?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Global rates of type 2 diabetes in children and adolescents have increased significantly over the past three decades. Type 2 diabetes is a relatively new disease in this age group, and there is a dearth of information about how to structure treatment programs to manage its comorbidities and complications. In this paper, we describe the design and implementation of a personalized multidisciplinary, family-centered, pediatric and adolescent type 2 diabetes program at a tertiary pediatric center in Hamilton, Ontario, Canada. We report the process of designing and implementing such a program, and show that this multidisciplinary program led to improvement in glycated hemoglobin (n=17, 8% at baseline versus 6.4% at 1 year, 95% confidence interval (0.1–0.28), P-value <0.0001) and stabilized body mass index, with lowered C-peptide and no change in fitness or metabolic biomarkers of lipid metabolism and liver function. As type 2 diabetes becomes more prevalent in youth, the need for programs that successfully address the complex nature of this disease is central to its management and to mitigate its long-term adverse outcomes.
Introduction
Over the past few decades, the global burden of disease has shifted from communicable to chronic noncommunicable diseases,Citation1,Citation2 and two of the main conditions in the latter group are obesity and type 2 diabetes (T2D). In a remarkable shift in global mortality trends, these two diseases and their complications cause more mortality today than famine.Citation1 The global epidemic of T2D is driven mainly by the obesity epidemic, and recently it was estimated that the number of people with T2D would increase over the next two decades to include nearly 600 million people.Citation3 Urgent action is needed to help reduce the impact of T2D on the lifespan and quality of life of people around the world.
Until recently, T2D was largely a disease of adults, yet in the past three decades its rates in children have risen by 10–30-fold in North America.Citation4,Citation5 While T2D has been mainly noted in certain ethnic groups and indigenous populations,Citation6–Citation8 several other pediatric populations are at risk of T2D, including children who are born small for gestational age, large for gestational age, infants of diabetic and obese mothers, as well as survivors of childhood cancer.Citation9–Citation20 An increasing number of adults with T2D will be diagnosed with this disease during childhood, and many patients diagnosed during adulthood have risk factors as children that predispose them to T2D. As the scale of the T2D epidemic becomes apparent, new approaches to managing T2D are warranted to limit its devastating impact on children, families, communities, and health care organizations worldwide.
There are several reasons to justify setting up specialized services for children and adolescents with T2D. An increasing number of children are being diagnosed with the disease and require comprehensive programs to manage both obesity and T2D.Citation4,Citation5,Citation21 Furthermore, T2D is a more aggressive disease in children than in adults, with diabetes-related complications and comorbidities presenting early in the course of the disease.Citation22–Citation26 Importantly, if good glycemic control is achieved early in the course of T2D, the risk of diabetes-related microvascular and macrovascular complications is markedly reduced in adults with good glycemic control,Citation27–Citation30 and while there are no natural history data for pediatric T2D, this may well be the case in children. Therefore, it is important to try to prevent or delay the onset of diabetes-related complications in children by maintaining adequate glycemic control.
On a mechanistic level, there are important considerations in T2D that differentiate it from the most common type of pediatric diabetes, ie, type 1 diabetes,Citation31–Citation33 and this is important because it may affect management approaches. Broadly, type 1 diabetes is an autoimmune disease caused by T-lymphocyte-mediated pancreatic β-cell destruction and insulin deficiency, and insulin therapy is mandatory for survival.Citation31–Citation34
On the other hand, T2D is predominantly a disease of insulin resistance in obese individuals, with insulin deficiency developing as demand outstrips pancreatic insulin production, and insulin treatment may or may not be required to maintain adequate control.Citation31–Citation33,Citation35 While obesity contributes to the risk of T2D via environmental factors including increased caloric intake and sedentary time, genetic and epigenetic factors also contribute to the genesis of T2D in children.Citation11,Citation36 Furthermore, immune activation and inflammation associated with obesity is an important driver of insulin resistance that ultimately leads to diabetes.Citation37,Citation38
One of the challenging aspects of managing pediatric T2D is related to the paucity of data regarding effective interventions. In a previous study including 129 children with T2D from Germany and Austria, dropout rates from the program were high and children had relatively high rates of comorbidities.Citation39 Recently, the Treatment Options for Type 2 diabetes in Adolescents and Youth (TODAY) study reported its findings.Citation26,Citation40 This was a large, randomized, prospective clinical trial in which 699 patients aged 10–17 years with T2D were randomized to three treatment choices including metformin alone, metformin plus intensive lifestyle intervention, and metformin plus rosiglitazone.Citation40 The lifestyle plan involved dietitian and activity specialist input to motivate the participants to adhere to treatment plans.Citation26,Citation40 This study demonstrated that metformin monotherapy is as effective as a combination of lifestyle plus metformin to manage diabetes, with stable glycemic control in almost 50% of participants over 3.9 years of treatment; there were also sex-specific and ethnic differences in outcomes.Citation40 While the combination of metformin and rosiglitazone was superior to the other two interventions, rosiglitazone has been on a restricted use policy until recently,Citation41 so its use is not likely to be extensive in the short term among children and adolescents.
