Abstract
Neuropathic pain (NeuP) is a syndrome that results from damaged nerves and/or aberrant regeneration. Common etiologies of neuropathy include chronic illnesses and medication use. Chronic disorders, such as diabetes and alcoholism, can cause neuronal injury and consequently NeuP. Certain medications with antineoplastic effects also carry an exquisitely high risk for neuropathy. These culprits are a few of many that are fueling the NeuP epidemic, which currently affects 7%–10% of the population. It has been estimated that approximately 10% and 7% of US adults carry a diagnosis of diabetes and alcohol disorder, respectively. Despite its pervasiveness, many physicians are unfamiliar with adequate treatment of NeuP, partly due to the few reviews that are available that have integrated basic science and clinical practice. In light of the recent Centers for Disease Control and Prevention guidelines that advise against the routine use of μ-opioid receptor-selective opioids for chronic pain management, such a review is timely. Here, we provide a succinct overview of the etiology and treatment options of diabetic and alcohol- and drug-induced neuropathy, three different and prevalent neuropathies fusing the combined clinical and preclinical pharmacological expertise in NeuP of the authors. We discuss the anatomy of pain and pain transmission, with special attention to key ion channels, receptors, and neurotransmitters. An understanding of pain neurophysiology will lead to a better understanding of the rationale for the effectiveness of current treatment options, and may lead to better diagnostic tools to help distinguish types of neuropathy. We close with a discussion of ongoing research efforts to develop additional treatments for NeuP.
Introduction
Neuropathic pain (NeuP) arises from aberrant or incomplete regeneration of damaged nerves, and is characterized by hyperalgesia and allodynia, enhanced sensitivity to pain, and exaggerated pain response to normal stimuli.Citation1 The prevalence of NeuP varies around the world, but has been cited as at a minimum of 3%,Citation2–Citation4 and true prevalence has been estimated to be around 7%–10%.Citation5 The incidence of NeuP also varies by type, as categorized by the mechanism of injury: diabetic neuropathy, alcoholic neuropathy, and medication-induced neuropathy.Citation6 A concerning trend in the US is the rise in diagnosis of neuropathies caused by type 2 diabetes.Citation7 This may in part be fueled by increased health care costs hampering proper management of diabetes (diabetes.org).Citation8 Alcoholic neuropathy in the US occurs in roughly 65% of patients diagnosed with an alcohol-use disorder.Citation9 Anticancer medications, especially taxanes, are also known to cause neuropathy. A study found that 100% of patients receiving paclitaxel developed symptoms of neuropathy.Citation10 On a clinical level, NeuP translates to a complex syndrome that is often multidrug-resistant and unresponsive to alternative therapies. Therefore, it is imperative that clinicians understand the etiology of NeuP and the mechanism and effectiveness of the current repertoire of treatments.
Significance and limitations
In this integrative review, an internal medicine physician and preclinical behavioral pharmacologist summarize differences and overlap in etiologies and treatment options for NeuP. This review focuses on three of the most common neuropathies – diabetic, alcohol-induced, and drug-induced neuropathy – describes the specific nerve fibers associated with each neuropathy, and lists recommended treatment options for NeuP. The review is purposely succinct, aimed at providing clinicians with insight into the etiology of NeuP and educating preclinical scientists on the diagnosis and choice of treatment for NeuP. The review favors conciseness over an extensive in-depth analysis of the available literature, thus limiting its scope.
Materials and methods
A PubMed search was performed to identify clinical and preclinical studies detailing etiology and treatment of NeuP. Emphasis was given to articles published in the last 10 years, with older articles used primarily to provide a frame of reference.
Basic anatomy of pain
Comprehending the pathophysiology of peripheral neuropathy and the mechanism of action for drugs requires a basic appreciation of the anatomy of the somatosensory system, especially with respect to pain. Noxious stimuli, such as thermal, chemical, and high-threshold mechanical stimuli, are detected in the periphery and conducted to the spinal cord via two types of small fibers. The C fibers are unmyelinated, slow-conducting, and localize pain poorly. The Aδ fibers are thinly myelinated, faster-conducting, and localize pain better.Citation11,Citation12 Larger and more thickly myelin-ated than Aδ fibers are Aα and Aβ fibers, which primarily transmit information about proprioception and vibration.Citation13 It is primarily the Aδ and C fibers that are indiscriminately affected in the different types of neuropathies (). Measuring which type of fibers are impacted is not trivial, but can be attempted by clinical examination; loss of tactile or vibratory skin sensation or tendon reflexes are indicative of large-fiber neuropathy, whereas alterations in lower-limb pinprick sensation and a visual analog scale pain score >40 suggest small-fiber neuropathy.Citation14 Small-fiber neuropathy can also be determined by measuring intraepidermal nerve-fiber density following biopsy.Citation15 Aδ-fiber neuropathy can be measured noninvasively by laser evoked-potentialCitation16,Citation17 and contact heat-evoked potential.Citation18,Citation19 Nerve-conducting studies are a useful technique for NeuP research, but less relevant for clinical studies, as they primarily measure Aβ-fiber function, which supersedes small-fiber neuropathyCitation20 and is laborious.Citation21
Figure 1 Overview of neuropathies affecting pain pathways.
Abbreviations: NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; TRP, transient receptor potential; CGRP, calcitonin gene-related peptide.
