Abstract
Objective
To investigate the functional connectivity (FC) and its variability in the primary somatosensory cortex (S1) of patients with low-back-related leg pain (LBLP) in the context of the persistent stimuli of pain and numbness.
Patients and Methods
We performed functional magnetic resonance imaging on LBLP patients (n = 26) and healthy controls (HCs; n = 34) at rest. We quantified and compared static FC (sFC) using a seed-based analysis strategy, with 6 predefined bilateral paired spherical regions of interest (ROIs) in the S1 cortex. Then, we captured the dynamic FC using sliding window correlation of ROIs in both the LBLP patients and HCs. Furthermore, we performed a correlational analysis between altered static and dynamic FC and clinical measures in LBLP patients.
Results
Compared with controls, the LBLP patients had 1) significantly increased static FC between the left S1back (the representation of the back in the S1) and right superior and middle frontal gyrus (SFG/MFG), between the left S1chest and right SFG/MFG, between right S1chest and right SFG/MFG, between the left S1face and right MFG, and between the right S1face and right inferior parietal lobule (P < 0.001, Gaussian random field theory correction); 2) increased dynamic FC only between the right S1finger and the left precentral and postcentral gyrus and between the right S1hand and the right precentral and postcentral gyrus (P < 0.01, Gaussian random field theory correction); and 3) a negative correlation between the Barthel index and the increased static FC between the left S1face and right inferior parietal lobule (P = 0.048).
Conclusion
The present study demonstrated the hyperconnectivity of the S1 cortex to the default mode and executive control network in a spatial pattern and an increase in the tendency for signal variability in the internal network connections of the S1 cortex in patients with LBLP.
Introduction
Low-back-related leg pain (LBLP) is one of the most common subgroups of low-back pain (LBP),Citation1,Citation2 but LBLP is usually associated with more serious disability and pain and poorer outcomes and recovery than LBP alone.Citation3 Additionally, LBLP patients also experience pain sensation and/or movement coordination impairment.Citation3–Citation5 Neuroimaging findings are considered to be evidence in support of this observation, that is, significant structural and functional alterations occur in chronic pain or chronic low-back pain, especially in the “pain matrix” brain regions, such as the anterior cingulate cortex,Citation6 medial prefrontal cortex,Citation7,Citation8 and primary somatosensory (S1) cortex.Citation9–Citation11 Therein, the S1 cortex, as an important sensory processing area, plays a prominent and highly regulatory role in pain perception, including localization and discrimination.Citation12–Citation15 Furthermore, the S1 cortex is involved in peripheral-stimulation-induced neuronal activity in downstream brain regions associated with pain signals.Citation9 This indicates that pain perception might not be only related to the altered processing within the “pain matrix”, but also related to the modulation of S1 itself. A number of studies have found that there is a structural and functional reorganization of S1 under long-term pain stimulation, such as migraine,Citation16 trigeminal neuropathic pain,Citation17 diabetic peripheral neuropathy.Citation18 However, a study has reported that pain per se is not associated with cortical plasticity, and the increased S1 inputs may be the key factor of cortical functional reorganization.Citation19
A functional and structural MRI study issued a new point that the plasticity of S1 is one of the causes of chronic pain, is not a simple and passive epiphenomenon after tissue/nerve injury as previously thought.Citation20 Moreover, previous study proposed a dynamic characteristic (variation across pain intensity) of FC in the S1 cortex in chronic LBP patients.Citation11 The results of this study indicated that not only altered FC between chronic LBP patients and healthy volunteers at S1 but also FC differs as chronic LBP patients experienced different levels of endogenous low-back pain (high-intensity pain condition showed increased FC). Furthermore, they also found brain structure differs between chronic LBP patients and healthy volunteers at S1. The combined changes of neuroanatomy and function of S1 suggest that this brain region may play an important role in the pathophysiological process of chronic LBP.Citation11
