwichCitation30 and lmtestCitation31 packages.
Ethics Statement
The NISAMI cohort study was conducted by the Federal University of Recôncavo da Bahia under the supervision of Professor Djanilson Barbosa dos Santos. At the time of conducting the research, there was no ethics review board established at this institution; therefore, the research had to be assessed regarding its ethics commitment at the Ethics and Research Committee of the Adventist Physiotherapy Faculty of Bahia (FAFIS), which is close to the Federal University of Recôncavo da Bahia. The research was approved under protocol #4369.0.000.070–10, and this study was conducted in accordance with the Declaration of Helsinki.
Pregnant women were informed about the research purpose, methodology, and confidentiality of the data through an informed consent form that contained explicit information about the nature and objectives of the study. For those aged < 18 years, their parents or guardians provided written informed consent for inclusion in the study. The participants were interviewed only after signing the form.
Results
Population Characteristics
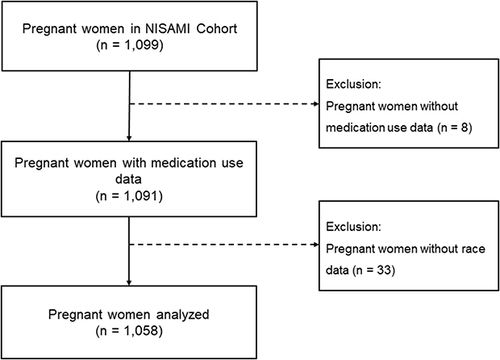
The NISAMI Cohort study enrolled 1099 pregnant women between June 2012 and February 2014. Among these, 1091 had data regarding medication use; 1058 (97.0%) who reported their race during the interviews were included in the study (). Most pregnant women were brown (47.26%), followed by black (37.43%), white (9.64%), Asian (3.69%), and Brazilian indigenous (1.98%). Maternal age ranged from 13 to 45 years (Table S2). The median age of pregnant women of each race was 21 years (IQR = 8) among Brazilian indigenous individuals, 23 years (IQR = 9.5) among Asians, 25 years (IQR = 9) among Black individuals, and 26 years among Brown (IQR = 9) and White (IQR = 10) individuals.
Although not statistically significant, Asian women were more likely to have 24 years or less (51.28%) and less than eight years of education (61.54%) than women of other races, while black (94.44%) and brown (96.60%) women were more likely to smoke during pregnancy. Regarding prenatal assistance and obstetric history, up to three prenatal consultations were more frequent among brown women (63.20%), and a history of miscarriage and health problems were more prevalent among Brazilian indigenous individuals (33.33% and 61.90%, respectively) ().
Table 1 Characteristics of the Study Population by Race. NISAMI Cohort Study, Brazil, 2012–2014 (N = 1058)
Medication Use During Pregnancy
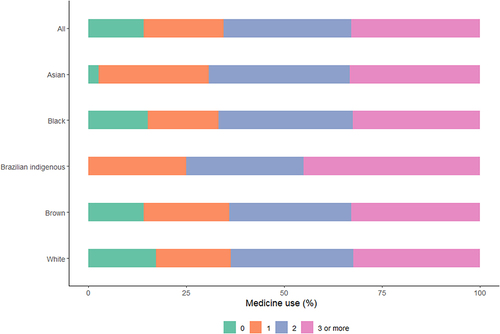
Overall, 84.31% of the women used at least one medication during pregnancy, with a median of 2.0 (IQR = 2) and a maximum of nine medications. However, pregnant white women reported a lower proportion of medication use than other women did (81.37%) (Supplementary Table S3).
shows the proportions of pregnant women who used one, two, or three or more medications at any time during pregnancy. Overall, 32.52% and 32.91% of pregnant women used two or three or more medications, respectively. A small proportion (20.51%) reported using only one medication. The proportion of white pregnant women using three or more medications was lower (32.32%) than that of Asian, black, Brazilian indigenous, and brown pregnant women.
Figure 2 Percentage of patients who used medication during pregnancy by race. NISAMI Cohort Study, Brazil, 2012–2014 (n = 1058).
According to the ATC classes, the most frequently reported class was antianemic preparations (B03), including iron preparations, vitamin B12, and folic acid, which were reported by 71.08% (95% CI 68.27–73.72%) of pregnant women.
Analgesics (N02) was the second most frequently reported class (21.74%; 95% CI 19.36–24.32%), followed by drugs for functional gastrointestinal (GI) disorders (A03) (18.81%; 95% CI 16.57–21.28%) and antihistamines for systemic use (R06) (8.60%; 95% CI 7.06–10.54%) (, ). Paracetamol was the most reported analgesic, while butylscopolamine, in combination with metamizole, was the main medicine used for functional GI disorders, and dimenhydrinate was used as an antihistamine for systemic use.
Table 2 Prevalence of the Use of at Least One Medication During Pregnancy According to Race According to the Anatomical Therapeutic Chemical Classification System (ATC) Level. NISAMI Cohort Study, Brazil, 2012–2014 (n = 892)
Figure 3 Percentage of the top medication classes taken during pregnancy by race. NISAMI Cohort Study, Brazil, 2012–2014 (n = 892).