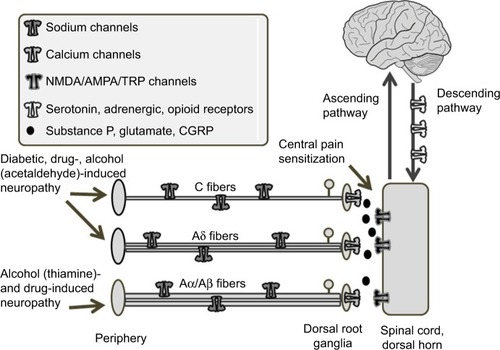
Receptors on primary sensory neurons convert environmental stimuli, such as pain, into an electrical signal that is transmitted to the dorsal root ganglia, with an important role for sodium channels.Citation22,Citation23 In the terminals of the dorsal root ganglia, neurons subsequently convert this electric signal into chemical signals by releasing neurotransmitters and neuropeptides, including glutamate, substance P, and calcitonin gene-related peptide into the dorsal horn (). A significant event that occurs during the development of a chronic pain state is central sensitization, where postsynaptic glutamate (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and N-methyl-d-aspartate [NMDA]) receptors become increasingly more adaptive in transmitting pain signals.Citation24–Citation28 Activation of presynaptic calcium channels can reduce the release of neurotransmitters and dampen central sensitization ().Citation29,Citation30 In the spinal cord, this nocicep-tive signal can be modulated by inhibitory interneurons using γ-aminobutyric acid (GABA) and glycine as their main neurotransmitters. Following the reception of a pain signal in the cortical structures of the brain, the experience of pain can still be suppressed by a descending system that originates from the brain stem. This efferent system attenuates the afferent signal via neurotransmitters, such as endogenous opioids, serotonin, and noradrenaline.Citation31–Citation33
Diagnosis of neuropathic pain
The diagnosis of NeuP is usually made on clinical grounds. Screening questionnaires are available for NeuP, including the Leeds Assessment of Neuropathic Symptoms and Signs,Citation34 PainDetect,Citation35 and Douleur Neuropathique 4 (DN4).Citation36 Of these, the DN4 has the higher sensitivity and specificity. The DN4 is a questionnaire that consists of four questions and incorporates both subjective and objective information, namely the patient’s perception of pain and the physician’s exam findings. It has been validated as a screening tool for different types of NeuP, including diabetic neuropathy, and has sensitivity and specificity of 80% and 92%, respectively, at a score cutoff of 4.Citation36 At a score cutoff of 3, sensitivity and specificity are 84%.Citation36 While these screening tools are useful, the diagnosis ultimately hinges on the clinician’s intuition from effective interviewing and physical examination. Symptoms of neuropathy can range from hypoalgesia and paresthesia (tingling) to hyperalgesia, especially prominent in the distal extremities, known as the “stocking and glove” distribution. Patients with neuropathy may exhibit a painful response to benign stimuli, such as light touch from a cotton swab or a finger. Patients may also exhibit an attenuated/exaggerated response to a pinprick. These phenomena are known as allodynia and hypo/hyperalgesia, respectively. As the neuropathy progresses, more severe symptoms, including burning sensations and electric shocks, can arise. Symptoms are aggravated during rest and prolonged weakness and sensory loss in extremities; particularly feet can culminate in gait impairment.Citation37 Because the diagnosis is based on subjective data, it can sometimes be challenging for the physician to track response to therapeutics. With this said, the threshold to treat NeuP is exceedingly low, given the good side-effect profile of most drugs. In our experience, most physicians would trial a serotonin–norepinephrine reuptake inhibitor (SNRI) if a patient complained of burning pain, even if no other findings were present.
Three major types of neuropathy
Diabetic neuropathy
Peripheral neuropathy is one of the most common microvascular complications of diabetes. It has been estimated that ~50% of diabetics suffer from peripheral neuropathy,Citation38 and 50% of these neuropathies are considered at least moderate in severity.Citation39–Citation41 Histological studies suggest that primarily small C fibers are affected by diabetes and glucose intolerance,Citation42–Citation45 although Aδ fibers have also been shown to be affected by type 1 and type 2 diabetes.Citation46–Citation48 Morbidities associated with diabetic neuropathy amount to more than $10 billion in the US.Citation49
Diabetes or glucose intolerance can impair vasodilation and lead to ischemia, which is thought to be central to the pathogenesis of peripheral neuropathy, including trigeminal neuralgia.Citation50,Citation51 A recent study demonstrated that patients with glucose intolerance, even without carrying the diagnosis of diabetes, exhibited C-fiber neuropathy, highlighting the devastating effect of prolonged hyperglycemia on neuronal health.Citation52 On a molecular level, there are at least five prevailing mechanisms of how hyperglycemia leads to different complications of diabetes, with the polyol and PARP pathways being the most relevant to neuronal death.Citation53 In the polyol pathway, the influx of glucose into the cell activates aldose reductase to convert glucose to sorbitol. Sorbitol is then converted to fructose via sorbitol dehydrogenase. Both of these steps generate oxidative species that contribute to neuronal injury.Citation53,Citation54 In Schwann cells, endothelial cells, and sensory neurons, PARP is stimulated by oxidative species and induces further oxidative stress in a feedback mechanism.Citation55–Citation59 PARP is a nuclear enzyme that can also alter gene expression, leading to impairment of neuronal conduction velocity, small-fiber neuropathy (), hyperalgesia, and allodynia, as well as other diabetic complications.Citation53,Citation55,Citation60–Citation64
Alcoholic neuropathy
The prevalence of alcohol-related peripheral neuropathy has been estimated to occur in two-thirds of chronic alcoholics.Citation9 Alcohol-related peripheral neuropathy is historically regarded as a large-fiber neuropathy from thiamine deficiency ().Citation65–Citation69 In contrast to small sensory fibers, large fibers are responsible for vibration and proprioception. However, advances in scientific techniques have reshaped the pathophysiology of alcoholic neuropathy. Observations that neuropathy can develop even in the setting of normal thiamine levelsCitation70 and that the early stages of alcoholic neuropathy are characterized by painful paresthesiaCitation71 have led scientists to postulate that alcohol and its metabolites have direct neurotoxic effects on small C fibers ().Citation72,Citation73 Acetaldehyde is a known neurotoxin that is formed when alcohol is metabolized by alcohol dehydrogenase. The precise mechanism underlying alcoholic neuropathy is yet to be fully elucidated. Some proposed explanations include direct neurotoxic effects of alcohol or its metabolite acetaldehydeCitation70 through activation of spinal cord microglia,Citation74 involvement of metabotropic glutamate 5 and opioid receptorsCitation74,Citation75 in the spinal cord, promotion of oxidative stress by the activity of alcohol-metabolizing enzymes in the liver,Citation76 and release of proinflammatory cytokines coupled with phosphorylation of protein kinase CCitation77 and extracellular signal-regulated kinases.Citation78 Taken together, these different initiating events may ultimately lead to DNA fragmentation and neuronal apoptosis.Citation79 Once formed, acetaldehyde is metabolized by ALDH into a much less harmful acetate. Interestingly, pharmacological and genetic data suggest that reducing ALDH activity can precipitate peripheral neuropathy, whereas increasing its activity may carry therapeutic potential. As such, disulfiram, an ALDH inhibitor, has the side effect of causing NeuP.Citation80