Here we describe the setup and implementation of a personalized, family-centered, multidisciplinary, pediatric and adolescent T2D management program in a tertiary pediatric center. We hypothesized that the implementation of personalized intensive intervention plans would improve glycated hemoglobin (HbA1c) levels in children and adolescents with T2D. Our primary objective was to determine the impact of this program on reducing HbA1c. Secondary objectives included evaluation of the impact of the intervention on improving fitness, stabilizing body mass index and fat mass, and improvement in the lipid profile, alanine aminotransferase (ALT), albumin-to-creatinine ratio (ACR), and C-peptide levels. In addition, we evaluated the conformation of the program to established quality indicators.
Materials and methods
Program design and implementation
This program was established at McMaster Children’s Hospital, a tertiary pediatric center in Hamilton, Ontario, Canada. The center serves a population of around 2.3 million people, about 25% of whom live in the city of Hamilton, and the rest reside in the surrounding regions. The hospital serves as a tertiary pediatric diabetes center caring for around 550 children with type 1 diabetes onsite, and is part of a regional diabetes program with several other satellite diabetes clinics in the region. At the time of establishing the T2D program, our center cared for six adolescents with T2D. The hospital also has a weight management program that cares for close to 400 obese children.
Needs assessment and staff engagement
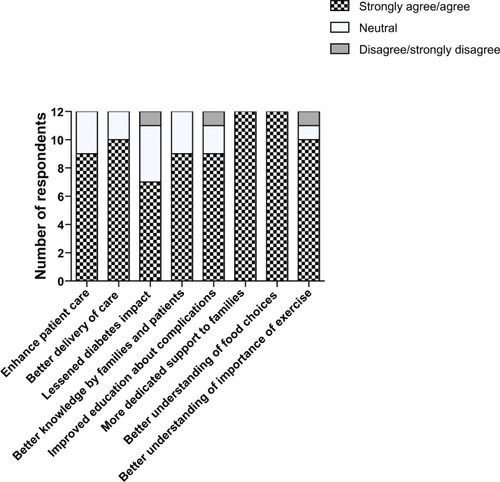
Setting up this new service required collaboration between several professional groups, including hospital management and multidisciplinary clinical teams. Several months before launching the T2D program, a needs assessment was conducted to assess the value of establishing a dedicated, multidisciplinary T2D program. An e-mail survey was sent to care providers, including general pediatricians, dietitians, kinesiologists, and nurses in the hospital. We enquired whether a dedicated T2D program was needed to deliver better care to patients and families. The respondents (n=12, 73% female, 70% aged 30–50 years, response rate 50%) identified setting up this program as an important step towards addressing an unmet need for patients with T2D, although there was a feeling that this intervention may or may not change long-term outcomes in these patients ().
In order to secure support from clinical leaders and hospital management, consultations with the head of clinical services, weight management and diabetes physician leads, and clinic managers took place to secure the infrastructure and resources necessary for the running of the program.
To get input from different stakeholders involved in the running of the program, a series of joint meetings including clinical staff, physician leads, and hospital managers were conducted. In these meetings, the program’s philosophy, mission, vision, values, and proposed structure were presented, and questions and suggestions were fielded from team members to finalize the design of the program. In order to communicate the setup of the new service to the wider medical community, we announced the launch of the program at a regional pediatric education conference attended by pediatricians, and also posted an announcement in a local newsletter for family physicians in our region.
Development of patient education materials
The development of the patient education manual was done in collaboration with the patient education department in the hospital, and involved inclusion of information needed by children and families to deal with T2D. The team members developed each section of the manual working in subcommittees of nutrition, physical activity, nursing, and social work/behavioral therapy. The manual includes information about diet, physical activity, sleep, stress, medications, screening for complications and comorbidities, and information about the roles and contact details of the team members, as it is felt that direct access of families to the team is critical in enhancing engagement and participation in the program. The patient education manual has been made available free of charge for use by the diabetes community.Citation42
We recognized that this document would need to be updated regularly, so it was created as a binder that can be modified by removing or adding documents as needed. Additional documentation regarding alcohol consumption, smoking, drug use, and sexual activity and precautions are added as needed to this binder and are discussed with adolescents during clinic visits. The online document is a live one that will be updated based on new knowledge and feedback received from patients, families, and staff.