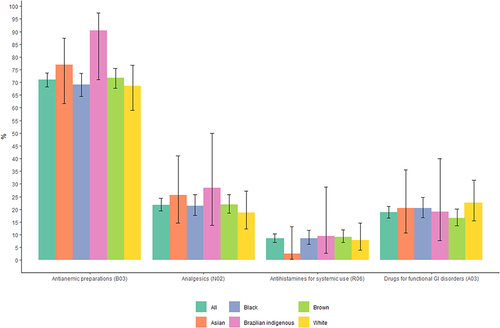
The use of antianemic preparations was less prevalent among white (68.63%; 95% CI 59.09–76.82%), black (69.19%; 95% CI 64.48–73.54%), and brown (71.80%; 95% CI 67.70–75.57%) pregnant women than among Asian (76.92%; 95% CI 61.66–87.35%) and Brazilian indigenous (90.48%; 95% CI 71.09–97.35%) pregnant women. Additionally, a lower frequency of analgesic consumption was observed among white women; more specifically, 18.63% (95% CI 12.26–27.27%) of white pregnant women used analgesics during pregnancy versus 21.46% (95% CI 17.70–27.77%) of black pregnant women, 22.00% (95% CI 18.59–25.84%) of brown pregnant women, 25.64% (95% CI 14.57–41.08%) of Asian pregnant women, and 28.57% (95% CI 13.81–49.96%) of Brazilian indigenous women.
Furthermore, white women reported the use of drugs for functional GI disorders more frequently. Remarkably, 22.55% (95% CI 15.52–31.57%) of white women took drugs for functional GI disorders, 20.51% (95% CI 10.78–35.53%) of Asian women, 20.45% (95% CI 16.77–24.70%) of black women, 19.05% (95% CI 7.67–40.00%) of Brazilian indigenous women, and 16.60% (95% CI 13.60–20.11%) of brown women. In contrast, the consumption of antihistamines for systemic use was greater among Brazilian indigenous (9.52%; 95% CI 2.65–28.91%), brown (9.20%; 95% CI 6.97–12.05%), and black (8.59%; 95% CI 6.21–11.76%) pregnant women than among white (7.84%; 95% CI 4.03–14.72%) and Asian (2.56%; 95% CI 0.45–13.18%) pregnant women (, ).
The proportion of self-reported medication use decreased during pregnancy from 75.78% in the first trimester to 6.17% in the third trimester (Supplementary Table S4).
Potentially Risky Medication Use During Pregnancy
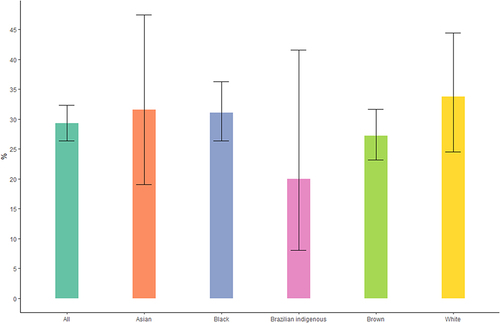
Among women who used medications during pregnancy, 29.26% (95% CI 26.37–32.33%) used at least one potentially risky medication. The most common potentially risky medications reported were butylscopolamine plus metamizole and isolated metamizole. Asian (31.58%; 95% CI 19.08–47.46%) and white (33.73%; 95% CI 24.48–44.42%) women used potentially risky medications during pregnancy more frequently than black (31.10%; 95% CI 26.33–36.30%), brown (27.19%; 95% CI 23.17–31.62%), and Brazilian indigenous women (20.00%; 95% CI 8.07–41.60%) ().
Figure 4 Percentage of potentially risky medication use during pregnancy by race. NISAMI Cohort Study, Brazil, 2012–2014 (n = 261).
Similarly to general medication use, potentially risky medication use during pregnancy decreased from 27.81% in the first trimester to 12.73% in the third trimester (Supplementary Table S5).
Factors Associated with Medication Use During Pregnancy
shows the crude prevalence ratios of medication use stratified by race. Thirty-six years or more, access to more than three prenatal consultations, and health problems were associated with medication use by pregnant black women. At least 24 years of age, more than 11 years of education, smoking during pregnancy, onset of prenatal care after the first trimester, more than three prenatal consultations, history of miscarriage, and health problems were associated with medication use by brown women. In contrast, only more than 11 years of education and more than three prenatal consultations were associated with the use of medication by white women.
Table 3 Crude Prevalence Ratio of Medication Use According to Race by Study Population Characteristics. NISAMI Cohort Study, Brazil, 2012–2014 (n = 892)
After the multivariate analysis, access to more than three prenatal consultations (PR 1.27; 95% CI 1.18–1.37) and health problems (PR 1.13; 95% CI 1.04–1.23) remained associated with medication use among black women. For brown women, more than 11 years of education (PR, 1.16; 95% CI 1.05–1.29), smoking during pregnancy (PR, 1.15; 95% CI 1.05–1.26), and more than three prenatal consultations (PR, 1.23; 95% CI 1.15–1.32) were associated with medication use in the multivariate analysis. For White women, the factors associated with medication use during pregnancy were more than 11 years of education (PR 1.18; 95% CI 1.01–1.38) and more than three prenatal consultations (PR 1.36; 95% CI 1.17–1.58) ().