Medication-induced neuropathy
Disulfiram is not unique in causing NeuP; in fact, many and more commonly prescribed drugs, which span the spectrum of chemotherapy to cardiovascular medications, are known to induce neuropathy. This section focuses on taxanes, with mention of statin-induced neuropathy. Paclitaxel and docetaxel are antineoplastic taxanes used to treat numerous types of solid tumors, including ovarian, breast, lung, and head and neck malignancies. Paclitaxel has its chemotherapeutic effect by promoting microtubule assembly in a disorganized manner, thereby prohibiting mitotic division. It is by this very same mechanism that paclitaxel causes peripheral neuropathy, one of the most common and limiting side effects of the drug. In vivo studies have demonstrated that paclitaxel causes abnormal microtubule accumulation, leading to demyelination,Citation81,Citation82 and inhibits the regenerative capacities of neurons.Citation83,Citation84 In a non-dose-dependent manner, paclitaxel causes hyperalgesia and allodynia without affecting motor performance.Citation85 Clinically, patients have complained of sensory neuropathy and decreased vibration and proprioception, indicating that both small C and Aδ fibers are affected ().Citation86–Citation89 One of the original articles on paclitaxel-induced neuropathy studied paclitaxel infusion at three doses, and found that neuropathy developed in >80% of the patients at all doses and was dose-limiting in 70% of patients at the highest dose.Citation90
It is noteworthy that diabetes is a predisposition to drug-induced neuropathy. In a retrospective study comparing the rates of taxane-induced neuropathy, chronic diabetics (defined by >5 years) developed neuropathy more frequently compared to nondiabetics.Citation91 With regard to treating chemotherapy-induced peripheral neuropathy, SNRIs have been shown to be superior to placebo.Citation92 These drugs are also used in the treatment of diabetic neuropathy. Together, these data suggest that taxanes and diabetes act differently but synergistically in causing peripheral nerve damage and NeuP.
Statins are prescribed to >40 million patients in the US alone (https://meps.ahrq.gov), and are frequently prescribed to diabetics to reduce their cardiovascular risk. It is interesting to note that there have been reports of statin-induced peripheral neuropathy.Citation93,Citation94 Though statins are demonstrated to have pleiotropic effects, preclinical studies suggest that they can attenuate NeuP by potentiating antioxidation.Citation95,Citation96 Promising data have also been found in human studies where rosuvastatin improved both the intensity of diabetic neuropathy pain and nerve conduction.Citation97 In combination with the rarity of statin-induced neuropathy, these data suggest that this potential side effect of statin should minimally influence the physician’s decision to prescribe statins for vascular protection.
Current neuropathic pain therapies
Based on a 2015 analysis of a systemic review and meta-analysis performed by the Neuropathic Pain Special Interest Group on clinical studies of NeuP pharmacotherapy, a new guideline for treatment of NeuP was recently proposed.Citation98 The guidelines highlight the difficulty in adequately treating NeuP, but recommend the use of tricyclic antidepressants (TCAs), SNRIs, pregabalin, and gabapentin as first-line treatment options for NeuP.Citation98 In the following section, we discuss these medications in more detail.
Tricyclic antidepressants
A number of randomized controlled trials have shown that TCAs may exert their NeuP-relieving effect via multiple mechanisms of action.Citation99,Citation100 The potential of TCAs in pain relief is primed by their inhibition of presynaptic reuptake of serotonin and norepinephrineCitation101,Citation102 and activity at NMDA receptors and sodium channels,Citation103 all of which are involved in pain transmission. Amitriptyline and nortriptyline are two of the oldest TCAs on the market. Although their use to treat depression has declined with the increased popularity of SNRIs and selective serotoninreuptake inhibitors, they continue to be used off-label to treat NeuP. However, according to recent Cochrane meta-analyses, no high-quality evidence exists to support the analgesic effect of both amitriptyline and nortriptyline, despite an extensive history of anecdotal success.Citation104,Citation105 A factor for the declining use of TCAs, whether for depressive disorders or NeuP, is that TCAs have a higher risk for fatal overdose and require careful dosing. Therefore, TCAs should not be advocated for use as first-line treatment of NeuP.
Serotonin–norepinephrine reuptake inhibitors
Venlafaxine and duloxetine are SNRIs that are prescribed to treat depression, anxiety, and NeuP. Serotonin and norepinephrine play integral parts in the descending pain pathway to suppress pain.Citation106–Citation108 Preclinical and clinical research has shown that drugs that increase serotonergic and noradrenergic neurotransmissions exert antinociceptive properties. For example, SNRIs significantly attenuated pain-related behaviors in the formalin model of persistent pain and the L5–L6 spinal nerve ligation model of NeuP in rats.Citation109 SNRIs are also efficacious in the treatment of pain and functional impairment associated with fibromyalgia, as per a number of randomized controlled trials.Citation110–Citation113
Pharmacological studies have demonstrated that for certain SNRIs, serotonin reuptake inhibition predominates at low drug concentration, whereas inhibition of norepinephrine reuptake occurs only at much higher doses.Citation114 Unlike treating depression, the treatment for NeuP with SNRIs is achieved at higher doses and more rapidly. For venlafaxine, the usual antidepressant dosage is much lower than what is needed for pain relief,Citation115 suggesting that norepinephrine contributes more strongly to attenuating pain. The importance of norepinephrine is further exemplified by the effect of clonidine and the adrenergic receptor agonist in alleviating pain.Citation116 However, a Cochrane review found that venlafaxine had limited efficacy compared to placebo.Citation32 Despite relatively similar pharmacology, duloxetine was effective for the relief of NeuP.Citation117 Duloxetine is a much more potent inhibitor of the serotonin- and norepinephrine reuptake transporters than venlafaxine.Citation114 Therefore, when choosing an SNRI, we would favor the use of duloxetine.