Funding
There was no dedicated funding available to hire new personnel for this program, therefore resources were reassigned from existing personnel and using existing infrastructure. This involved agreements with hospital management and clinical service directors that resources from both the weight management and type 1 diabetes programs are dedicated to the new T2D program.
Space and infrastructure
The program runs in a 2,800 square foot dedicated clinical setting in the hospital that is used for weight management clinics, with several clinic rooms available for use by team members. The clinic space also includes two exercise testing rooms which have cycle ergometers, treadmills, metabolic carts, and spirometry equipment used by the exercise physiologist and kinesiologist. We also use the InBody520 scan (GE Healthcare, UK Ltd, Little Chalfont, UK) for measuring weight and body fat percentage.
Program structure
The program started accepting patients 7 months after initiating the consultation process, and the design of the program is shown in . The main source of referrals of these patients is family physicians, with occasional presentation to the emergency department or endocrine team in the hospital.
Figure 2 Type 2 diabetes program clinic structure and patient evaluation plans.
Abbreviations: C-peptide, endogenous insulin; GAD, glutamic acid decarboxylase autoantibodies; ICA, islet cell autoantibodies; IAA, insulin autoantibodies; HbA1c, glycated hemoglobin; ALT, alanine aminotransferase; ACR, albumin to creatinine ratio; US, ultrasound; PCOS, polycystic ovarian syndrome; OSA, obstructive sleep apnea; MD, physician; RD, registered dietitian; RN, registered nurse OGTT, oral glucose tolerance test.
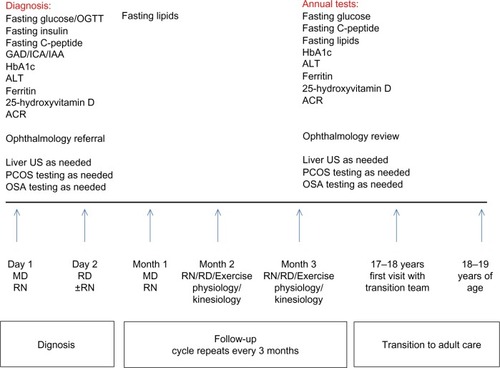
The program included patients who were younger than 18 years of age, with diabetes diagnosed as per current guidelines.Citation43,Citation44 Autoantibody testing is done at presentation, and further investigations and clinical evaluations are done at baseline and annually as outlined in .
The clinic structure is shown in . The clinic structure runs on one morning per month, and this ensures that members of diabetes and weight management teams dedicate their time to dealing only with T2D patients. The program was set up to include monthly visits for patients with team members. Transition to adult care begins at 17–18 years of age, and is completed at 18–19 years of age.
The envisaged structure of the monthly visits involved meeting with the physician and diabetes nurse educator on month 1, then seeing the dietitian on month 2, followed by a meeting with the exercise physiologist to perform exercise testing every 3 months, and meeting with the kinesiologist on month 3 to help with planning activities with some joint visits with team members. A meeting with the social worker was organized for new patients and a behavioral therapist was consulted if the team felt there was a need to help the patient overcome barriers to compliance with regard to managing their diabetes.
During the clinic visits, a review of the management plan including medications, dietary plans, exercise patterns, sleep habits, stress, screen time goals, and progress over the past few weeks was completed. This frequency of visits allowed the team to make suggestions on a regular basis to help patients navigate their management plan, and was important in providing support for patients and their families. Importantly, patients and families also had direct access to all team members between visits if they had any questions. The responsibilities of the team members are defined below.
Personnel
Diabetes nurse educator
The nurse was involved in educating newly diagnosed T2D patients and families, including but not restricted to education about blood glucose checks, insulin administration and dose adjustment if needed, management of sick days, hyperglycemia and hypoglycemia, and metformin use and side effects. The nurse educator served as the case manager for the patient, providing the main “go-to” person for families to communicate with the team.
Registered dietitian
The dietitian focus was on analyzing dietary intake and eating behaviors to try to understand what drives dietary choices in T2D patients. The dietitian also helped educate families around reading of food labeling and provided guidance on meal planning with controlled portion sizes and provision of the right mix of foods per meal.Citation45 The advice provided focused on increasing fruit and vegetable intake and eliminating high fat/high carbohydrate-containing food and sugary drinks, as well as addressing eating behaviors that precipitate increased food intake.
