Abstract
Objective
Chemokine CXCL12 and its receptor CXCR4 have been reported to play a critical role in neurogenesis and neuronal differentiation. Recently, some reports have implicated this chemokine signaling in the pathogenesis of many kinds of pain. However, its role in neuropathic pain (NP) is still largely unclear. This study explored the distribution and function of CXCR4 in spinal cord (SC) dorsal horn (DH) in a rat L5 spinal nerve ligation (SNL) model.
Methods
Rats received repeated intrathecal injection of CXCR4 antagonist AMD3100. Behavioral assessments were conducted using a traditional “up–down” method. The spinal CXCL12 contents were measured by enzyme linked immunosorbent assay. The expression and distribution of CXCR4 in the SC were determined by immunoflurescence and Western blot. GlyRα3 expressions were also measured by Western blot or immunofluorescence.
Results
SNL induced CXCL12–CXCR4 activation in the spinal DH. Intrathecal administration of AMD3100 alleviated the chronic NP against SNL (P<0.01). CXCR4 was colocalized with GlyRα3-positive neurons in the spinal DH at ratio >97%. Meanwhile, AMD3100 rescued the decrease of GlyRα3 expression (P<0.01 vs the SNL group on Day 14 and Day 21).
Conclusion
CXCR4 antagonist can elicit analgesic effects and restore the inhibitory neurotransmission such as GlyRα3 against NP.
Introduction
Neuropathic pain (NP) is a pain caused by a lesion or disease of the somatosensory system.Citation1 It is an intractable clinical disorder mainly characterized by hyperalgesia, allodynia, and spontaneous pain.Citation2 The current therapy for NP is not satisfactory. Although pharmacological management (such as antidepressants, anticonvulsants, and opoids) is widely used, more than two-thirds of NP patients obtain insufficient pain relief.Citation2
After peripheral nerve injuries, nonneuronal cells such as microglial cells or astrocytes release numerous chemical mediators, which modulate the pain sensation by acting on neurons or other glial cells.Citation3 Chemokines are a family of small secreted molecules (8–14 kDa) with verified roles in the many biological functions modulation, such as leukocyte chemoattractant and cytokine activation.Citation4,Citation5 CXCL12 and its major receptor CXCR4 are widely existed in the nervous system.Citation6 Recently, some reports have implicated this chemokine signaling in the pathogenesis of inflammation pain,Citation7 opiate-induced hyperalgesia,Citation8 diabetic neuropathy,Citation9 and bone cancer pain.Citation10 CXCL12/CXCR4 signaling was demonstrated to mediate the release of proinflammatory cytokineCitation11 and neurotransmittersCitation12 (such as glutamic acid and prostaglandins) from glial cells, which are involved in the maintenance of NP. GlyRα3 is distinctly expressed in superficial spinal lamina and selectively involved in pathological pain.Citation13,Citation14 GlyRα3 is regarded as a typical element in the inhibitory neurotransmission circuit.Citation14 Whether CXCL12/CXCR4 affects GlyRα3 expression and contributes to the allodynia after peripheral nerve injury remains largely unknown. The present study therefore was performed to study 1) the distribution and expression of CXCR4 in spinal cord (SC) in a rat model of L5 spinal nerve ligation (SNL) model; 2) whether administration of CXCR4 antagonist AMD3100 would alleviate the allodynia against SNL; and 3) whether AMD3100 would affect the expression of GlyRα3 expression after SNL.
Methods
Animals
Adult male Sprague Dawley (SD) rats (weight: 150–180 g) were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd, Shanghai Branch, Experimental Animal Center of Chinese Academy of Sciences (Shanghai, People’s Republic of China). The rats were acclimated in a SPF room and provided with a standard diet at the animal center in Shanghai Xinhua Hospital for 5 days at baseline. All animal procedures in this study were performed according to the Guide for the Care and Use of Laboratory Animals of the International Association for the Study of Pain. The whole protocol was approved by the Animal Care and Use Committee of Xinhua Hospital Experimental Animal Center.