Calcium-channel blockers
Changes in the expression and activity of voltage-gated calcium channels are known to modulate neuronal excitability and synaptic plasticity in the dorsal horn, culminating in pain processing.Citation118–Citation120 The Cavα2δ1subunit, an important accessory subunit for calcium channels, plays an important role in NeuP development, based on reports of increased expression in the dorsal root ganglia and spinal neurons during NeuP states.Citation121–Citation123 Further supporting evidence shows that blockade of the Cavα2δ1 subunit could reverse tactile allodynia in nerve-injured animals.Citation124,Citation125 Interestingly, the Cavα2δ1subunit is the binding site for pregabalin and gabapentin.Citation126 Pregabalin and gabapentin were developed and US Food and Drug Administration (FDA) approved for treatment of epilepsy, but have become first-line treatments for NeuP.
A Cochrane review using randomized double-blind trials found pregabalin to be effective for the treatment of NeuP. Pregabalin at doses of >300 mg provided moderate pain relief (50% improvement from baseline) in different types of pain.Citation127 Another Cochrane review investigated the efficacy of gabapentin on NeuP using randomized double-blind controlled studies, and concluded that 1,200 mg daily was needed to achieve 50% pain relief. This effect was found in 35% of study participants compared to 21% in the placebo group.Citation128 To avoid sedating effects, gabapentin is divided into three doses, and patients are routinely instructed to titrate the dose, starting at 300 mg daily. However, compliance is a major issue in patients on gabapentin, usually as they dismiss the medications as ineffective and discontinue the medications before reaching the therapeutic dose. Therefore, it is prudent to educate patients of this therapeutic range. Moreover, a recent study has shown that the endogenous lipid palmitoylethanolamide has synergistic effects with gabapentin to relieve chemotherapy-induced allodynia in mice, which makes it possible to reduce the dosage of gabapentin and lower its side effects.Citation13
To illustrate further the vital role of calcium channels in pain transmission, ziconotide is a selective calcium-channel blocker and potent analgesic. Ziconotide is FDA approved for the treatment of refractory chronic pain.Citation129 As ziconotide is a large peptide that cannot readily cross the blood–brain barrier, it can thus only be administered intrathecally. Intrathecal drug delivery can be used to manage chronic pain effectively, and may provide the most targeted approach with the fewest side effects.Citation130–Citation133
Opioids and drug development
Although opioids are intended for short-term use for acute pain, they have repeatedly been used to treat chronic pain. For example, tramadol has been used to treat chronic pain, in part due to its dual action as a μ-opioid agonist and SNRI.Citation98 Patients frequently cite failures of different adjunctive therapies to alleviate their pain, and revert to the use of opioids. In light of the rapid increase in patients suffering from opioid-dependence/use disorders, the Centers for Disease Control and Prevention (CDC) has recently published guidelines to avoid routinely prescribing narcotics for the management of chronic pain. In addition to dependence, opioids also cause other serious side effects, including tolerance, ileus, and respiratory depression. The latter side effect explains the high hospitalization and mortality rate associated with opioid overdose, which has increased concomitantly with the rise in opioid dependence. Moreover, prolonged use of (escalating doses of) opioids can lead to paradoxical pain, also known as opioid-induced hyperalgesia, and discontinuation of opioids leads to withdrawal of hyperalgesia. While the CDC guidelines are helpful for guiding narcotic use, it will be challenging to unearth the culture of pain management that is heavily rooted in narcotics. The CDC currently excludes these guidelines from patients with active malignancy, which remains a challenge to treat, despite rapidly escalating doses of opioids. Clearly, safer and more potent and selective therapeutics are necessary and overdue. Although the CDC raised concerns regarding the use of μ-opioids in chronic pain, it is important not to dismiss completely their analgesic potential for acute pain and palliative care.Citation134 Importantly, other opioid-receptor subtypes like μ are also expressed along descending pain pathways, and increasing research efforts have identified these non-μ-opioid receptors as potential analgesic targets for chronic pain.
While current analgesic opioids target μ-opioid receptors, there are three other opioid-receptor subtypes. One of the most intriguing new developments in the use of opioids for NeuP comes from work focused on δ-opioid receptors (DORs), κ-ORs (KORs), and nociception ORs (NORs). DORs, KORs, and NORs are expressed in several levels of pain pathways, including the periphery, spinal cord, and supraspinal regions.Citation135–Citation141 The expression of opioid-peptide messenger RNA also increases under conditions of chronic pain.Citation142–Citation144 Preclinical evidence has demonstrated that inhibition of DORs or KORs via either opioid antagonists or genetic ablation in mice enhances allodynia and hyperalgesia following spinal cord injury.Citation145–Citation148 Additionally, both DOR and KOR agonists have elicited antinociceptive and antiallodynic effects in animal models of NeuP.Citation142,Citation149–Citation151 An intriguing recent study identified 6-methoxyflavanone as a positive allosteric modulator of GABAA channels that can alleviate streptozotocin-induced diabetic NeuP in female rats in a naloxone-reversible manner, potentially via direct interaction with DORs and KORs.Citation152 In contrast, the role of NORs in nociception is less linear: analgesic actions of the nociceptin system in rodents are bidirectional, depending upon the doses and assays.Citation153–Citation156 Encouragingly, the activation of NORs produces only attenuated and not intensified pain in primates regardless of experimental conditions,Citation157,Citation158 and thus their therapeutic potential in humans remains.