Table 4 Factors Associated with Medication Use During Pregnancy According to Race in the Study Population. NISAMI Cohort Study, Brazil, 2012–2014
Factors Associated with Potentially Risky Medication Use During Pregnancy
Variables statistically associated with potentially risky medication use during pregnancy by black women in the bivariate analysis were maternal age between 25 and 29 years and between 30 and 35 years, onset of prenatal care during the first trimester, and more than prenatal consultations. For brown women, the factors associated with the use of these medications in the bivariate analysis were maternal age between 25 and 29 years, age between 30 and 35 years, age 36 years or older, employment status, smoking during pregnancy, and more than three prenatal consultations. In addition, two or more previous pregnancies and a history of miscarriage were associated with potentially risky medication use during pregnancy among white women ().
Table 5 Crude Prevalence Ratio of Potentially Risky Medication Use During Pregnancy According to Race According to Study Population Characteristics. NISAMI Cohort Study, Brazil, 2012–2014 (n = 261)
A greater prevalence of potentially risky medication use during pregnancy among black women aged between 25 and 29 years (PR 1.52; 95% CI 1.01–2.30) and 30 to 35 years (PR 1.74; 95% CI 1.18–2.56) was found in the multivariate analysis. Among brown women, those aged between 25 and 29 years (PR 1.70; 95% CI 1.14–2.53), 30 to 35 years (PR 1.68; 95% CI 1.09–2.58), and 36 years or more (PR 2.37; 95% CI 1.41–3.96) and those who smoked during pregnancy (PR 2.27; 95% CI 1.34–3.84) were identified via multivariate analysis. Moreover, two or more previous pregnancies were associated with potentially risky medication use during pregnancy among white women (PR 2.34; 95% CI 1.34–4.08) ().
Table 6 Factors Associated with Potentially Risky Medication Use During Pregnancy According to Race in the Study Population. NISAMI Cohort Study, Brazil, 2012–2014
Discussion
The use of medication was highly prevalent in this prospective cohort of pregnant Brazilian women. Approximately 84% of women reported using at least one medication during pregnancy, and approximately one-third reported using at least three medications at any time during pregnancy. Overall, white women reported a lower proportion of medication consumption than Asian, black, Brazilian indigenous, and brown women. The most commonly reported medications were antianemic preparations, analgesics, drugs for functional GI disorders, and antihistamines for systemic use. Additionally, almost 29.3% of women took potentially risky medications during pregnancy, and Asian and White women used potentially risky medications more frequently than black, Brazilian indigenous, and brown women did. Factors significantly associated with the use of medications during pregnancy included a greater number of prenatal consultations, a higher education level, health problems, and smoking. Additionally, maternal age above 25 years, smoking status, and two or more previous pregnancies were associated with potentially risky medication use during pregnancy.
The overall prevalence of medication use reported by pregnant women in this study was lower than that previously reported in another Brazilian study. Lutz et alCitation9 observed that 92.5% of pregnant women used at least one medication in the Pelotas Birth Cohort Study in southern Brazil. The variation in prevalence between studies may be related to differences in study designs and ways to collect and analyze data. Additionally, region-specific healthcare, socioeconomic, and cultural characteristics may be important when comparing different Brazilian regions. Worldwide, the variations in medication use by pregnant women may be even greater, with the prevalence of at least one medicine used reaching 97% in the United States.Citation8 In Europe, a Norwegian population-based cohort revealed that 60% of pregnant women used prescription medications between 2005 and 2015.Citation5 In contrast, a Chinese population-based cohort revealed that only 11.1% of women used at least one medicine during pregnancy.Citation32
The lower proportion of women who consumed medication during pregnancy among white women found in our study is different from the findings of several previous studies.Citation8,Citation9,Citation22 Studies have previously demonstrated that limited health literacy and reduced access to healthcare lead to increased self-medication,Citation33,Citation34 which is commonly practiced by pregnant women due to better accessibility, affordability, and availability of medicines.Citation35 In this study, medication use included both prescribed drugs and self-medication. Consequently, the higher medication use rates among black and brown women may be related to greater self-medication due to limited access to healthcare and health literacy, which are typically lower among black and brown individuals than among white individuals.Citation36–38
Nonetheless, racial differences in medication use prevalence with lower medication use in pregnancy among black women than among women of other races were observed in Brazil and the United States.Citation8,Citation9,Citation22 Racism leads to socioeconomic disadvantages that extrapolate and extend to health inequities, making access to health services more difficult and increasing the mortality of minority groups.Citation39 Moreover, institutional racism in health services predominantly affects black peopleCitation14 and may result in lower use of medications by black women during pregnancy.