Study design
Rats were performed SNL surgery on Day 1. AMD3100 was administrated once daily from Days 1 to 14. This study consisted of three phases. In Phase I (total of 34 rats), the distribution (n=3 for every point in time) and expression (n=5 for every point in time) of CXCR4 in the ipsilateral SC after SNL were studied at baseline on Day 14 and Day 21. And the ipsilateral spinal CXCL12 expressions were assayed at baseline on Day 7 and Day 10 (n=5 for every point in time). In Phase II (total of 18 rats), CXCR4 antagonist AMD3100 was used to assess the effects against NP. Rats were randomly assigned to three experimental groups, the sham group, the control (SNL) group, and the AMD group. Behavioral assessments were performed at baseline, on Day 7, Day 14, and Day 21, separately (n=6 for every group). In Phase III (total of 63 rats), SC specimen was collected and the protein expressions of GlyRα3 were measured by Western blot at baseline on Day 14 and Day 21 (n=5 for every time point in every group). The distribution and signals of GlyRα3 were studied at baseline and on Day 14 by immunoflurescence (n=3 for each time point in every group). The schematic diagram for study design is shown in .
Figure 1 The schematic diagram for study schedule.
Abbreviations: ELISA, enzyme linked immunosorbent assay; IF, immunofluorescence; it, intrathecal; LC, lumbar catheterization; SNL, spinal nerve ligation; WB, Western blot.
Drugs and drugs’ administration
Specific CXCR4 antagonist AMD3100 was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in phosphate-buffered saline (PBS) at the concentration of 0.5 μg μL−1 and injected (1 μg rat−1 day−1, intrathecally [it]) once daily from Days 1 to 14 using a lumbar catheterization technique. The day of SNL and lumbar catheterization surgeries was referred to as Day 1. The doses of the drugs were chosen according to the results of our preliminary experiments and previous studies in the literature.Citation8–Citation10
The drug administration using a lumbar catheterization technique was carried out in accordance with previous studies described.Citation15,Citation16 Briefly, rats were anesthetized with pentobarbital sodium (50 mg kg−1 intraperitoneally). The lumbar region was disinfected with 75% (v/v) ethanol after hair shaving. An incision was made over L4–L5 lumbar vertebrae. The intervertebral foramen was exposed after cutting the intervertebral ligament. Then, we inserted a PE-10 catheter filled with sterile 0.9% saline into the subarachnoid space. The correct intrathecal localization was confirmed by easy insertion of a catheter, by a tail-flick or a paw retraction, or occasionally by leakage of cerebrospinal fluid. After finishing the experiments, lidocaine (15 μL, 20 μg μL–1) followed by 10 μL of saline was injected through the catheters to verify an intraspinal location. An immediate motor paralysis of the hind part of the animal (within 15 s) lasting for 20–30 min indicated a correct intraspinal location. Finally, the catheter was secured. The paravertebral muscles and the skin incision were sutured. The external portion of the catheter was coiled into a protector made of tinsel plates.Citation16 Animals were returned to the individual cages for recovery after surgery.
Surgical procedures of L5 SNL
Surgical L5 SNL model for NP was performed just like our previous study.Citation14 Rats received anesthesia by 50 mg kg−1 sodium pentobarbital (injected intraperitoneally). A midline incision was made along the rat’s back. After blunt separation of the paraspinal muscles, the L5 nerve was exposed and tightly ligated with a 3–0 silk thread. Thereafter, the incision was cleansed with saline and was sutured. Sham-operated animals received the lumbar catheterization procedure and the same operation of paraspinal muscles incision and the L5 spinal nerve exposure without nerve ligation.