Summary
NeuP results from nerve damage, and can be classified based on the inciting factors, such as hyperglycemia (as in diabetes), toxins from alcohol metabolism, and drugs like chemotherapy. In basic pain anatomy, noxious stimuli are detected in the periphery, and are transmitted via small fibers to the central nervous system, where they are converted into the experience of pain (). This afferent system is modulated by an efferent system via GABAergic neurons and neurotransmitters, such as serotonin and norepinephrine. Opioids that act on GABAergic neurons have been used in pain relief for millennia. However, the side effects of opioids, especially their addictive properties, have recently led the CDC to advise against their use in chronic pain. Therefore, an understanding of adjunctive therapies for NeuP is essential. Meta-analyses show the most promising efficacy in calcium-channel ligands and SNRIs over older TCAs, but these medications still leave a number of patients untreated. Sodium-channel blockers may represent a broadly applicable strategy for many types of NeuP.Citation159–Citation162 However, improved diagnosis of symptoms paired with increased understanding and detection of the exact fibers affected by disease-specific neuropathies may guide the development of more precise therapeutics.Citation163 Targeting receptors or ion channels that are uniquely expressed in Aβ, Aδ, or C fibers, including DORs and transient receptor-potential (TRPV, TRPA1) channelsCitation164–Citation166 may represent a new direction for NeuP treatment.
Acknowledgments
RMvR receives funding from the National Institute on Alcohol Abuse and Alcoholism (R00AA020539) and the Ralph W and Grace M Showalter Research Trust.
Disclosure
The authors report no conflicts of interest in this work.
References
- JensenTSBaronRHaanpääMA new definition of neuropathic painPain2011152102204220521764514
- GustorffBDornerTLikarRPrevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative surveyActa Anaesthesiol Scand200852113213617976220
- BouhassiraDLanteri-MinetMAttalNLaurentBTouboulCPrevalence of chronic pain with neuropathic characteristics in the general populationPain2008136338038717888574
- TorranceNSmithBHBennettMILeeAJThe epidemiology of chronic pain of predominantly neuropathic origin: results from a general population surveyJ Pain20067428128916618472
- van HeckeOAustinSKKhanRASmithBHTorranceNNeuropathic pain in the general population: a systematic review of epidemiological studiesPain2014155465466224291734
- BaronRForsterMBinderASubgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approachLancet Neurol20121111999100523079556
- MenkeACasagrandeSGeissLCowieCCPrevalence of and trends in diabetes among adults in the United States, 1988–2012JAMA2015314101021102926348752
- American Diabetes Association Available from: http://diabetes.org/Accessed January 13, 2017
- AmmendolaATataMRAurilioCPeripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjectsAlcohol Alcohol200136327127511373267
- LoprinziCLReevesBNDakhilSRNatural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1J Clin Oncol201129111472147821383290
- BeissnerFBrandauAHenkeCQuick discrimination of Adelta and C fiber mediated pain based on three verbal descriptorsPloS One201059e1294420886070
- McGloneFReillyDThe cutaneous sensory systemNeurosci Biobehav Rev201034214815919712693
- HoitsmaEReulenJPde BaetsMDrentMSpaansFFaberCGSmall fiber neuropathy: a common and important clinical disorderJ Neurol Sci2004227111913015546602
- LefaucheurJPCreangeANeurophysiological testing correlates with clinical examination according to fibre type involvement and severity in sensory neuropathyJ Neurol Neurosurg Psychiatry200475341742214966158
- LauriaGMorbinMLombardiRAxonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathiesNeurology200361563163612963753
- MagerlWAliZEllrichJMeyerRATreedeRDC- and Aδ-fiber components of heat-evoked cerebral potentials in healthy human subjectsPain199982212713710467918
- TreedeRDLorenzJBaumgartnerUClinical usefulness of laser-evoked potentialsNeurophysiol Clin200333630331414678844
- GranovskyYMatreDSokolikALorenzJCaseyKLThermoreceptive innervation of human glabrous and hairy skin: a contact heat evoked potential analysisPain2005115323824715911150
- ChenACNiddamDMArendt-NielsenLContact heat evoked potentials as a valid means to study nociceptive pathways in human subjectsNeurosci Lett20013162798211742720
- BreinerALovblomLEPerkinsBABrilVDoes the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients?Diabetes Care20143751418142424574353
- EnglandJDGronsethGSFranklinGDistal symmetric polyneuropathy: a definition for clinical researchNeurology200564219920715668414
- RushAMCumminsTRWaxmanSGMultiple sodium channels and their roles in electrogenesis within dorsal root ganglion neuronsJ Physiol2007579Pt 111417158175
- WangWGuJLiYQTaoYXAre voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain?Mol Pain201171621345196
- WoolfCJThompsonSWThe induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity statesPain19914432932991828878
- UlteniusCLinderothBMeyersonBAWallinJSpinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the ratNeurosci Lett20063991–2859016469445
- LuYSunYNWuXRole of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunit GluR1 in spinal dorsal horn in inflammatory nociception and neuropathic nociception in ratBrain Res20081200192618289517
- ParkJSYasterMGuanXRole of spinal cord α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund’s adjuvant-induced inflammatory painMol Pain200846719116032
- LatremoliereAWoolfCJCentral sensitization: a generator of pain hypersensitivity by central neural plasticityJ Pain200910989592619712899
- JørumEWarnckeTStubhaugACold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine – a double-blind, cross-over comparison with alfentanil and placeboPain2003101322923512583865
- ChizhBAGöhringMTrösterAQuarteyGKSchmelzMKoppertWEffects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteersBr J Anaesth200798224625417251214
- Al-HasaniRBruchasMRMolecular mechanisms of opioid receptor-dependent signaling and behaviorAnesthesiology201111561363138122020140