Functional connectivity (FC), one of the most widely used and reliable statistical methods, is used to describe the temporal correlation of the low-frequency blood-oxygenation-level-dependent (BOLD) signal oscillations between different brain areas or networks. Several studies found decreased static FC (sFC, stationary connectivity at the time) between the S1 subregion activated by an experimental pain stimulus and other S1 subregions.Citation21,Citation22 Moreover, a recent study based on a large-sample-size investigation found that the abnormal cross-network sFC in LBLP patients possessed S1 somatotopic specificity.Citation23 However, these studies are based on the assumption of the “static” brain and neglect the change in BOLD signal variability at various time scales (eg, short-term state versus long-term trait) between multiple brain areas or networks. Resting-state dynamic functional connectivity (dFC) could be a useful tool to study differences in intrinsic network dynamics between individuals and whether those differences are associated with pain-related experiences or behavior. In more recent studies, dFC or networks have been investigated broadly, which can provide some information about the variability of the brain in terms of the strength of the spatial dynamic organization.Citation24
Hence, for LBLP patients, chronic and persistent abnormal sensory input, including pain and numbness, might affect the patient’s sensory processing. Recent study puts forward a new standpoint: regional BOLD signal variability represents a new dynamic perspective to observe how individuals process and cope with pain.Citation25 In this study, we hypothesize that abnormalities in S1 cortex FC (both static and dynamic FC), are susceptible to the effects of the persistent stimuli of pain and numbness on LBLP patients. Motivated by this hypothesis, we first used a seed-based correlational analysis to examine the sFC of the S1 cortex. dFC was measured by a sliding window correlational analysis for each region of interest (ROI), and a coefficient of variation (CV) map was calculated over time to quantify the temporal variations of dFC. Next, the associations between FC and the clinical assessments of tactile spatial resolution and pain intensity were evaluated.
Patients and Methods
Participants
The participants included 30 patients with LBLP and 37 healthy controls (HCs; gender: 15 women/15 men vs 19 women/18 men, χ2 = 0.912; mean ± SD age: 53.67 ± 7.46 ± years vs 54.41 ± 5.51, t = 0.296). Patients with LBLP were recruited from the First Affiliated Hospital of Nanchang University, and HCs were recruited from the community from Oct. 2016 to Jan. 2018. The inclusion criteria for the patients were as follows: (1) age 35–65 years and voluntary participation in the study; (2) a clear diagnosis of discogenic compression on a lumbar CT and/or MRI (>1 ruptured annulus fibrosus with compressed soft tissue); (3) the sensation of radiating pain in the buttock(s) and lower limb(s) for more than 3 months with scores on the visual analog scale (VAS) above 4; and (4) ineffective conservative treatment with medications (eg, anti-inflammatory drugs (Motrin, Advil and Naproxen) and acetaminophen (eg, Tylenol) without opioids, exercise and physical therapy). The exclusion criteria for the LBLP group were a history of head or spinal cord injury or a major systemic disease; a history of spinal stenosis due to calcifications on the spinal protrusions, lateral recess stenosis, spinal stenosis, pyriformis syndrome, or sciatica; or a history of significant cardiac events.
All participants provided informed written consent to procedures approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University in accordance with the Declaration of Helsinki.
Clinical Assessment
Subjects were assessed in a series of clinical evaluations, including the VAS (0–10) for pain intensity, the Japanese Orthopaedic Association (JOA) Back Pain Evaluation questionnaire (−6 to 29) to examine the impact of neuropathic or nociceptive pain on quality of life,Citation26 the Fugl-Meyer assessment for sensorimotor impairment measurement, the Barthel index (0–100) for performance in activities of daily and the 2-point tactile discrimination (2PD) test was used to assess the tactile spatial resolution ability.
Imaging Data Acquisition
All participants in the study underwent an MRI (3.0T, Trio Tim, Siemens Medical Systems, Munich, Germany) scan. Data acquisition included a high-resolution 3D, T1-weighted, magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (repetition time (TR)/echo time (TE) = 1900 ms/2.26 ms, field of view (FOV) = 215 mm × 230 mm, matrix = 240 × 256, thickness/gap = 1.0/0 mm and 176 sagittal slices) and rs-fMRI scan (TR/TE = 2000/30 ms, matrix = 64 × 64, FOV = 210 × 210 mm, 30 interleaved axial slice, 4 mm thickness, interslice gap of 1.2 mm, and 240 volumes over 8 min). During data acquisition, participants received the following instruction: “Keep your eyes closed, do not think about anything and do not fall asleep.”
To determine anatomical brain abnormalities, T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences were collected. For diagnoses of the lumbar spine, additional conventional sagittal and axial T1-weighted and T2-fat suppression sequences were performed (Supplementary 1).