Furthermore, a greater prevalence of use of three or more medications among Brazilian indigenous women was observed in this study. This is possibly related to the increasing biomedical hegemony and overvaluation of the use of medications in Brazilian indigenous health care,Citation40 contributing to this finding.
Antianemic preparations were the most commonly used medications for pregnant women, reflecting the adoption of international and national prenatal care recommendations. The supplementation of folic acid, iron, and vitamins during pregnancy, in addition to the adjustment of diet pattern and quality, is important for fulfilling increased nutritional demands during pregnancy, preventing adverse pregnancy outcomes, such as maternal anemia and low birth weight, and promoting fetal development.Citation41,Citation42
The present study revealed a lower prevalence of antianemic preparations among pregnant white and black women. As long as white women have a lower risk of developing anemia during pregnancy,Citation43 this may have impacted the reduced prescription of these supplements to those women. In addition, these results may suggest lower access to health services and inadequate prenatal care among Black women. These findings are concerning, especially for pregnant black women, who are more likely to have anemia than women of other racesCitation44 due to genetic or unfavorable socioeconomic conditions.Citation39 Previous studies have reported differences in iron preparations, folic acid, and vitamin use during pregnancy according to race, where black and Hispanic women present lower rates of intake of these supplements.Citation22,Citation45 Iron supplements, folic acid, and vitamins may improve pregnancy outcomes and minimize the illness burden and cost.Citation46 Therefore, further research must be conducted to assess the outcomes related to the lower prevalence of use of these medicines by pregnant women of different races.
Analgesics were used by 21.74% of pregnant women, and paracetamol was the most commonly reported medicine in this group, either alone or in combination. Pregnant women widely use analgesic medications, particularly paracetamol, to treat fever and pain. Despite controversies in the reporting literature about the long-term effects of paracetamol,Citation47 it remains a safe analgesic and antipyretic recommended during pregnancy and breastfeeding.Citation48
Additionally, a greater frequency of analgesic consumption was observed among Brazilian indigenous women. As discussed before, this may be associated with the medicalization of Brazilian indigenous healthcare in the last few years. Furthermore, since these medications are available by prescription and over the counter, this finding may be related to self-medication, which may pose risks to the mother and the fetus.
Almost 19% of the women in this cohort used drugs for functional GI disorders, mainly butylscopolamine and metamizole. Butylscopolamine is used to relieve colic, abdominal, and pelvic pain, which is common during pregnancy. Similar to the findings of this research, a high prevalence of butylscopolamine use by pregnant women was previously described in Brazilian studies.Citation4,Citation49
An interesting finding in our study was the relatively low prevalence of antihistamines of systemic use. These medications, predominantly dimenhydrinate, were used by 8.6% of pregnant women. Antihistamines are used to treat allergies, relieve indigestion, and prevent nausea and vomiting during pregnancy.Citation50 Dimenhydrinate is commonly used to manage nausea and vomiting during pregnancy and is commonly used as an over-the-counter medication. It acts by blocking H1 receptors in the gastrointestinal tract and decreases stimulation of the vomiting reflex by acting indirectly on the body’s vestibular center.Citation51 A significant variation in the prevalence of antihistamine use during pregnancy has been reported, with antihistamine consumption during pregnancy of 4.5% in Denmark,Citation52 13.7% in Norway,Citation5 17.0% in the United States,Citation22 and 23.3% in Brazil.Citation9
Approximately 29% of the women analyzed used at least one potentially risky medication during pregnancy, mainly butylscopolamine combined with metamizole and metamizole alone. Metamizole is an analgesic that is extensively consumed in Brazil but is not available in some European countries or North America because of its association with serious adverse events.Citation26 Additionally, a similar prevalence of the use of potentially risky medications during pregnancy has been reported. For example, web-based research conducted in South America, Europe, North America, and Australia reported that 28% of women used potentially risky medication during pregnancy.Citation53 Likewise, an Ethiopian cross-sectional study using clinical charts reported a prevalence of potentially risky medication dispensing of 20%.Citation54 Nonetheless, interpretation of the prevalence of consumption of risky medications during pregnancy must be made in light of how the information on medications was obtained (through questionnaires and clinical charts), cultural differences, and the adopted risk classification systems. In addition, surveillance is needed considering the lack of studies on the safety, rationality, and risk-benefit associated with its use by pregnant women.
With regard to medication consumption throughout pregnancy, a decrease in all the medications analyzed was observed. Nonetheless, the scientific literature regarding this finding remains controversial. Similar to our results, a Brazilian prospective cohort revealed a reduction in medication use during pregnancy, from 54.2% to 53.2% and 47.4% during the first, second, and third trimesters, respectively.Citation9 In contrast, Trønnes et alCitation53 reported a prevalence of medication consumption of 5.4% in the first trimester, 4.5% in the second trimester, and 6.0% in the third trimester among pregnant women in Europe. Moreover, an Italian cross-sectional study reported that 23.8% of women used some medication in the first trimester, 40.3% in the second trimester, and 14.2% in the third trimester.Citation55
In this study, medication use was more prevalent among Black, Brown, and White women who had access to more than three prenatal consultations. This is an expected finding, as more consultations may lead to more access to prescriptions and, consequently, facilitate access to medications. However, a careful interpretation of the rationality of medication use is needed. For example, butylscopolamine combined with metamizole is classified as a potentially risky medication, and despite being labeled as a prescription medication in Brazil, it is usually acquired without a prescription. Further studies need to be conducted to understand whether these medications are prescribed by a health professional or acquired for self-medication.