Exercise physiologist
The exercise physiologist was involved in fitness testing using a cycle ergometer. Graded progressive exercise to volitional exhaustion on the bike was used to assess each patient’s peak mechanical power. The test protocol is based on the McMaster All-Out Progressive Cycling Test.Citation46
Kinesiologist
Based on the results of fitness testing, the kinesiologist designs an exercise plan tailored to the ability, resources, and goals of the individual patient. This involves recommendations about enhancing physical activity within the usual daily routines and recommendations for more specific exercise routines based on patient preference, and include resistance training, stretching, using an exercise ball, and aerobic activities.
Social worker
The social worker meets new families at or shortly after diagnosis, and conducts detailed assessments regarding family structure, dynamics, and resources, and screens for stressors in the child’s life.
Behavioral therapist
The behavioral therapist works closely with some patients to define strategies for implementation of the treatment plans, and helps children and their families adjust to the diagnosis.
Administrative staff
Support staff are involved in making clinic appointments and directing patient queries to the appropriate staff member.
Physician
During consultations, the physician implements motivational interviewing.Citation47–Citation49 This approach engages the patient by asking their permission to discuss issues related to their health and allows the patient to set goals and decide on changes needed to manage their diabetes.
Other collaborations
Partnerships with other clinical services including sleep clinic, adolescent medicine, and child psychiatry have been established. All patients get referrals to the ophthalmology service initially and then are reviewed annually, and the nephrology service is involved if the patient has proteinuria or hypertension.
Implementation of management plans
The clinic’s structure and care plans are shown in , and the clinical management pathway used in the program is shown in . The protocol was generated based on an extensive review of the literature, and several evidence-based guidelines were consulted including those of the Canadian Diabetes Association,Citation44 International Society of Pediatric and Adolescent Diabetes,Citation50 and American Academy of Pediatrics,Citation51 along with data demonstrating the value of early insulin use in those with metabolic decompensation to reverse islet glucotoxicity and lipotoxicity.Citation52
Figure 3 Type 2 diabetes program treatment pathway.
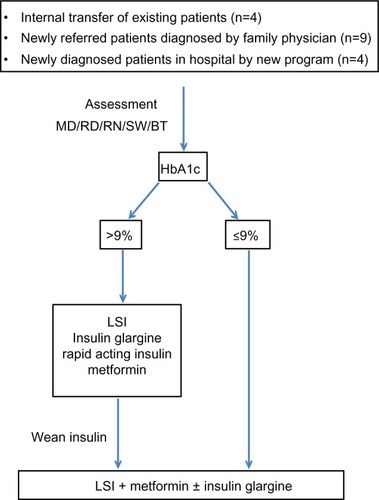
Given that lifestyle intervention alone has about a 10% success rate in T2D,Citation53–Citation55 the treatment plan for patients with newly diagnosed T2D involved implementation of pharmacotherapy early in the course of the disease depending on metabolic status and the presence of complications.Citation51,Citation56 As noted in , we started patients on insulin at a dose of 0.3 units/kg/day if HbA1c was >9%, and doses were adjusted to achieve glycemic control. We implemented multiple daily injection regimens of insulin glargine (Lantus®; Sanofi-Aventis, Bridgewater, NJ, USA) and rapid-acting insulin including insulin lispro (Eli Lilly and Company, Indianapolis, IN, USA) or aspart (Novo Nordisk A/S, Bagsværd, Denmark), with blood glucose checks at least four times daily. Insulin was weaned once blood glucose levels were stabilized, and metformin was added for those on insulin within 4–12 weeks of diagnosis and maintained after insulin was stopped.
For those who were 10 years of age or older with HbA1c ≤9% and metabolic stability, metformin was initiated at a dose of 500 mg once daily and increased by 500 mg every week to a maintenance dose of 1,000 mg twice daily.
In cases where compliance was a concern (n=6) we used Glumetza® (Valeant Pharmaceuticals International, Inc., Laval, QC, Canada), a slow-release version of metformin used once daily. Blood glucose checks were recommended twice daily, but adherence to this recommendation has been poor, despite reported good compliance with oral hypoglycemic therapy.
Lifestyle intervention began immediately, with consultations with the dietitian followed by a consultation with the exercise physiologist to measure fitness and the kinesiologist to provide recommendations regarding physical activity.