Preprocessing of fMRI Data
The preprocessing of all fMRI data was performed using the toolbox for Data Processing & Analysis of Brain ImagingCitation27 (DPABI v4.2; http://rfmri.org/dpabi), which is based on Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and runs on the MATLAB2014b platform (MathWorks, Natick, MA, USA). The following preprocessing steps were applied: (1) the first 10 volumes (20 s) of the rs-fMRI scan were discarded; (2) slice timing, head motion correction and the individual registration of high-resolution T1 images to echo-planar imaging images were performed with the segmentation from the high-resolution T1 template of the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolkit; followed by (3) spatial normalization and transformation to Montreal Neurological Institute Space (MNI) space, (4) resampling the image to 3 mm isotropic voxels and spatially smoothing (6 mm full-width at half-maximum kernel), and (5) detrend linear drift and nuisance linear regression, including the white matter, cerebrospinal fluid (CSF) and head motion parameters, according to the Friston-24 model;Citation28 followed by (6) temporal bandpass filtering (0.01–1 Hz). In addition, we chose only subjects with head motion estimation parameters of <2.0 mm maximal translation and <2.0° maximal rotation for the final analysis. Notably, in the case of possible distortions of intrinsic functional connectivity at the group level and increased negative correlations, we did not perform the global signal regression.
Definition of Seeds
We used seed-based correlational analysis to examine the FC of the S1 cortex. Based on previous study,Citation29 we chose a total of 12 ROIs, and seed location within the S1 cortex included the leg (MNI: x = ± 8, y = −38, z = 68), back (MNI: x = ± 18, y = −44, z = 64), chest (MNI: x = ± 18, y = −36, z = 64), hands (MNI: x = ± 28, y = −30, z = 50), fingers (MNI: x = ± 50, y = −16, z = 50), and face (MNI: x = ± 60, y = −14, z = 40). Then, we created a 4-mm radius sphere and extracted the average time signal of the spherical seed area.
Static Functional Connectivity
sFC was measured between each seed of the S1 cortex and other voxels in the whole brain and was then transformed by Fisher r-to-z transformation to fit the normal distribution for statistical analysis. A two-sample t-test was performed between the LBLP group and the HCs (two-tailed, voxel-level P < 0.001, Gaussian random field theory correction (GRF) with cluster-level P < 0.005).
Dynamic Functional Connectivity Analysis
Dynamic FC was measured by a sliding window correlational analysis for each ROI, and the coefficient of variation (CV: SD/mean) map was computed across time windows. In brief, the dFC analysis was performed as follows: (1) the time-series signals from each ROI were extracted; (2) a rectangular sliding window length of 30 TR (60 s) and a step of 1 TR was selected based on previous studies;Citation30 (3) within each window (201 windows in total), the temporal correlation coefficient to other voxels throughout the whole brain was computed for each ROI (in total of 12), and a correlation coefficient map was created for each participant; and (4) the CV map was calculated over time to quantify temporal variations of dFC. To improve the normality of the correlation distribution, a Z-standardization was applied to all maps. Notably, each map was spatially smoothed by a full-width half-maximum kernel of 6 mm. A two-sample t-test was calculated to compare the group difference between patients with LBLP and HCs (two-tailed, voxel-level P < 0.01, GRF correction with cluster-level P<0.05), and a group mask was constructed. The mean of each time series of each ROI was extracted, and a two-sample t-test was performed in SPSS, with age and gender as covariates, to assess the between-group differences of each time series.
Relationship with Clinical Measures
We analyzed clinical data by partial correlational analyses between the sFC or dFC values of the region, with alteration and clinical evaluation at the group level and with age and gender as covariates (P < 0.05), using SPSS software (version 21.0; IBM, Armonk, NY, USA).