Behavioral test
Behavioral assessments were performed at baseline (before SNL) on Day 7, Day 14, and Day 21. The test chamber consisted of six Plexiglas box (30 cm×30 cm×30 cm) with a clear floor. Each rat was placed in its own testing chamber. About 30 min after acclimation, rats were assessed on test days. The mechanical withdrawal thresholds were determined using the “up–down method” by a series of Von Frey filaments (Stoelting Co, Wood Dale, IL, USA). Generally, we started the first test with the filament of 2 g force. If paw withdrawal was observed, the next lesser force was applied. Otherwise, if no movement was observed, the next greater force was used. Every force was applied three times with 5 s intervals. A 50% mechanical threshold was calculated as described previously.Citation17 The range of scores was between 0.25 g and 26 g. All behavioral assessments were performed by the same author (LD) who was not aware of the group assignment.
Western blot analysis
Animals were sacrificed with 10% chloral hydrate and, then, transcardially perfused with PBS, and the L5 lumbar SC segments were dissected. Then, the protein samples were quantified by a bicinchoninic acid protein assay kit (Pierce Biotechonology, Rockford, IL, USA). A total of 40 μg ipsilateral L5 spinal sample proteins were electrophoresed through a 10% sodium dodecyl sulfate-polyacrylamide gel and electrotransferred to 0.4 mm polyvinylidene fluoride membranes. The blots were first incubated overnight at 4°C with one of the following primary antibodies (CXCR4: 1:1000, rabbit; Abcam, Cambridge, UK; GlyRα3: 1:1000, rabbit; EMD Millipore, Billerica, MA, USA) and then with a horseradish peroxide-conjugated secondary antibody (1:4000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). An enhanced chemiluminescence detection system (Pierce Biotechonology) was applied for protein detection. An anti-GAPDH antibody (1:1000, rabbit; Cell Signaling Technology, Danvers, MA, USA) was used for normalization. The intensity of protein bands was analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescent detection
After deep anesthesia with pentobarbital sodium, rats were sequentially perfused with saline and 4% paraformaldehyde (pH 7.2–7.4, 4°C) through the heart. The L5 spinal segments were postfixed overnight and dehydrated at 4°C. After mounted in optical coherence tomography compound, the SP sections (30 μm) were cut using a cryostat (Leica CM1900 UV; Leica Microsystems, Wetzlar, Germany) for further immunofluorescent detection. Free-floating SP sections were first incubated overnight at 4°C with the following primary antibodies: neuronal nuclei (NeuN): 1:500, mouse (EMD Millipore); GFAP: 1:500, mouse (EMD Millipore); CXCR4: 1:500, rabbit (Abcam); and GlyRα3: 1:500, rabbit (EMD Millipore). After washing, sections were incubated with conjugated secondary antibodies (1:250; Jackson ImmunoResearch Laboratories, Inc.) in the dark for 60 min. Images were photographed by confocal microscope (Leica TCS SP5 II). The fluorescent images were analyzed by Leica AF. For the assessment of immunoflurescence, six sections from L5 SP segments were randomly selected in every animal (n=3 in each group).
Statistical analyses
All data were presented as mean±SD. SPSS 10.0 was used for statistical analyses. A P<0.05 was considered statistically significant. ELISAs and Western blot assays were analyzed by a one-way analysis of variance (ANOVA) followed by the Tukey test. Behavioral data were analyzed first by a two-way repeated measures ANOVA and, then, by multivariate ANOVA for further comparison among groups at the same time.
Results
SNL induces CXCL12–CXCR4 activation in the spinal dorsal horn (DH)
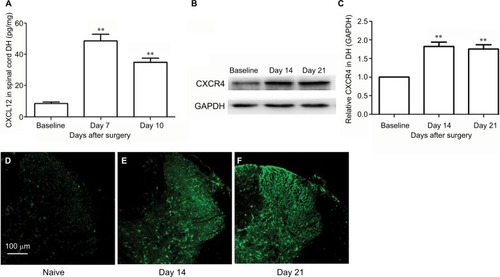
SNL induced significant CXCL12 activation (, main effect of SNL F(2,14)=204.95, P<0.01). The expressions of CXCR4 in DH after SNL were examined using immunofluorescence and Western blot. There was a low basal CXCR4 protein expression in the spinal DH at baseline, which significantly increased on Day 14 and Day 21 after SNL (, main effect of SNL F(2,14)=66.9, P<0.01). shows representative immunofluorescent pictures of CXCR4 signals in the L5 spinal DH after SNL.