- GallagherHCGallagherRMButlerMBuggyDJHenmanMCVenlafaxine for neuropathic pain in adultsCochrane Database Syst Rev20158CD01109126298465
- BasbaumAIFieldsHLEndogenous pain control systems: brainstem spinal pathways and endorphin circuitryAnnu Rev Neurosci198473093386143527
- BennettMThe LANSS pain scale: the Leeds assessment of neuropathic symptoms and signsPain2001921–214715711323136
- FreynhagenRBaronRGockelUTolleTRPainDetect: a new screening questionnaire to identify neuropathic components in patients with back painCurr Med Res Opin200622101911192017022849
- SpalloneVMorgantiRD’AmatoCGrecoCCacciottiLMarfiaGAValidation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathyDiabet Med201229557858522023377
- HawleyRJKurtzkeJFArmbrustmacherVWSainiNManzHThe course of alcoholic-nutritional peripheral neuropathyActa Neurol Scand19826655825896293240
- BoultonAJVinikAIArezzoJCDiabetic neuropathies: a statement by the American Diabetes AssociationDiabetes Care200528495696215793206
- DaviesMBrophySWilliamsRTaylorAThe prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetesDiabetes Care20062971518152216801572
- DiBonaventuraMDCappelleriJCJoshiAVAssociation between pain severity and health care resource use, health status, productivity and related costs in painful diabetic peripheral neuropathy patientsPain Med201112579980721481171
- SadoskyASchaeferCMannRBurden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional surveyDiabetes Metab Syndr Obes20136799223403729
- OrstavikKNamerBSchmidtRAbnormal function of C-fibers in patients with diabetic neuropathyJ Neurosci20062644112871129417079656
- PittengerGLRayMBurcusNIMcNultyPBastaBVinikAIIntraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patientsDiabetes Care20042781974197915277426
- PolydefkisMHauerPShethSSirdofskyMGriffinJWMcArthurJCThe time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathyBrain2004127Pt 71606161515128618
- SmithAGRamachandranPTrippSSingletonJREpidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathyNeurology20015791701170411706115
- ChaoCCTsengMTLinYJPathophysiology of neuropathic pain in type 2 diabetes: skin denervation and contact heat-evoked potentialsDiabetes Care201033122654265920841612
- LøsethSStålbergEVLindalSOlsenEJordeRMellgrenSISmall and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5-year follow-up studyJ Peripher Nerv Syst2016211152126663481
- JagodicMMPathirathnaSNelsonMTCell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neuronsJ Neurosci200727123305331617376991
- GordoisAScuffhamPShearerAOglesbyATobianJAThe health care costs of diabetic peripheral neuropathy in the USDiabetes Care20032661790179512766111
- Gordon SmithARobinson SingletonJIdiopathic neuropathy, prediabetes and the metabolic syndromeJ Neurol Sci20062421–291416448668
- XuZZhangPLongLHeHZhangJSunSDiabetes mellitus in classical trigeminal neuralgia: a predisposing factor for its developmentClin Neurol Neurosurg2016151707227816028
- GreenAQKrishnanSFinucaneFMRaymanGAltered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose toleranceDiabetes Care201033117417620040675
- EdwardsJLVincentAMChengHTFeldmanELDiabetic neuropathy: mechanisms to managementPharmacol Ther2008120113418616962
- BrownleeMBiochemistry and molecular cell biology of diabetic complicationsNature2001414686581382011742414
- ObrosovaIGDrelVRPacherPOxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisitedDiabetes200554123435344116306359
- SouthanGJSzaboCPoly(ADP-ribose) polymerase inhibitorsCurr Med Chem200310432134012570705
- DuXMatsumuraTEdelsteinDInhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cellsJ Clin Invest200311271049105714523042
- SorianoFGVirágLJagtapPDiabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activationNat Med20017110811311135624
- HaHCHesterLDSnyderSHPoly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in gliaProc Natl Acad Sci USA20029953270327511854472
- IlnytskaOLyzogubovVVStevensMJPoly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathyDiabetes20065561686169416731831
- LiFDrelVRSzabóCStevensMJObrosovaIGLow-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathyDiabetes20055451514152215855340
- ObrosovaIGLiFAbatanOIRole of poly(ADP-ribose) polymerase activation in diabetic neuropathyDiabetes200453371172014988256
- PacherPLiaudetLSorianoFGMableyJGSzabóESzabóCThe role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetesDiabetes200251251452111812763
- ZhengLSzaboCKernTSPoly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κBDiabetes200453112960296715504977
- AbeTItokawaYEffect of ethanol administration on thiamine metabolism and transketolase activity in ratsInt J Vitam Nutr Res1977474307314591201
- FrankOLuisada-OpperASorrellMFThomsonADBakerHVitamin deficits in severe alcoholic fatty liver of man calculated from multiple reference unitsExp Mol Pathol19711521911975111801
- HoyumpaAMJrMechanisms of thiamin deficiency in chronic alcoholismAm J Clin Nutr19803312275027616254354
- LeevyCMBakerHTenhoveWFrankOCherrickGRB-complex vitamins in liver disease of the alcoholicAm J Clin Nutr196516433934614278632
- TomasuloPAKaterRMIberFLImpairment of thiamine absorption in alcoholismAm J Clin Nutr19682111134113445699730
- KoikeHMoriKMisuKPainful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine statusNeurology200156121727173211425941
- KoikeHIijimaMSugiuraMAlcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathyAnn Neurol2003541192912838517
- ChenXLevineJDMechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the ratMol Pain20073517319957
- ChopraKTiwariVAlcoholic neuropathy: possible mechanisms and future treatment possibilitiesBr J Clin Pharmacol201273334836221988193
- NaritaMMiyoshiKSuzukiTInvolvement of microglia in the ethanol-induced neuropathic pain-like state in the ratNeurosci Lett20074141212517284346
- MiyoshiKNaritaMTakatsuMSuzukiTmGlu5 receptor and protein kinase C implicated in the development and induction of neuropathic pain following chronic ethanol consumptionEur J Pharmacol2007562320821117349994
- McDonoughKHAntioxidant nutrients and alcoholToxicology20031891–2899712821285
- DinaOABarlettaJChenXKey role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the ratJ Neurosci200020228614861911069970