Statistical analysis
Sociodemographic and anthropometric variables are reported as the mean ± standard deviation or percentage as noted. Data were checked for normality using the Shapiro–Wilk test, and multiple imputations were used for missing data. Variables were log-transformed if not normally distributed for analysis; this included body mass index, fitness, percent fat mass, HbA1c, lipids, C-peptide, ALT, and urine ACR. The paired-samples t-test was used to evaluate the differences between variables at baseline and 1 year.
To measure the percent overweight change over 1 year, we used the following equation:Citation40
The statistical significance level for data analysis was set at a P-value of <0.05. Data analysis was done using Statistical Package for the Social Sciences version 22 software (IBM Corp, Armonk, NY, USA).
Results
Ethical approval for data collection and setting up the database was obtained from Hamilton Integrated Research Ethics Board. The patient anthropometric details are reported in . During the first year we acquired 17 patients, including four diagnosed having been referred for management of obesity; this is in addition to four out of the six patients already diagnosed before the setup of the program who were transitioned to the new program. The majority of newly diagnosed patients were referred for further management from the family physician with the diagnosis already established (n=9; ).
Table 1 Anthropometric parameters and comorbidities and complications in participants (n=17) in the type 2 diabetes program
The ethnic composition of our group included six patients from the aboriginal community, six Caucasian Europeans, two South Asians, two of mixed Caucasian/Afro-Caribbean descent, and one Hispanic subject. The average age at diagnosis was 14.0±1.6 years (n=4 male). The average birth weight was 3,760±718 g, and the reported age of menarche was 11.6±1.7 years.
One patient had positive anti-GAD antibodies (1.6 units/mL; normal reference for the laboratory is <1); this patient was diagnosed by their family physician 18 months before referral and was managed with oral hypoglycemic therapy and did not need insulin for the first 2 years while on metformin, and then needed insulin glargine at a dose of 0.06 units/kg/day with no rapid-acting insulin. By the time she was referred to our program, this patient was noted to be obese and had dyslipidemia, proteinuria, fatty liver disease, and acanthosis nigricans. Her genetic testing for maturity onset diabetes of the young (MODY) was negative, and her C-peptide levels were in the mid-normal range. While we did consider the potential diagnosis of latent autoimmune diabetes of the young (LADY), the low GAD antibody titer, normal C-peptide levels and the other features of insulin resistance led us to classify this patient as T2D. All others were negative for anti-GAD antibodies and none of the patients had detectable anti-insulin or islet cell autoantibodies. One patient presented in diabetic ketoacidosis requiring admission to the intensive care unit.
Of note, data available on 15 mothers and 14 fathers showed that eight (53%) mothers had diabetes compared to three (21%) of fathers at the time their child was diagnosed with T2D; 11 (73%) mothers and four (28%) fathers reported being overweight or obese at presentation, and those mothers reported being obese when pregnant with their diabetic child.
Comorbidities and complications
documents the T2D-associated comorbidities. All patients were obese (body mass index ≥95th percentile for age and sex), and the majority had nonalcoholic fatty liver disease diagnosed on measurement of ALT and liver ultrasound. The majority had dyslipidemia, and a quarter of the subjects were hypertensive or had proteinuria requiring therapy. Menstrual irregularities and polycystic ovarian syndrome based on a history of oligomenorrhea and hormonal profile with or without ovarian cysts on ultrasound was noted in about half of the female patients. Few had obstructive sleep apnea documented on sleep studies and needing continuous positive airway pressure therapy. None of our patients had peripheral neuropathy, and peripheral pulses were normal in all patients. A pediatric ophthalmologist did detailed annual evaluations, and none of our patients had evidence of retinopathy. Developmental and mental health concerns were noted in some patients, including anxiety (n=3), depression (n=5), and learning disability (n=2).
Evaluation of T2D program outcomes
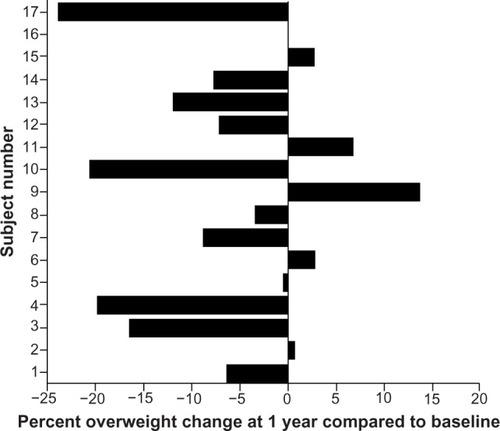
Over the first 12 months, the average HbA1c dropped from 8.0%±2.1% at baseline to 6.4%±0.9% (95% confidence interval (0.1–0.28), P<0.0001, ). In addition, the percent overweight change at 1 year compared to baseline was −6.2% (SD 10.4) (n=16; ).Citation40 Over the course of the year, body mass index and adiposity levels stabilized, with no further gain noted; all patients had low fitness levels at baseline and this did not improve with intervention (). Subjects had lower endogenous insulin (C-peptide) production (P=0.004), but did not show significant changes in metabolic biomarkers including fasting lipids, liver function (ALT), or urinary protein excretion (ACR, ).