Figure 2 SNL induces CXCL12 and CXCR4 upregulation in spinal cord.
Abbreviations: DH, dorsal horn; SNL, spinal nerve ligation.
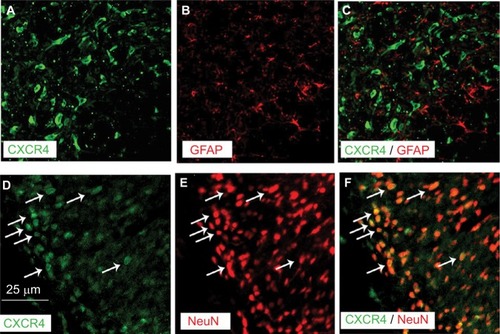
Double immunofluorescence of CXCR4 with different cell markers was then performed to define its distribution in spinal DH. CXCR4 was primarily coexpressed with NeuN (a neuronal marker, ) but not GFAP (an astrocytic marker, ).
Figure 3 Double staining of immunoflurescence shows that CXCR4 is predominantly colocalized with NeuN, a marker for neurons (D–F) but not with GFAP, a marker for astrocytes (A–C) in SNL rats.
Abbreviations: GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; SNL, spinal nerve ligation.
Intrathecal administration of CXCR4 antagonist AMD3100 alleviates the chronic NP induced by SNL
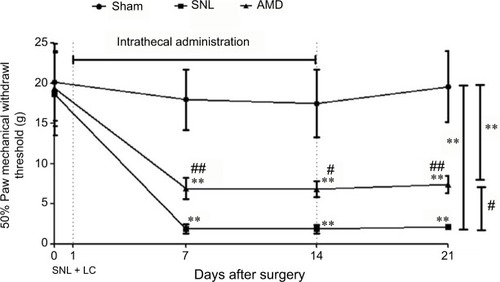
Behavioral assessments were performed on Days 0, 7, 14, and 21 separately. After the sphericity assumption test, we got an epsilon coefficient >0.7, so the Bonferroni test was used for post hoc comparisons. The results showed that ipsilateral 50% mechanical threshold substantially decreased in the SNL and AMD groups than in the sham group (main effect of drug F(2,17)=61.69, P<0.01, interaction F(6,60)=11.52, P<0.01, n=6 every group, ). AMD3100 at 1 μg day−1 repeated for 2 weeks remarkably alleviated the allodynia induced by SNL (P=0.02, ).
Figure 4 Effects of intrathecal injection of CXCR4 antagonist AMD3100 on SNL-induced mechanical allodynia.
Abbreviations: ANOVA, analysis of variance; LC, lumbar catheterization; SNL, spinal nerve ligation.