Results
Clinical Characteristics and Indices
Among the subjects, 2 LBLP patients and 3 HCs were excluded for excessive head motion. Furthermore, 2 LBLP patients were excluded due to vascular malformation and infarction. Ultimately, 26 LBLP patients and 34 HCs participated in the study, and the detailed clinical characteristics and indices are summarized in . Among the LBLP patients, lower scores of JOA Back Pain Evaluation (14.38 ± 4.92) indicated an effect on the quality of life due to neuropathic or nociceptive pain, and high VAS scores (5.65 ± 1.04) indicated tolerable or moderate pain. The mean Fugl-Meyer score was 20.12 ± 1.90 (range from 15.0 to 22.0). The higher Barthel index scores (LBLP: 86.15 ± 12.59) could mean a better ability to live independently. In those LBLP patients, decreased performance on the 2PD test was observed in the right feet (28.88 ± 7.65 mm), left feet (29.31 ± 6.11 mm), right hands (23.77 ± 4.74 mm) and left hands (24.27 ± 4.74 mm), which indicate cortical reorganization of somatosensory cortices. Finally, LBLP patients and HCs group exhibited no significant differences in age (P = 0.249), or gender (χ2 test, P = 0.271).
Table 1 Demographic Data and Clinical Characteristics of the LBLP and HCs Groups
sFC of the S1 Cortex
Static connectivity spatial distributions are shown in and S1. The results from LBLP patients showed that a portion of the somatotopic S1 subregions (left back, bilateral chest) demonstrated increased sFC only to the right superior and middle frontal gyrus (SFG/MFG) (-, ); LBLP patients also demonstrated increased sFC of the left S1face (the representation of the face in the primary somatosensory) to the right MFG (, ) and increased sFC of the right S1face to the right inferior parietal lobule (IPL) (, ), when compared with the HCs group. In the LBLP patients, there were no significant differences in several S1 subregions (bilateral leg, hand, finger and right back) compared to HCs.
Table 2 Differences in the Alterations of sFC of the S1 Cortex Between the LBLP and HCs Groups (Two-Sample t-Test, Two-Tailed, Voxel-Level P < 0.001, GRF Correction, Cluster-Level P < 0.005)
Figure 1 Static functional connectivity spatial distributions were observed at the group level for LBLP patients and healthy controls (the left somatotopic S1 subregions).
Abbreviations: sFC, static functional connectivity; S1, primary somatosensory cortex; LBLP, low-back-related leg pain; HCs, healthy controls.
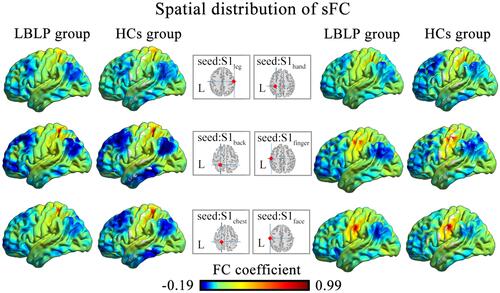
Figure 2 Differences in the alterations of sFC between the LBLP patients and healthy controls (two-sample t-test, two-tailed, voxel-level P < 0.001, GRF correction, cluster-level P < 0.005).
Notes: (A–C) Patients with LBLP exhibited increased sFC to the right SFG/MFG. (D) Patients with LBLP exhibited increased sFC to the right MFG. Values of sFC are the mean ± SEM.
Abbreviations: sFC, static functional connectivity; LBLP, low-back-related leg pain; HCs, healthy controls; SFG, superior frontal gyrus; MFG, middle frontal gyrus; S1back_L, representation of the left back in the primary somatosensory cortex.
dFC of the S1 Cortex
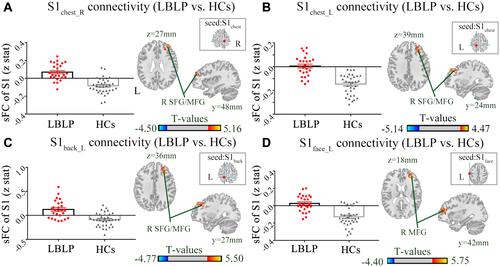
and show the alterations of the dFC of the S1 cortex in LBLP patients with P < 0.01 with GRF correction. The dFC of the right S1hand to the left precentral and postcentral gyrus (PRG/POG) was increased (). Furthermore, the dFC of the right S1finger to the right PRG/POG in the LBLP group was increased ().
Table 3 Differences in the Alterations of dFC of the S1 Cortex Between the LBLP and HCs Groups (Two-Sample t-Test, Two-Tailed, Voxel-Level P < 0.01, GRF Correction, Cluster-Level P < 0.05)
Figure 3 Differences in the alterations of dFC between LBLP patients and healthy controls (two-sample t-test, two-tailed, voxel-level P < 0.01, GRF correction, cluster-level P < 0.05).