Table 2 Change in primary and secondary outcomes from baseline at 1 year, with mean difference and confidence interval data reported for log-transformed variables (n=17)
Insulin was initiated in five patients presenting to the new program at diagnosis, and bedtime Lantus® (Sanofi-Aventis) added in another patient who was inadequately controlled on metformin monotherapy. Of the five patients started on insulin at diagnosis, one required 0.6 units/kg/day for 9 months and another patient required 0.85 units/kg/day for 11 months to remain euglycemic. The two patients were noncompliant with their treatment plan and had mental health problems that contributed to intermittent compliance with medications and lifestyle intervention plans; another patient remained on Lantus and rapid-acting insulin at 1 year.
Quality indicators
Quality indicators for this program were measured using a tool proposed by the International Society of Pediatric and Adolescent Diabetes to test quality of processes and outcomes in pediatric diabetes care with some modifications.Citation57 A summary of the modified quality indicators is given in . Our data show that this program fulfills the measured quality indicators.
Table 3 Quality indicators of the type 2 diabetes program
Discussion
Here, we describe the setup of a personalized multi disciplinary T2D management program in a tertiary pediatric center. One of the distinctive strengths of this program is the pooling of finite resources and creating partnerships between clinical programs that bring in complementary skill sets to provide excellent care. In addition, forging collaborations with subspecialist services including nephrology, ophthalmology, adolescent medicine, child psychiatry, sleep clinic, and others has enabled delivery of comprehensive care to these children and families. Collaboration between clinicians and clinical staff with hospital management to support the delivery of innovative care has been instrumental for the realization of this program.
The process of setting up the program involved carefully designed steps to gather staff input, synthesize the information into a workable plan, negotiate resources including personnel and space, design educational material, and maintain constant communication with team members. The clinical team meets regularly with management to discuss progress and address any operational issues.
We have demonstrated that the implementation of this program resulted in a significant reduction of HbA1c, coupled with a reduction in percent overweight. While our sample size is small, our results are concordant with those of larger studies showing similar effects of lifestyle intervention plus metformin compared with metformin monotherapy,Citation43 and argues for the role of pharmacotherapy as an important intervention for adequate glycemic control in pediatric and adolescent patients with T2D in addition to lifestyle intervention.
An important aspect of our program is the early use of multiple daily insulin injections in those patients with HbA1c >9%. Previous reports suggested marked improvement in glycemic control within weeks of initiating insulin therapy.Citation52 Our regimen may be perceived as a more demanding routine than twice-daily regimens by patients and families, and close contact with families in the early weeks of initiation of therapy and rapid withdrawal of insulin if adequate control is achieved may help reduce the duration of insulin use and improve compliance.
We also demonstrated that children with T2D have multiple comorbidities that require subspecialist services to enhance their management. In addition, our program meets the quality indicators of pediatric diabetes programs, including screening for comorbidities and their appropriate management. The attendance rates have been realistic considering that this population is known for its low attendance rates.Citation39,Citation40
One important note is that despite having better diabetes control, these patients continued to be morbidly obese, and their physical fitness as well as biomarkers of lipid metabolism and hepatic and renal health did not change significantly with intervention. One notable change was a reduction in C-peptide levels, potentially indicating reduced insulin resistance.
There was a significant number of obese and diabetic mothers in our cohort, and this may argue for epigenetic effects of maternal in utero environment driving fetal programming that allows evolution of obesity and T2D in the offspring, but may also affect the response to interventions and outcomes. This is an intriguing possibility and will require a larger sample size to define the answers. The main benefit for implementing lifestyle interventions in these patients may be to target the cardiometabolic risk factors and comorbidities of T2D and not to exclusively manage diabetes.Citation9–Citation18,Citation58
One of the challenges after setting up a clinical service is consolidation of current structures, sustaining resources, and planning for future growth. We are advocating for the provision of funding that will allow the program to grow its services, including organization of youth retreats, adding further dietetic services, group education sessions for patients and families, T2D outreach programs, and providing psychology services as part of the team, as this is currently an added referral for these patients.