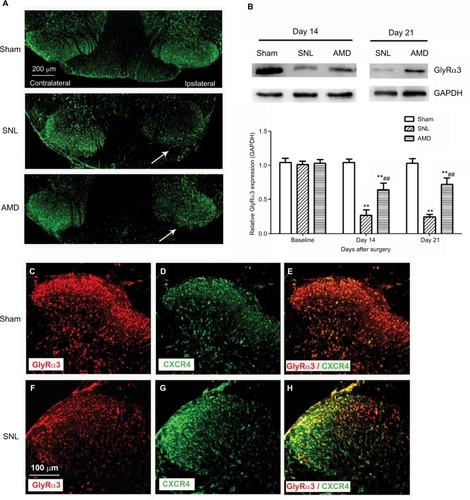
Intrathecal administration of AMD3100 rescues the decrease of GlyRα3 expression in spinal DH
According to our results, SNL significantly decreased the GlyRα3 signals in the ipsilateral spinal DH without affecting the contralateral DH (). The Western blot assays also revealed that the GlyRα3 protein expression was remarkably inhibited on Day 14 and Day 21 in the SNL group than in the sham group. Furthermore, the decrease of GlyRα3 expression was dramatically rescued by administration of CXCR4 antagonist AMD3100 (main effect of treatment on Day 14 F(2,14)=123.43, P<0.01; main effect of treatment on Day 21 F(2,14)=162.83, P<0.01, ). Another interesting finding was that CXCR4 was colocalized with GlyRα3 in the spinal DH at ratio >97% ().
Figure 5 Effects of AMD3100 on GlyRα3 expression in spinal DH.
Abbreviations: DH, dorsal horn; SNL, spinal nerve ligation.
Discussion
We reported the distribution, expression, and possible function of the receptor of chemokine CXCL12, CXCR4, in the SC DH in an in vivo rat SNL model. Our results reveal that 1) SNL induces both CXCL12 and CXCR4 upregulation in spinal DH, and CXCR4 antagonist AMD3100 can elicit analgesic effect against allodynia and decrease CXCR4 expression induced by SNL and 2) CXCR4 is predominantly expressed in neurons but not astrocytes in SC DH and coexpressed with GlyRα3. SNL induced depression of GlyRα3, which could be rescued by CXCR4 antagonist AMD3100. These findings suggest that CXCL12/CXCR4 might play a cardinal role in the development of NP.
Increasing evidence implied CXCL12/CXCR4 to be important in nociceptive signal processing. Miller and his colleagues reported that CXCL12 and glycoprotein 120 elicited allodynia after paw injection.Citation18 CXCR4 antagonists may be important for therapeutic interventions in the pain that is associated with HIV-1 infection and inflammation.Citation18 According to Bai et al’sCitation19 and Shen et al’sCitation10 studies, CXCL12/CXCR4 sensitized neurons and activated glial cells, which substantially contributed to the maintenance of NP and bone cancer pain. CXCL12/CXCR4 signaling was also reported to mediate enhanced calcium influx and excitability in DRG neurons and pain hypersensitivity in diabeticCitation9 and toxic neuropathy.Citation20 Our present study studied the distribution and expression of CXCR4 in spinal DH after SNL in a rat in vivo model. According to our results, SNL induces both CXCL12 (7–10 days after SNL) and CXCR4 (14–21 days after SNL) upregulation in SC DH and CXCR4 antagonist AMD3100 could elicit analgesic effect against mechanical allodynia and decrease CXCR4 expression induced by SNL.
AMD3100 is a synthetic and highly specific inhibitor of the CXCR4 receptor. It disrupts binding of CXCL12 to CXCR4 by competing binding site, thus blocking the physiological function of CXCL12/CXCR4 axis.Citation21 Hatse et alCitation21 showed that inhibition by AMD3100 is strictly restricted to CXCR4 but not to any other “CXC” or “CC” chemokine receptors. Currently, there are two major kinds of CXCR4 antagonist. One is T140 and its analogs, and the other is non-T140-related such as AMD3100. CXCR4 is a G-protein-coupled receptor with seven transmembrane regions. AMD3100 and its analogs bind to the amino acid residues in the central hydrophobic core of the receptor, whereas T140 binds to the residues in extracellular domains of the hydrophobic core.Citation21–Citation23 In our study, we used AMD3100 as CXCR4 antagonist because it was widely used in other CXCR4 studies. The dose in other studies was 1–5 μg intrathecal. In our preliminary study, normal control rats showed mechanical thresholds between 15 and 26 g. AMD3100 at the dose of 5 μg for >5 days would cause pain hypersensitivity itself in rats. Therefore, we used AMD3100 at the dose of 1 μg for a longer time (14 days) that low dose of AMD3100 did not affect the pain sensation compared with the normal rats.