Furthermore, more than 11 years of education increased the prevalence of medication use during pregnancy by 16% (95% CI 1.05–1.29) among brown women and 18% (95% CI 1.01–1.38) among white women in this cohort. Previous studies have also shown higher rates of medication use during pregnancy among women with higher education.Citation8,Citation56,Citation57 In addition, an increase in education can increase socioeconomic status, allowing women to increase their medication consumption.Citation56 However, the remaining question is related to whether those who need access to essential medication during pregnancy have access to them.
Additionally, higher rates of medication use during pregnancy have been associated with health problems,Citation3 mainly due to the increase in women with chronic diseases.Citation58 The present study revealed that a higher prevalence of medication use was observed in black women with health problems (PR 1.13; 95% CI 1.04–1.23). In addition, we observed a high prevalence of pain management in this population, which is usually due to acute conditions.
Among brown women, smoking during pregnancy increased the prevalence of medication use by 15% (95% CI 1.05–1.26). A population-based cohort from Canada between 2002 and 2011 with 225,973 women reported comparable findings. The authors observed that smoking during pregnancy increased the odds of prescription medication use during pregnancy (OR 1.17; 95% CI 1.13–1.21).Citation3
Regarding potentially risky medication during pregnancy, it was observed that a greater prevalence of the use of these medications increases with maternal age among black and brown women. A previous study elucidated the influence of age on the use of any potentially inappropriate medication during pregnancy (category D and X medications only), primarily among women aged at least 35 years.Citation59 This is probably related to the increased prevalence of preexisting chronic diseases, such as diabetes, hypertension, asthma, and depression, and increased risk of obstetric complications, such as nausea and vomiting, gestational diabetes, and preeclampsia, that require pharmacotherapy and may lead to the use of potentially risky medications during pregnancy.Citation60
Moreover, among white women, two or more previous pregnancies were associated with potentially risky medication use during pregnancy. This finding may suggest more experience with common pregnancy symptoms and less concern about risks associated with medication use during pregnancy.Citation61
An unexpected finding was the apparent lack of association between the use of potentially risky medications during pregnancy and health problems (comorbidities). This finding differs from those of previous studies, in which pregnant women with health problems, especially chronic diseases, were more likely to use potentially risky medications during pregnancy than those without these problems.Citation53,Citation62 Therefore, further examination must be conducted to identify the specific needs and medicines prescribed for women with comorbidities.
The strengths of our study are the control of the quality of the questionnaires, standardization of data collection, and data validation. Another difference of this study was its prospective design, in which interviews were conducted during the prenatal period rather than only at delivery.
Furthermore, another strength of this study is the adoption of Poisson regression with robust variance, which provides correct estimates and is a better alternative for the analysis of cross-sectional data with binary outcomes than logistic regression since the prevalence ratio is more interpretable and easier to communicate than the odds ratio.Citation63
Nevertheless, these limitations cannot be ruled out. Considering that medications may be consumed by prescription and/or self-medication, medication use during pregnancy may be underestimated owing to recall bias and underreporting. In addition, women may not correctly report self-medication and may try to answer what is socially acceptable.
Furthermore, information on the medications’ clinical indications was not collected; therefore, there is a possibility of indication bias in the study. In addition, medication use during pregnancy in the present study may not indicate better healthcare because data on pregnant women’s previous health status were unavailable.
Additionally, this study used the FDA Pregnancy Risk Categories to categorize potentially risky medications during pregnancy. This classification is still used by ANVISA despite the recommendation to be discontinued in 2015. This categorization may be inappropriate for our population and may not reflect the risks to which pregnant women are exposed.
In addition, because this study was conducted exclusively in the urban area of the city due to the challenges encountered in collecting data from rural areas and only included pregnant women who visited basic health units, it may not be representative of all pregnant women in the city.
To promote health equity in prenatal care, it is crucial to address systemic racism and socioeconomic disparities, which are significant barriers to accessing health care services. Strategies are needed to address unconscious biases among health care workers, improve health literacy, and increase access to prenatal care. It is also important to enhance monitoring and education on drug safety, provide in-depth medication counseling, and promote counseling with healthcare professionals before initiating or discontinuing medication during pregnancy. Public health campaigns and educational programs should target pregnant women to educate them on decision-making regarding the use of medicines and promote safe medication practices.