Notes: (A) Increased dFC between the right S1hand cortex and the left PRG/POG in LBLP patients. (B) Increased dFC between the right S1finger cortex and the right PRG/POG in LBLP patients.
Abbreviations: dFC, dynamic functional connectivity; LBLP, low-back-related leg pain; HCs, healthy controls; PRG, precentral gyrus; POG, postcentral gyrus; S1hand_R, representation of the right hand in the primary somatosensory cortex; values of sFC are the mean ± SEM.
Relationship with Clinical Measures in cLBLP Patients
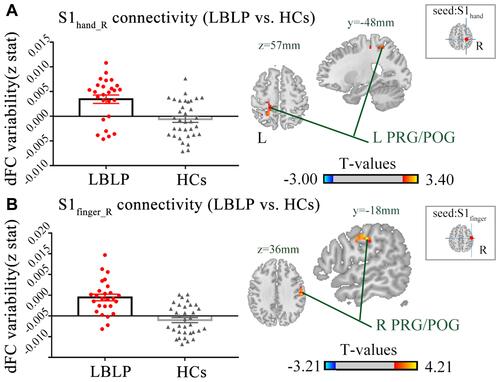
Moreover, a negative correlation was found between the increased sFC of the right S1face and the Barthel index (ρ= 0.408, P = 0.048) (). However, the other S1 subregion connectivity changes did not show a significant correlation with the clinical index (P = 0.054 ~ 0.978) (Table S1). Similarly, there was no significant correlation found between dFC and clinical parameters (P = 0.115 ~ 0.963) (Table S2).
Figure 4 (A) Patients with LBLP exhibited increased sFC to the right IPL. Values are the mean ± SEM (two-sample t-test, two-tailed, voxel-level P < 0.001, GRF correction, cluster-level P < 0.005). (B) Partial correlational analysis between sFC alterations and the Barthel index in LBLP patients.
Abbreviations: sFC, static functional connectivity; LBLP, low-back-related leg pain; IPL, inferior parietal lobule; S1face_R, representation of the right face in the primary somatosensory cortex.
Discussion
This study provides novel insights into the pain perception features of LBLP patients by the alterations of sFC and dFC, which represent the functional reorganization of the S1 cortex. Our key findings can be summarized as follows: (1) alterations of the sFC of the S1 cortex were mostly exhibited in default mode network (DMN) regions (the right SFG/MFG and IPL); (2) the dFC of the S1 cortex was increased in sensorimotor network regions (PRG/POG); and (3) the Barthel index was related to abnormalities of sFC between the right IPL and the right S1face. Collectively, our study indicates that our sFC and dFC findings are distinct in patients with LBLP.
Increased Static S1 Connectivity Mostly Within the DMN in LBLP Patients
We performed a comparison between groups to detect alterations of sFC in LBLP patients. Interestingly, alterations of the sFC of the S1 cortex were mostly exhibited in the DMN and partly located in the dorsolateral prefrontal cortex (dlPFC). The S1 cortex is a critical component of sensory processing and receives and processes sensory to encode the intensity of nociceptive stimuli and pain.Citation12 Furthermore, the dlPFC is a key node of the executive control network (ECN), has been implicated a central role in top-down pain processing, and it has extensive connections with sensory and motor cortices and contribute to regulating thought, attention, and action.Citation31 Previous study proposed that left dlPFC exhibited a negative correlation with pain affect, but the right dlPFC primarily displayed an association with a weakened relationship with both pain intensity and pleasantness.Citation32 Another study indicated that pain-related activity within the dlPFC was associated with pain catastrophizing and its relationship was related to the pain intensity.Citation33 Moreover, this study also proposed pain catastrophizing has no relationship with the activity of the area related to sensory-discriminative aspects of pain, such as the primary or secondary somatosensory cortex. However, a dynamic causal modeling study observed both nociceptive and neuropathic pain patients’ groups revealed an additional forward and backward connection between the somatosensory cortex and right dlPFC when compared to HCs.Citation34 This could reflect that chronic patients have paid more attention towards pain and might be explained by the higher levels of pain catastrophizing in these patients. Consistent with this finding, our study also indicated increased sFC between the right S1 (left back, bilateral chest subregions) cortex and right dlPFC, suggesting that pain catastrophizing might be related to the progression or persistence of chronic pain.