According to recent studies, there are several possible mechanisms of CXCL12/CXCR4 involved in NP. The upregulation of CXCR4 triggers the downstream GPCR transduction pathway, induces an increase in Ca2+ in neurons, which leads to hyperalgesia.Citation24 CXCL12/CXCR4 signaling activates an ERK-dependent Nav1.8 upregulation.Citation25 CXCL12 stimulates the astrocytes to release the glutamic acid and prostaglandins, which are involved in the process of sensitization.Citation12 CXCL12 also participates the opoids tolerance, decreasing the endogenous analgesic effects.Citation26,Citation27 Concomitant with an increased activation of excitatory neurotransmitter receptors after NP, there is a decline in inputs of inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA) and glycine.Citation28 Previous studies mostly focused on the effects of CXCR4 on microglial cells.Citation8–Citation10 However, no one has reported the effect of CXCL12/CXCR4 on spinal inhibitory neurotransmitters.
Glycine is a ubiquitous amino acid, and it is the second most important fast inhibitory neurotransmitter in the central nerve system.Citation29 There are four α subunits (Gly α1–4) and one β subunit of glycine receptor. Subunit α1 (GlyRα1) is the most abundant in the adult nervous system, which is more localized to deeper layers of the spinal DH (laminae III–IV).Citation30 GlyRα3 is not a widely expressed subunit, but it is distinctly expressed in the more superficial laminae of the spinal DH, where nociceptive Aδ- and C-fiber afferents terminate.Citation13,Citation30 GlyRα1 serves in the control of spinal motor circuit, whereas GlyRα3 is more specifically involved in spinal nociceptive processing.Citation13,Citation30,Citation31 All GlyRα3 subunits’ immunoreactivity was found to colocalize with gephyrin, which functions with GlyRs and GABAA at postsynaptic sites.Citation13,Citation14 Therefore, GlyRα3 is regarded as a typical element in the inhibitory neurotransmission circuit. Furthermore, a recent study of Lv found that this Glyα3-associated inhibitory circuit serves as a gate control, which is the prime factor to separate the innocuous mechanoreceptive pathway to the nociceptive pathway.Citation14 These studies suggested that nerve injury-induced inhibition of the GlyRα3 inhibitory is vital in mechanical allodynia in NP. In our study, we found that SNL induced inhibition of GlyRα3 expression in the ipsilateral spinal DH but not affected the contralateral one. We report for the first time that CXCR4 is colocalized with GlyRα3 and further shows that an administration of CXCR4 antagonist AMD3100 can rescue the inhibition of GlyRα3 in ipsilateral spinal DH. It remains unclear how exactly CXCL12/CXCR4 signaling affects the GlyRα3 or inhibitory neurotransmission circuit. This protective effect may be direct, indirect, or even both. A further study on this topic may need coimmunoprecipitation to clarify the relationship between CXCL12/CXCR4 and GlyRα3.