- DinaOAGearRWMessingROLevineJDSeverity of alcohol-induced painful peripheral neuropathy in female rats: role of estrogen and protein kinase (A and Cε)Neuroscience2007145135035617204374
- JungMEGatchMBSimpkinsJWEstrogen neuroprotection against the neurotoxic effects of ethanol withdrawal: potential mechanismsExp Biol Med (Maywood)2005230182215618121
- ChenCHFerreiraJCGrossERMochly-RosenDTargeting aldehyde dehydrogenase 2: new therapeutic opportunitiesPhysiol Rev201494113424382882
- RöyttäMHorwitzSBRaineCSTaxol-induced neuropathy: short-term effects of local injectionJ Neurocytol19841356857016150968
- RöyttäMRaineCSTaxol-induced neuropathy: chronic effects of local injectionJ Neurocytol19861544834962427662
- VuorinenVRoyttaMRaineCSThe acute effects of taxol upon regenerating axons after nerve crushActa Neuropathol198876126342899374
- VuorinenVRoyttaMRaineCSThe acute response of Schwann cells to taxol after nerve crushActa Neuropathol198876117252899373
- PolomanoRCMannesAJClarkUSBennettGJA painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxelPain200194329330411731066
- LiptonRBApfelSCDutcherJPTaxol produces a predominantly sensory neuropathyNeurology19893933683732564647
- XiaoWHBennettGJChemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitinePain2008135326227017659836
- CataJPWengHRBurtonAWVillarealHGiraltSDoughertyPMQuantitative sensory findings in patients with bortezomib-induced painJ Pain20078429630617175202
- FlattersSJBennettGJStudies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunctionPain2006122324525716530964
- PostmaTJVermorkenJBLieftingAJPinedoHMHeimansJJPaclitaxel-induced neuropathyAnn Oncol1995654894947669713
- KusTAktasGKalenderMETaxane-induced peripheral sensorial neuropathy in cancer patients is associated with duration of diabetes mellitus: a single-center retrospective studySupport Care Cancer20162431175117926279147
- SmithEMPangHCirrincioneCEffect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trialJAMA2013309131359136723549581
- GaistDJeppesenUAndersenMRodriguezLAHallasJSindrupSHStatins and risk of polyneuropathy: a case-control studyNeurology20025891333133712011277
- LoYLLeohTHLohLMTanCEStatin therapy and small fibre neuropathy: a serial electrophysiological studyJ Neurol Sci20032081–210510812639733
- BhallaSSinghNJaggiASStatins: do they aggravate or ameliorate neuropathic pain?J Pain201415111069108025086324
- PathakNNBalaganurVLingarajuMCAtorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levelsNeurochem Int2014681924513038
- Hernandez-OjedaJRoman-PintosLMRodriguez-CarrizalezADEffect of rosuvastatin on diabetic polyneuropathy: a randomized, double-blind, placebo-controlled Phase IIA studyDiabetes Metab Syndr Obes2014740140725214797
- FinnerupNBAttalNHaroutounianSPharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysisLancet Neurol201514216217325575710
- BoyleJErikssonMEGribbleLRandomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of lifeDiabetes Care201235122451245822991449
- JoseVMBhansaliAHotaDPandhiPRandomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathyDiabet Med200724437738317335465
- MaxMBCulnaneMSchaferSCAmitriptyline relieves diabetic neuropathy pain in patients with normal or depressed moodNeurology19873745895962436092
- SindrupSHEjlertsenBFrølandASindrupEHBrøsenKGramLFImipramine treatment in diabetic neuropathy: relief of subjective symptoms without changes in peripheral and autonomic nerve functionEur J Clin Pharmacol19893721511532792168
- SindrupSHOttoMFinnerupNBJensenTSAntidepressants in the treatment of neuropathic painBasic Clin Pharmacol Toxicol200596639940915910402
- MooreRADerrySAldingtonDColePWiffenPJAmitriptyline for neuropathic pain and fibromyalgia in adultsCochrane Database Syst Rev20157CD008242
- DerrySWiffenPJAldingtonDMooreRANortriptyline for neuropathic pain in adultsCochrane Database Syst Rev20151CD01120925569864
- BasbaumAIBautistaDMScherrerGJuliusDCellular and molecular mechanisms of painCell2009139226728419837031
- JuliusDBasbaumAIMolecular mechanisms of nociceptionNature2001413685220321011557989
- BasbaumAISpinal mechanisms of acute and persistent painReg Anesth Pain Med199924159679952097
- IyengarSWebsterAAHemrick-LueckeSKXuJYSimmonsRMEfficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in ratsJ Pharmacol Exp Ther2004311257658415254142
- ArnoldLMLuYCroffordLJA double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorderArthritis Rheum20045092974298415457467
- ArnoldLMPritchettYLD’SouzaDNKajdaszDKIyengarSWernickeJFDuloxetine for the treatment of fibromyalgia in women: pooled results from two randomized, placebo-controlled clinical trialsJ Womens Health (Larchmt)20071681145115617937567
- FishbainDADetkeMJWernickeJChappellASKajdaszDKThe relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorderCurr Med Res Opin200824113105311518828958
- GendreauRMThornMDGendreauJFEfficacy of milnacipran in patients with fibromyalgiaJ Rheumatol200532101975198516206355
- MarksDMShahMJPatkarAAMasandPSParkGYPaeCUSerotonin-norepinephrine reuptake inhibitors for pain control: premise and promiseCurr Neuropharmacol20097433133620514212
- RowbothamMCGoliVKunzNRLeiDVenlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled studyPain2004110369770615288411
- NeilMJClonidine: clinical pharmacology and therapeutic use in pain managementCurr Clin Pharmacol20116428028721827389
- LunnMPHughesRAWiffenPJDuloxetine for treating painful neuropathy, chronic pain or fibromyalgiaCochrane Database Syst Rev20141CD00711524385423
- MatthewsEADickensonAHEffects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathyPain2001921–223524611323145
- KatoAOhkuboTKitamuraKAlgogen-specific pain processing in mouse spinal cord: differential involvement of voltage-dependent Ca(2+) channels in synaptic transmissionBr J Pharmacol200213551336134211877344
- WoolfCJSalterMWNeuronal plasticity: increasing the gain in painScience200028854721765176910846153
- RumoreMMAronSMHirossEJA review of mechanism of action of aspirin and its potential as an immunomodulating agentMed Hypotheses19872243874002438544
- BauerCSNieto-RostroMRahmanWThe increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalinJ Neurosci200929134076408819339603
- LuoZDChaplanSRHigueraESUpregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured ratsJ Neurosci20012161868187511245671