Meanwhile, increased sFC of the S1 cortex were mostly found in DMN (including the dorsal medial prefrontal cortex and IPL), which were generally identified because of their consistent deactivation, focusing attention on pain.Citation35 A previous study found an increased intensity of pain in LBP patients following increased blood flow in the S1 cortex,Citation36 and structural alterations, for example, increased S1 cortical thickness, were also found in patients with chronic low-back pain when compared with HCs.Citation21 Furthermore, the dysfunction of the DMN in chronic back painCitation37,Citation38 and alterations in regional homogeneity in evoked, experimental pain stimulus-induced low-back pain have been reported,Citation39 together emphasizing the effect of persistent pain and behavioral and cognitive deterioration following chronic pain. Recently, rs-fMRI studies demonstrated increased DMN connectivity to the S1back in patients with chronic low-back pain, implying a greater intrinsic transfer of pain information between self-referential processing areas and S1.Citation23 Combined with previous studies, results from LBLP patients demonstrated that increased static S1 connectivity with the DMN may be related to increased intrinsic transfer of pain information between DMN and S1 cortex. Otherwise, in this study, increased connectivity with the DMN was not only found in the S1 subregions of the back. These results may provide evidence that sustained chronic back pain promotes the threshold value of somatosensory processing, and we reasoned that alterations in intrinsic activity at baseline might lead to LBLP patients developing persistent pain and numbness, even after disc decompression procedures.
Increased Dynamic Internal Network Connections of the S1 Cortex in LBLP Patients
dFC is a measure of fluctuations correlated over time. Compared with HCs, we also demonstrated increased dFC of the S1 cortex to the PRG/POG in patients with LBLP. The higher signal variability of internal network connections of sensorimotor network regions may reveal alterations in the internal structure of the S1 cortex and the increased transmission of neural signals. To the best of our knowledge, several studies have revealed the functional and structural plasticity in the S1 cortex associated with chronic low-back pain.Citation20 In this study, increased BOLD signal variability may indicate the functional plasticity of the S1 cortex in LBLP patients. Furthermore, higher BOLD signal variability might reveal increased low-frequency oscillations in the S1 cortex compare to HCs. The low-frequency BOLD fluctuations have physiological significance and are related to the neural spontaneous activity.Citation40 Our previous studies found that LBLP patients showed hyperamplitude of low-frequency oscillations in the pain matrix and information-processing regions and its intrinsic functional plasticity affects the amplitude of fluctuations of the pain matrix and sensory-processing regions.Citation41,Citation42 Besides, a previous study proposed that low-frequency oscillations play an important part in the context of identifying and assessing dFC.Citation43 In the present study, higher BOLD signal variability might be associated with the functional reorganization of the S1 cortex and greater low-frequency signal oscillations.
Meanwhile, previous studies have also found that S1 is activated during pain experienceCitation10,Citation20,Citation22,Citation23,Citation44 and that the dysfunction of the S1 cortex in migraineurs may affect nociception pathways.Citation45 Furthermore, peripheral nerve injury can indeed elicit rapid and dynamic neural circuit rewiring in the S1 area.Citation9,Citation46 Functional BOLD MRI measures the hemodynamic response, a correlate of neural activity that is relevant to blood flow.Citation47 However, no articles have revealed alterations in the blood flow of the S1 cortex in LBLP patients. Interestingly, we found that dFC was altered only in the sensorimotor internal network, which may indicate that the variability in the BOLD signal may be more specific in revealing alterations of structure and the increased transmission of neural signals of the S1 cortex in patients with chronic back pain. Although this study found alterations of dFC only at the threshold of P < 0.01 with GRF correlation, when the statistical P value threshold was set at 0.001 without correlation, we also did not find significantly different coefficients of variation between the two groups.
Association of an Altered Connectivity Index with Clinical Measures in LBLP Patients
For LBLP patients, we found that only the increased sFC of the left S1face to the IPL was negatively associated with the Barthel index. A recent study found that brain structural changes following peripheral vestibulocochlear lesions and functional impairments of daily living were negatively correlated with a relative GMV increase in the IPL.Citation48 As a brain region for attention control and cognitive control, the IPL plays a regulatory role in risk decision-making. We hypothesize that the reorganization of functional connectivity may be associated with the aggravation of pain perception after repeated thinking and action in patients with persistent pain. In addition, there were no correlations between the sFC of other subregions of the S1 cortex or the dFC of the S1 cortex with the clinical variables (the duration of disease, JOA, VAS, 2PD, etc.) in LBLP patients, implying the lower clinical relevance of connectivity in these regions.