Conclusion
This study revealed a high prevalence of medication use during pregnancy, supporting the existing scientific literature suggesting a pattern of medicalization among pregnant women. However, this prevalence was lower among white women, which may be related to increased self-medication due to limited health literacy and reduced access to healthcare among black and brown women. Nonetheless, black and brown women used medications indicated during pregnancy as antianemic preparations less frequently, indicating lower access to health services and adequate prenatal care. Therefore, these findings suggest that race is a factor of inequity in prenatal care, demanding public policies to mitigate inequalities.
Ethical Approval
Maternal Risk Factors for Low Birth Weight, Prematurity, and Intrauterine Growth Retardation in Recôncavo da Bahia research was approved by the Adventist Physiotherapy Faculty of Ethics and Research Committee of the Adventist Physiotherapy Faculty Bahia (FAFIS) (Protocol No. 4369.0.000.070-10).
Consent to Participate
Informed consent was obtained from all the participants.
Pregnant women were informed about the confidentiality of the data and were informed about the purpose of the research and its methodology. The interviews were performed only with pregnant women who agreed to participate in the study and signed the informed consent form, which contained explicit information about the study objectives and nature.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The authors thank Prof. Debra Jackson, who kindly revised the manuscript.
References
- Stanley AY, Durham CO, Sterrett JJ, Wallace JB. Safety of over-the-counter medications in pregnancy. MCN Am J Matern Nurs. 2019;44(4):196. doi:10.1097/NMC.0000000000000537
- Lunardi-Maia T, Schuelter-Trevisol F, Galato D. Uso de medicamentos no primeiro trimestre de gravidez: avaliação da segurança dos medicamentos e uso de ácido fólico e sulfato ferroso. Rev Bras Ginecol E Obstetrícia. 2014;36(12):541–547. doi:10.1590/So100-720320140005051
- Smolina K, Hanley GE, Mintzes B, Oberlander TF, Morgan S, Fischer G. Trends and determinants of prescription drug use during pregnancy and postpartum in british columbia, 2002–2011: a population-based cohort study. PLoS One. 2015;10(5):e0128312. doi:10.1371/journal.pone.0128312
- Costa DB, Coelho HLL, Santos DB. Utilização de medicamentos antes e durante a gestação: prevalência e fatores associados. Cad Saúde Pública. 2017;33:e00126215. doi:10.1590/0102-311X00126215
- Engeland A, Bjørge T, Klungsøyr K, Hjellvik V, Skurtveit S, Furu K. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf. 2018;27(9):995–1004. doi:10.1002/pds.4577
- Bérard A, Abbas-Chorfa F, Kassai B, et al. The French Pregnancy Cohort: medication use during pregnancy in the French population. PLoS One. 2019;14(7):e0219095. doi:10.1371/journal.pone.0219095
- Donald S, Sharples K, Barson D, Horsburgh S, Parkin L. Patterns of prescription medicine dispensing before and during pregnancy in New Zealand, 2005–2015. PLoS One. 2020;15(6):e0234153. doi:10.1371/journal.pone.0234153
- Haas DM, Marsh DJ, Dang DT, et al. Prescription and other medication use in pregnancy. Obstet Gynecol. 2018;131(5):789. doi:10.1097/AOG.0000000000002579
- Lutz BH, Miranda VIA, Silveira MPT, et al. Medication use among pregnant women from the 2015 Pelotas (Brazil) birth cohort study. Int J Environ Res Public Health. 2020;17(3):989. doi:10.3390/ijerph17030989
- Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4(2):e004365. doi:10.1136/bmjopen-2013-004365
- Havard A, Barbieri S, Hanly M, et al. Medications used disproportionately during pregnancy: priorities for research on the risks and benefits of medications when used during pregnancy. Pharmacoepidemiol Drug Saf. 2021;30(1):53–64. doi:10.1002/pds.5131
- Goes EF, Ferreira AJF, Ferreira AJF. Desigualdades raciais em saúde e a pandemia da Covid-19. Trab Educ E Saúde. 2020;18(3):e00278110. doi:10.1590/1981-7746-sol00278
- Araújo EM, Caldwell KL, Santos MPA, et al. Morbimortalidade pela Covid-19 segundo raça/cor/etnia: a experiência do Brasil e dos Estados Unidos. Saúde Em Debate. 2021;44(spe4):191–205. doi:10.1590/0103-11042020E412
- Kalckmann S, Santos CG, Batista LE, Cruz VM. Racismo institucional: um desafio para a eqüidade no SUS? Saúde E Soc. 2007;16:146–155. doi:10.1590/S0104-12902007000200014
- López LC. O conceito de racismo institucional: aplicações no campo da saúde. Interface Comun Saúde Educ. 2012;16(40):121–134. doi:10.1590/S1414-32832012005000004
- Fonseca JM, Rocha PRH, Rocha PRH, et al. Racial inequality in perinatal outcomes in two Brazilian birth cohorts. Braz J Med Biol Res. 2021;54(1):e10120. doi:10.1590/1414-431X202010120
- Guimarães JMN, Yamada G, Barber S, et al. Racial inequities in self-rated health across Brazilian cities: does residential segregation play a role? Am J Epidemiol. 2022;191(6):1071–1080. doi:10.1093/aje/kwac001