- LiCYSongYHHigueraESLuoZDSpinal dorsal horn calcium channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodyniaJ Neurosci200424398494849915456823
- BoroujerdiAKimHKLyuYSInjury discharges regulate calcium channel alpha-2-delta-1 subunit upregulation in the dorsal horn that contributes to initiation of neuropathic painPain2008139235836618571852
- MaraisEKlugbauerNHofmannFCalcium channel α2δ subunits-structure and gabapentin bindingMol Pharmacol20015951243124811306709
- MooreRAStraubeSWiffenPJDerrySMcQuayHJPregabalin for acute and chronic pain in adultsCochrane Database Syst Rev20093CD00707619588419
- DonvitoGWilkersonJLDamajMILichtmanAHPalmitoylethanolamide reverses paclitaxel-induced allodynia in miceJ Pharmacol Exp Ther2016359231031827608657
- MooreRAWiffenPJDerrySToelleTRiceASGabapentin for chronic neuropathic pain and fibromyalgia in adultsCochrane Database Syst Rev20144CD00793824771480
- McGivernJGZiconotide: a review of its pharmacology and use in the treatment of painNeuropsychiat Dis Treat2007316985
- PopeJEDeerTRBruelBMFalowskiSClinical uses of intrathecal therapy and its placement in the pain care algorithmPain Pract Epub2016223
- McDowellGC2ndPopeJEIntrathecal ziconotide: dosing and administration strategies in patients with refractory chronic painNeuromodulation201619552253226856969
- SanfordMIntrathecal ziconotide: a review of its use in patients with chronic pain refractory to other systemic or intrathecal analgesicsCNS Drugs20132711989100223999971
- HayekSMHanesMCIntrathecal therapy for chronic pain: current trends and future needsCurr Pain Headache Rep201418138824338701
- LaursenLPalliative care: the other opioid issueNature20165357611S16S1727410527
- SchepersRJMahoneyJLShippenbergTSInflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociceptionPain2008136332033017764840
- HurleyRWHammondDLThe analgesic effects of supraspinal μ and δ opioid receptor agonists are potentiated during persistent inflammationJ Neurosci20002031249125910648729
- FieldsHState-dependent opioid control of painNat Rev Neurosci20045756557515208698
- SteinCSchaferMMachelskaHAttacking pain at its source: new perspectives on opioidsNat Med2003981003100812894165
- VanderahTWDelta and kappa opioid receptors as suitable drug targets for painClin J Pain201026Suppl 10S10S1520026960
- MaFXieHDongZQWangYQWuGCExpression of ORL1 mRNA in some brain nuclei in neuropathic pain ratsBrain Res200510431–221421715862535
- ChenYSommerCNociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processingJ Peripher Nerv Syst200611323224016930285
- KabliNCahillCMAnti-allodynic effects of peripheral delta opioid receptors in neuropathic painPain20071271–2849316963185
- BrisciniLCorradiniLOnginiEBertorelliRUp-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injuryEur J Pharmacol20024471596512106803
- SunRQWangYZhaoCSChangJKHanJSChanges in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic painNeurosci Lett20013111131611585556
- NadalXBanosJEKiefferBLMaldonadoRNeuropathic pain is enhanced in δ-opioid receptor knockout miceEur J Neurosci200623383083416487163
- AitaMByersMRChavkinCXuMTrigeminal injury causes kappa opioid-dependent allodynic, glial and immune cell responses in miceMol Pain20106820109235
- ObaraIMikaJSchaferMKPrzewlockaBAntagonists of the κ-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligationBr J Pharmacol2003140353854612970097
- XuMPetraschkaMMcLaughlinJPNeuropathic pain activates the endogenous κ opioid system in mouse spinal cord and induces opioid receptor toleranceJ Neurosci200424194576458415140929
- MikaJPrzewlockiRPrzewlockaBThe role of δ-opioid receptor subtypes in neuropathic painEur J Pharmacol20014151313711245849
- PetrilloPAngeliciOBinghamSEvidence for a selective role of the δ-opioid agonist [8R-(4bS*,8aα,8aβ,12bβ)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexa-hydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]isoquinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responsesJ Pharmacol Exp Ther200330731079108914551288
- AbrahamKEBrewerKLMcGintyJFOpioid peptide messenger RNA expression is increased at spinal and supraspinal levels following excitotoxic spinal cord injuryNeuroscience200099118919710924963
- AkbarSSubhanFKarimN6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic painBiomed Pharmacother20168496297127764759
- MogilJSGriselJEZhangsGBelknapJKGrandyDKFunctional antagonism of μ-, δ- and κ-opioid antinociception by orphanin FQNeurosci Lett19962142–31311348878101
- MabuchiTMatsumuraSOkuda-AshitakaEAttenuation of neuropathic pain by the nociceptin/orphanin FQ antagonist JTC-801 is mediated by inhibition of nitric oxide productionEur J Neurosci20031771384139212713641
- ScotoGMAricoGIemoloARonsisvalleSParentiCInvolvement of the nociceptin/orphanin FQ-NOP receptor system in the ventrolateral periaqueductal gray following mechanical allodynia in chronic painLife Sci2009855–620621019523963
- SchieneKTzschentkeTMSchröderWChristophTMechanical hyperalgesia in rats with diabetic polyneuropathy is selectively inhibited by local peripheral nociceptin/orphanin FQ receptor and μ-opioid receptor agonismEur J Pharmacol2015754616525697471
- DingHHayashidaKSutoTSupraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primatesBr J Pharmacol2015172133302331225752320
- KoMCNaughtonNNAntinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeysJ Pain200910550951619231294
- KalsoESodium channel blockers in neuropathic painCurr Pharm Des200511233005301116178759
- BhattacharyaAWickendenADChaplanSRSodium channel blockers for the treatment of neuropathic painNeurotherapeutics20096466367819789071
- DrayANeuropathic pain: emerging treatmentsBr J Anaesth20081011485818511441
- BrochuRMDickIETarpleyJWBlock of peripheral nerve sodium channels selectively inhibits features of neuropathic pain in ratsMol Pharmacol200669382383216301337
- EisensteinMNeuropathy: a name for their painNature20165357611S10S1127410524
- Le PichonCECheslerATThe functional and anatomical dissection of somatosensory subpopulations using mouse geneticsFront Neuroanat201482124795573
- BenarrochEEIon channels in nociceptors: recent developmentsNeurology201584111153116425713000