Limitations
The limitations of this study should not be ignored. First, this is a relatively small-sample-size study in which only individuals < 65 years old participated. Age is also known to alter brain function; therefore, our study design does not permit the analysis of factors related to age, such as brain atrophy. Second, another potential confounding factor is that the patients were not required to stop taking medications; the potential physiological effects of these medications on the BOLD fMRI signal are unknown in this study. There are differences in p-value between static functional connectivity and dynamic functional connectivity analysis. It may be arbitrary to use different p-value. For that, we added the effect size for statistical power analysis, and high strength of association between variables or the degree of difference in this study (> 1.0). Finally, the connectivity between the S1 subregions was not evaluated, though a previous study found that a pain stimulus could reduce the sFC between the S1 subregion activated by that stimulus and other S1 subregions.Citation22
Conclusion
In conclusion, the present study demonstrated hyperconnectivity of the S1 cortex to the DMN and ECN in a spatial pattern and an increased tendency for signal variability in the internal network connections of the S1 cortex in patients with LBLP. This finding might provide novel insight into the capture of neural plasticity feature effects by the persistent stimuli of pain and numbness in LBLP patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank all of the participants in this study. This study was supported by the National Natural Science Foundation of China (81560284 and 81771808), the Key Science and Technology Financing Projects of Jiangxi Provincial Education Department (GJJ170003), and the Distinguished Young Scholars of Jiangxi Province (20171BCB23089). The funders had no role in the study design, data collection and analysis; the decision to publish; or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work. None of the authors have any personal or financial involvement with organizations that have a financial interest in the content of this manuscript.
References
- Stynes S, Konstantinou K, Dunn KM. Classification of patients with low back-related leg pain: a systematic review. BMC Musculoskel Dis. 2016;17(1):226. doi:10.1186/s12891-016-1074-z
- Kongsted A, Kent P, Albert H, Jensen TS, Manniche C. Patients with low back pain differ from those who also have leg pain or signs of nerve root involvement-a cross-sectional study. BMC Musculoskel Dis. 2012;13(1):236. doi:10.1186/1471-2474-13-236
- Konstantinou K, Hider SL, Jordan JL, Lewis M, Dunn KM, Hay EM. The impact of low back-related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clin J Pain. 2013;29(7):644–654. doi:10.1097/AJP.0b013e31826f9a52
- Elgueta-Cancino E, Schabrun S, Hodges P. Is the organisation of the primary motor cortex in low back pain related to pain, movement and/or sensation? Clin J Pain. 2018;34(3):207–216. doi:10.1097/AJP.0000000000000535
- Pranata A, Perraton L, El-Ansary D, et al. Trunk and lower limb coordination during lifting in people with and without chronic low back pain. J Biomech. 2018;71:257–263. doi:10.1016/j.jbiomech.2018.02.016
- Koga K, Descalzi G, Chen T, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85(2):377–389. doi:10.1016/j.neuron.2014.12.021
- Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi:10.1038/nn.3153
- Metz AE, Yau H, Centeno MV, Apkarian AV, Martina M, Merzenich MM. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA. 2009;106(7):2423–2428. doi:10.1073/pnas.0809897106
- Eto K, Wake H, Watanabe M, et al. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 2011;31(21):7631–7636. doi:10.1523/JNEUROSCI.0946-11.2011
- Vrana A, Meier ML, Hotz-Boendermaker S, Humphreys BK, Scholkmann F. Cortical sensorimotor processing of painful pressure in patients with chronic lower back pain-an optical neuroimaging study using fNIRS. Front Hum Neurosci. 2016;10:578. doi:10.3389/fnhum.2016.00578
- Kong J, Spaeth B, Wey H, et al. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain. 2013;9(1):43. doi:10.1186/1744-8069-9-43
- Bushnell MC, Duncan GH, Hofbauer RK, et al. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96(14):7705–7709. doi:10.1073/pnas.96.14.7705