- Silva NN, Favacho VBC, Boska GA, Andrade EC, Merces NP, Oliveira MAF. Access of the black population to health services: integrative review. Rev Bras Enferm. 2020;73(4):e20180834. doi:10.1590/0034-7167-2018-0834
- Oliveira KA, Araújo EM, Casotti CA, Silva CAL, Santos DB, Santos DBD. Associação entre raça/cor da pele e parto prematuro: revisão sistemática com meta-análise. Rev Saúde Pública. 2018;52:26. doi:10.11606/S1518-8787.2018052000406
- Sachdeva P, Patel BG, Patel BK. Drug Use in Pregnancy; a Point to Ponder! Indian J Pharm Sci. 2009;71(1):1–7. doi:10.4103/0250-474X.51941
- Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Network Open. 2020;3(5):e205734. doi:10.1001/jamanetworkopen.2020.5734
- Vafai Y, Yeung EH, Sundaram R, et al. Racial/ethnic differences in prenatal supplement and medication use in low-risk pregnant women. Am J Perinatol. 2022;39(6):623–632. doi:10.1055/s-0040-1717097
- Bachhav SS, Kshirsagar NA. Systematic review of drug utilization studies & the use of the drug classification system in the WHO-SEARO Region. Indian J Med Res. 2015;142(2):120–129. doi:10.4103/0971-5916.164223
- Instituto Brasileiro de Geografia e Estatística. Cidades: Santo Antônio de Jesus. IBGE; 2011. Available from: https://cidades.ibge.gov.br/brasil/ba/santo-antonio-de-jesus/panorama. Accessed May 30, 2024.
- Castro CT, Pereira M, Santos DB. Association between paracetamol use during pregnancy and perinatal outcomes: prospective NISAMI cohort. PLoS One. 2022;17(4):e0267270. doi:10.1371/journal.pone.0267270
- Costa DB, Castro CT, Gama RS, Santos DB. Drug use according to risk classification and associated factors among pregnant women: results from NISAMI cohort. Res Soc Dev. 2020;9(12):e43691211247–e43691211247. doi:10.33448/rsd-v9i12.11247
- WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology & WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Introduction to Drug Utilization Research. World Health Organization; 2003. Available from: https://apps.who.int/iris/handle/10665/42627. Accessed May 30, 2024.
- Agência Nacional de Vigilância Sanitária. Resolução Da Diretoria Colegiada No 60, de 17 de Dezembro de 2010. ANVISA; 2010.
- R Core Team. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2023. Available from: https://www.R-project.org. Accessed May 30, 2024.
- Zeileis A, Lumley T, Graham N, Koell S. sandwich: robust covariance matrix estimators; 2023. Available from: https://cran.r-project.org/web/packages/sandwich/index.html. Accessed April 20, 2024.
- Hothorn T, Zeileis A. lmtest: testing linear regression models; 2022. Available from: https://cran.r-project.org/web/packages/lmtest/index.html. Accessed April 20, 2024.
- Zhang J, Ung COL, Guan X, Shi L. Safety of medication use during pregnancy in mainland China: based on a national health insurance database in 2015. BMC Pregnancy Childbirth. 2019;19(1):459. doi:10.1186/s12884-019-2622-y
- Persell SD, Karmali KN, Lee JY, et al. Associations between health literacy and medication self-management among community health center patients with uncontrolled hypertension. Patient Prefer Adherence. 2020;14:87–95. doi:10.2147/PPA.S226619
- Pagán JA, Ross S, Yau J, Polsky D. Self-medication and health insurance coverage in Mexico. Health Policy. 2006;75(2):170–177. doi:10.1016/j.healthpol.2005.03.007
- Tuha A, Faris AG, Mohammed SA, Gobezie MY. Self-medication and associated factors among pregnant women attending antenatal care at kemisie general hospital, North East Ethiopia. Patient Prefer Adherence. 2020;14:1969–1978. doi:10.2147/PPA.S277098
- Chaudhry SI, Herrin J, Phillips C, et al. Racial disparities in health literacy and access to care among patients with heart failure. J Card Fail. 2011;17(2):122–127. doi:10.1016/j.cardfail.2010.09.016
- Ylitalo KR, Meyer MRU, Lanning BA, During C, Laschober R, Griggs JO. Simple screening tools to identify limited health literacy in a low-income patient population. Medicine. 2018;97(10):e0110. doi:10.1097/MD.0000000000010110
- Rikard RV, Thompson MS, McKinney J, Beauchamp A. Examining health literacy disparities in the United States: a third look at the National Assessment of Adult Literacy (NAAL). BMC Public Health. 2016;16(1):975. doi:10.1186/s12889-016-3621-9
- Pacheco VC, Silva JC, Mariussi AP, Lima MR, Silva TR. As influências da raça/cor nos desfechos obstétricos e neonatais desfavoráveis. Saúde Em Debate. 2018;42:125–137. doi:10.1590/0103-1104201811610
- Diehl EE, Almeida LK. Medicamentos em contexto local indígena: a “farmácia caseira” Xokleng, Santa Catarina. Rev Antropol UFSCar. 2012;4(1):189–206. doi:10.52426/rau.v4i1.70
- Liu D, Cheng Y, Dang S, et al. Maternal adherence to micronutrient supplementation before and during pregnancy in Northwest China: a large-scale population-based cross-sectional survey. BMJ Open. 2019;9(8):e028843. doi:10.1136/bmjopen-2018-028843
- Tuncalp Ö, Rogers LM, Lawrie TA, et al. WHO recommendations on antenatal nutrition: an update on multiple micronutrient supplements. BMJ Glob Health. 2020;5(7):e003375. doi:10.1136/bmjgh-2020-003375
- Frayne J, Pinchon D. Anemia in pregnancy. Aust J Gen Pract. 2019;48:125–129.