A fundamental question that will not be answered immediately is whether this intervention will prevent or delay adverse long-term outcomes or ameliorate their severity; furthermore, the long-term cost-effectiveness of this program will not be evident immediately. However, as T2D is an aggressive disease in children and adults with multiple comorbidities, and as it is occurring early in the case of children, it seems reasonable to treat it early and aggressively.
In summary, we report the development of a new multidisciplinary, personalized, family-centered T2D program for children and adolescents at a tertiary pediatric center. Longitudinal follow-up of patients is mandatory to see if these initial gains are maintained and long-term outcomes are improved.
Acknowledgments
We would like to thank the patients and families attending the program for their participation and feedback. We also acknowledge the contribution of members of the diabetes team, weight management team, clinical managers, physicians, and administrative staff in their support of this program. Special thanks are due to Ms Karen Murray for providing organizational and administrative support.
Disclosure
The authors report no conflicts of interest in this work.
References
- LimSSVosTFlaxmanADA comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592224226023245609
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- WhitingDRGuarigualaLWeilCShawJIDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030Diabetes Res Clin Pract201194331132122079683
- Pinhas-HamielOZeitlerPThe global spread of type 2 diabetes mellitus in children and adolescentsJ Pediatr2005146569370015870677
- RosenbloomALJoeJRYoungRSWinterWEEmerging epidemic of type 2 diabetes in youthDiabetes Care199922234535410333956
- Fazeli FarsaniSvan der AaMPvan der VorstMMKnibbeCAde BoerAGlobal trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approachesDiabetologia20135671471148823677041
- DabeleaDPettittDJJonesKLArslanianSAType 2 diabetes mellitus in minority children and adolescents. An emerging problemEndocrinol Metab Clin North Am1999284709729 viii10609116
- NadeauKDabeleaDEpidemiology of type 2 diabetes in children and adolescentsEndocr Res2008331–2355819156573
- PorthaBChaveyAMovassatJEarly-life origins of type 2 diabetes: fetal programming of the beta-cell massExp Diabetes Res2011201110507622110471
- BeltrandJLevy-MarchalCFetal origins of type 2 diabetesArch Pediatr2008155537539 French18582664
- BarkerDJThe fetal origins of type 2 diabetes mellitusAnn Intern Med19991304 Pt 132232410068392
- LehnenHZechnerUHaafTEpigenetics of gestational diabetes mellitus and offspring health: the time for action is in early stages of lifeMol Hum Reprod201319741542223515667
- BrensekeBPraterMRBahamondeJGutierrezJCCurrent thoughts on maternal nutrition and fetal programming of the metabolic syndromeJ Pregnancy2013201336846123476780
- DabeleaDPettittDJIntrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibilityJ Pediatr Endocrinol Metab20011481085109111592564
- PettittDJLawrenceJMBeyerJAssociation between maternal diabetes in utero and age at offspring’s diagnosis of type 2 diabetesDiabetes Care200831112126213018694977
- DabeleaDMayer-DavisEJLamichhaneAPAssociation of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control StudyDiabetes Care20083171422142618375420
- DabeleaDThe predisposition to obesity and diabetes in offspring of diabetic mothersDiabetes Care200730Suppl 2S169S17417596467
- OngKKDungerDBThrifty genotypes and phenotypes in the pathogenesis of type 2 diabetes mellitusJ Pediatr Endocrinol Metab200013Suppl 61419142411202218
- SohnYBKimSJParkSWThe metabolic syndrome and body composition in childhood cancer survivorsKorean J Pediatr201154625325921949520
- ChemaitillyWBouladFOeffingerKCSklarCADisorders of glucose homeostasis in young adults treated with total body irradiation during childhood: a pilot studyBone Marrow Transplant200944633934319308039
- SamaanMCManagement of pediatric and adolescent type 2 diabetesInt J Pediatr2013201397203424260037
- MaahsDMSnivelyBMBellRAHigher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth studyDiabetes Care200730102593259817630264
- KershnarAKDanielsSRImperatoreGLipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth StudyJ Pediatr2006149331431916939739
- LiuLLLawrenceJMDavisCPrevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth studyPediatr Diabetes201011141119473302
- RodriguezBLDabeleaDLieseADPrevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth studyJ Pediatr20101572245251. e120394942
- ZeitlerPEpsteinLGreyMTreatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetesPediatr Diabetes200782748717448130
- [No authors listed]Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318548659742977
- [No authors listed]Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318378539742976
- [No authors listed]Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study GroupBMJ199831771607137209732338
- [No authors listed]Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study GroupBMJ199831771607037139732337
- SamaanMCThe macrophage at the intersection of immunity and metabolism in obesityDiabetol Metab Syndr2011312922035457
- CnopMWelshNJonasJCJörnsALenzenSEizirikDLMechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes many differences, few similaritiesDiabetes200554Suppl 2S97S10716306347
- KnipMSiljanderHAutoimmune mechanisms in type 1 diabetesAutoimmun Rev20087755055718625444
- WherrettDHuotCMitchellBPacaudDType 1 diabetes in children and adolescents. Canadian Diabetes Association Clinical Practice Guidelines Expert CommitteeCan J Diabetes201337S153S16224070940
- KahnSEHullRLUtzschneiderKMMechanisms linking obesity to insulin resistance and type 2 diabetesNature2006444712184084617167471
- DrongALindgrenCMcCarthyMThe genetic and epigenetic basis of type 2 diabetes and obesityClin Pharmacol Ther201292670771523047653
- MaretteAMolecular mechanisms of inflammation in obesity-linked insulin resistanceInt J Obes Relat Metab Disord200327Suppl 3S46S4814704744
- ShoelsonSEHerreroLNaazAObesity, inflammation, and insulin resistanceGastroenterology200713262169218017498510
- ReinehrTSchoberERothCLWiegandSHollRType 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centersHorm Res200869210711318059091
- ZeitlerPHirstKPyleLA clinical trial to maintain glycemic control in youth with type 2 diabetesN Engl J Med2012366242247225622540912
- TuckerMEFDA panel advises easing restrictions on rosiglitazoneBMJ2013346f376923752055
- McMaster Children’s HospitalThe pediatric & adoloscent type 2 diabetes programHamilton Health Sciences patient eduction92013 Available from http://www.hamiltonhealthsciences.ca/documents/Patient%20Education/AdolescentType2DiabetesProgram-lw.pdfAccessed May 29, 2014
- International Diabetes Federation/International Society for Pediatric and Adolescent DiabetesThe Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence2011 Available from: http://www.idf.org/global-idfispad-guideline-diabetes-childhood-and-adolescenceAccessed May 7, 2014
- Canadian Diabetes AssociationClinical Practice Guidelines for the Prevention and Management of Diabetes in CanadaCan J Diabetes201337Suppl 1S1S21224070926
- Health CanadaEating well with Canada’s food guide Available from: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.phpAccessed May 7, 2014
- Bar-OrORowlandTPediatric Exercise Medicine: From Physiologic Principles to Health Care ApplicationChampaign, IL, USAHuman Kinetics2004
- SuarezMMullinsSMotivational interviewing and pediatric health behavior interventionsJ Dev Behav Pediatr200829541742818852613
- SchwartzRP1HamreRDietzWHOffice-based motivational interviewing to prevent childhood obesity: a feasibility studyArch Paediatr Adolesc Med20071615495501
- TreasureJMotivational interviewingAdv Psychiatr Treat2004105331337
- RosenbloomALSilversteinJHAmemiyaSZeitlerPKlingensmithGJType 2 diabetes mellitus in the child and adolescentPaediatr Diabetes200895512526
- CopelandKCSilversteinJMooreKRManagement of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescentsPediatrics2013131236438223359574
- SellersEADeanHJShort-term insulin therapy in adolescents with type 2 diabetes mellitusJ Pediatr Endocrinol Metab200417111561156415570994
- SilversteinJHRosenbloomALType 2 diabetes in childrenCurr Diab Rep200111192712762953
- DaaboulJJSiversteinJHThe management of type 2 diabetes in children and adolescentsMinerva Pediatr200456325526415252375
- MillerJLSilversteinJHThe management of type 2 diabetes mellitus in children and adolescentsJ Pediatr Endocrinol Metab200518211112315751600
- GeorgeMMCopelandKCCurrent treatment options for type 2 diabetes mellitus in youth: today’s realities and lessons from the TODAY studyCurr Diab Rep2013131728023065368
- PihokerCForsanderGWolfsdorfJKlingensmithGJThe delivery of ambulatory diabetes care to children and adolescents with diabetesPediatr Diabetes200910Suppl 12587019754619
- NadeauKJKlingensmithGZeitlerPType 2 diabetes in children is frequently associated with elevated alanine aminotransferaseJ Pediatr Gastroenterol Nutr2005411949815990637