Acknowledgments
We acknowledge the assistance of Dr Ting Zhao (Howard Hughes Medical Institute) for polishing the manuscript. The study was financially supported by Postdoctoral Science Foundation of Jiangsu Province (Grant no 1302019B) and Natural Science Foundation of China (Grant no 81371246). X Liu and H Liu are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- JensenTSBaronRHaanpääMA new definition of neuropathic painPain20111522204220521764514
- AttalNBouhassiraDPharmacotherapy of neuropathic pain: which drugs, which treatment algorithms?Pain2015156Suppl 1S104S11425789426
- TiwariVGuanYRajaSNModulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveatsNeurosci Biobehav Rev201445192724820245
- GaoYJJiRRChemokines, neuronal-glial interactions, and central processing of neuropathic painPharmacol Ther20101261566820117131
- AbbadieCBhangooSDe KoninckYMalcangioMMelik-ParsadaniantzSWhiteFAChemokines and pain mechanismsBrain Res Rev200960112513419146875
- LiMRansohoffRMMultiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiologyProg Neurobiol200884211613118177992
- GosselinRDDansereauMAPohlMChemokine network in the nervous system: a new target for pain reliefCurr Med Chem200815272866287518991641
- WilsonNMJungHRipschMSMillerRJWhiteFACXCR4 signaling mediates morphine-induced tactile hyperalgesiaBrain Behav Immun201125356557321193025
- MenichellaDMAbdelhakBRenDShumAFrietagCMillerRJCXCR4 chemokine receptor signaling mediates pain in diabetic neuropathyMol Pain2014104224961298
- ShenWHuXMLiuYNCXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cordJ Neuroinflammation2014117524735601
- HanYHeTHuangDRPardoCARansohoffRMTNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytesJ Clin Invest200110842543511489936
- CalìCBezziPCXCR4-mediated glutamate exocytosis from astrocytesJ Neuroimmunol2010224132120580441
- HarveyRJDepnerUBWässleHGlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitizationScience2004304567288488715131310
- LvYDongHGaoYA feed-forward spinal cord glycinergic neural circuit gates mechanical allodyniaJ Clin Invest201312394050406223979158
- StørksonRVKjørsvikATjølsenAHoleKLumbar catheterization of the spinal subarachnoid space in the ratJ Neurosci Methods19966521671728740594
- XuFLiTZhangBAn improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of ratsJ Neurosci Methods2009183211411819559050
- LiuXLiuHXuSSpinal translocator protein alleviates chronic neuropathic pain behavior and modulates spinal astrocyte-neuronal function in rats with L5 spinal nerve ligation modelPain2016157110311626307860
- OhSBTranPBGillardSEHurleyRWHammondDLMillerRJChemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neuronsJ Neurosci200121145027503511438578
- BaiLWangXLiZUpregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in ratsNeurosci Bull2016321274026781879
- BhangooSKRenDMillerRJCXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviraltoxic neuropathyBrain Behav Immun200721558159117292584
- HatseSPrincenKBridgerGDe ClercqEScholsDChemokine receptor inhibition by AMD3100 is strictly confined to CXCR4FEBS Lett20025271–325526212220670
- WongRSYBodartVMetzMLabrecqueJBridgerGFrickerSPComparison of the potential multiple binding modes of bicyclam, monocyclam, and noncyclam small-molecule CXC chemokine receptor 4 inhibitorsMol Pharmacol2008741485149518768385
- TrentJOWangZXMurrayJLLipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonistsJ Biol Chem200327847471364714412958314
- Reaux-Le GoazigoARivatCKitabgiPPohlMMelik ParsadaniantzSCellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevanceEur J Neurosci20123652619263122694179
- YangFSunWYangYSDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulationJ Neuroinflammation20151221926597700
- RivatCSebaihiSVan SteenwinckelJSrc family kinases involved in CXCL12-induced loss of acute morphine analgesiaBrain Behav Immun201438385224263070
- LinCPKangKHTuHJCXCL12/CXCR4 signaling contributes to the pathogenesis of opioid tolerance: a translational studyAnesth Analg2017124397297928212183
- ZeilhoferHUWildnerHYévenesGEFast synaptic inhibition in spinal sensory processing and pain controlPhysiol Rev201292119323522298656
- LynchJWMolecular structure and function of the glycine receptor chloride channelPhysiol Rev20048441051109515383648
- BerrocalYAAlmeidaVWPuentesRLoss of central inhibition: implications for behavioral hypersensitivity after contusive spinal cordinjury in ratsPain Res Treat2014201417827825180088
- ZeilhoferHUThe glycinergic control of spinal pain processingCell Mol Life Sci200562182027203515968463