- Harrison RK, Lauhon SR, Colvin ZA, McIntosh JJ. Maternal anemia and severe maternal morbidity in a US cohort. Am J Obstet Gynecol MFM. 2021;3(5). doi:10.1016/j.ajogmf.2021.100395
- Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143(4):486–492. doi:10.3945/jn.112.169987
- Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134(6):1234. doi:10.1097/AOG.0000000000003557
- Kennon-McGill S, McGill MR. Extrahepatic toxicity of Acetaminophen: critical evaluation of the evidence and proposed mechanisms. J Clin Trans Res. 2017;3(3):297–310.
- Gou X, Wang Y, Tang Y, et al. Association of maternal prenatal Acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: a meta-analysis. Aust N Z J Psychiatry. 2019;53(3):195–206. doi:10.1177/0004867418823276
- Mengue SS, Schenkel EP, Duncan BB, Schmidt MI. Uso de medicamentos por gestantes em seis cidades brasileiras. Rev Saúde Pública. 2001;35:415–420. doi:10.1590/S0034-89102001000500002
- Hansen C, Desrosiers TA, Wisniewski K, Strickland MJ, Werler MM, Gilboa SM. Use of antihistamine medications during early pregnancy and selected birth defects: the national birth defects prevention study, 1997–2011. Birth Defects Res. 2020;112(16):1234–1252. doi:10.1002/bdr2.1749
- Garcia Saborio OE, Hines BK, Wesselman J. Safe management of nausea and vomiting during pregnancy in the emergency department. Adv Emerg Nurs J. 2019;41(4):336. doi:10.1097/TME.0000000000000258
- Volqvartz T, Vestergaard AL, Aagaard SK, et al. Use of stimulants, over-the-counter and prescription drugs among Danish pregnant women. Basic Clin Pharmacol Toxicol. 2020;127(3):205–210. doi:10.1111/bcpt.13396
- Trønnes JN, Lupattelli A, Nordeng H. Safety profile of medication used during pregnancy: results of a multinational European study. Pharmacoepidemiol Drug Saf. 2017;26(7):802–811. doi:10.1002/pds.4213
- Alema NM, Semagn G, Melesse S, et al. Patterns and determinants of prescribed drug use among pregnant women in Adigrat general hospital, northern Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):624. doi:10.1186/s12884-020-03327-7
- Navaro M, Vezzosi L, Santagati G, Angelillo IF, Group CW. Knowledge, attitudes, and practice regarding medication use in pregnant women in Southern Italy. PLoS One. 2018;13(6):e0198618. doi:10.1371/journal.pone.0198618
- Mengue SS, Schenkel EP, Schmidt MI, Duncan BB. Fatores associados ao uso de medicamentos durante a gestação em seis cidades brasileiras. Cad Saúde Pública. 2004;20:1602–1608. doi:10.1590/S0102-311X2004000600018
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e1–51.e8. doi:10.1016/j.ajog.2011.02.029
- Admon LK, Winkelman TNA, Moniz MH, Davis MM, Heisler M, Dalton VK. Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstet Gynecol. 2017;130(6):1319. doi:10.1097/AOG.0000000000002357
- Dillon P, O’Brien KK, McDonnell R, et al. Prevalence of prescribing in pregnancy using the Irish primary care research network: a pilot study. BMC Pregnancy Childbirth. 2015;15(1):67. doi:10.1186/s12884-015-0489-0
- Ayad M, Costantine MM. Epidemiology of medications use in pregnancy. Semin Perinatol. 2015;39(7):508–511. doi:10.1053/j.semperi.2015.08.002
- Beyene KG, Beza SW. Self-medication practice and associated factors among pregnant women in Addis Ababa, Ethiopia. Trop Med Health. 2018;46(1):10. doi:10.1186/s41182-018-0091-z
- Blotière PO, Damase-Michel C, Weill A, Maura G. Dispensing of potentially harmful prescription drugs in 1.8 million pregnant women in France: a nationwide study based on two risk classification systems. Drug Saf. 2021;44(12):1323–1339. doi:10.1007/s40264-021-01117-4
- Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):21. doi:10.1186/1471-2288-3-21