- Willis WD, Zhang X, Honda CN, Giesler GJ. A critical review of the role of the proposed VMpo nucleus in pain. J Pain. 2002;3(2):79–94. doi:10.1054/jpai.2002.122949
- Craig ADB. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26(1):1–30. doi:10.1146/annurev.neuro.26.041002.131022
- Ogino Y, Nemoto H, Goto F. Somatotopy in human primary somatosensory cortex in pain system. Anesthesiology. 2005;103(4):821–827. doi:10.1097/00000542-200510000-00021
- DaSilva AFM, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–1995. doi:10.1212/01.wnl.0000291618.32247.2d
- Chao TH, Chen J, Yen C. Plasticity changes in forebrain activity and functional connectivity during neuropathic pain development in rats with sciatic spared nerve injury. Mol Brain. 2018;11(1):55. doi:10.1186/s13041-018-0398-z
- Selvarajah D, Wilkinson ID, Fang F, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes. 2019;68(4):796–806. doi:10.2337/db18-0509
- Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32(43):14874–14884. doi:10.1523/JNEUROSCI.1733-12.2012
- Kim W, Kim SK, Nabekura J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J Neurochem. 2017;141(4):499–506. doi:10.1111/jnc.14012
- Kim J, Loggia ML, Cahalan CM, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395–1405.
- Kim J, Loggia ML, Edwards RR, Wasan AD, Gollub RL, Napadow V. Sustained deep-tissue pain alters functional brain connectivity. Pain. 2013;154(8):1343–1351. doi:10.1016/j.pain.2013.04.016
- Kim J, Mawla I, Kong J, et al. Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain. 2019;160(7):1594–1605. doi:10.1097/j.pain.0000000000001541
- Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20(3):353–364. doi:10.1038/nn.4502
- Rogachov A, Cheng JC, Erpelding N, Hemington KS, Crawley AP, Davis KD. Regional brain signal variability. Pain. 2016;157(11):2483–2492. doi:10.1097/j.pain.0000000000000665
- Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the Japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26(17):1890–1894. doi:10.1097/00007632-200109010-00014
- Chao-Gan Y, Yu-Feng Z. DPARSF: a matlab toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi:10.3389/fnsys.2010.00009
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement‐related effects in fMRI time‐series. Magn Reson Med. 1996;35(3):346–355. doi:10.1002/mrm.1910350312
- Kim J, Loggia ML, Cahalan CM, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395–1405.
- Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage. 2017;15(160):41–54. doi:10.1016/j.neuroimage.2016.12.061
- Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18(9):1027–1035. doi:10.1016/j.jpain.2017.03.008
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(5):1079–1091. doi:10.1093/brain/awg102
- Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120(3):297–306. doi:10.1016/j.pain.2005.11.008
- Lisa Goudman DMFV, De Smedt EHKP, Iris Coppieters MM. The influence of nociceptive and neuropathic pain states on the processing of acute electrical nociceptive stimulation: a dynamic causal modeling study. Brain Res. 2020;1733:146728. doi:10.1016/j.brainres.2020.146728
- Igelström KM, Graziano MSA. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. doi:10.1016/j.neuropsychologia.2017.01.001
- Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115(2):364–374. doi:10.1097/ALN.0b013e318220e880
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi:10.1523/JNEUROSCI.4123-07.2008
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485(1):26–31. doi:10.1016/j.neulet.2010.08.053
- Zhang S, Wu W, Liu Z, Huang G, Guo S, Yang J. Altered regional homogeneity in experimentally induced low back pain: a resting-state fMRI study. J Neuroeng Rehabil. 2014;11(1):115. doi:10.1186/1743-0003-11-115
- Xiang A, Yu Y, Jia X, et al. The low-frequency BOLD signal oscillation response in the insular associated to immediate analgesia of ankle acupuncture in patients with chronic low back pain. J Pain Res. 2019;12:841–850. doi:10.2147/JPR.S189390
- Zhou F, Zhao Y, Zhu L, et al. Compressing the lumbar nerve root changes the frequency-associated cerebral amplitude of fluctuations in patients with low back/leg pain. Sci Rep. 2019;9(1):2246. doi:10.1038/s41598-019-38721-5
- Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res. 2018;11:165–176. doi:10.2147/JPR.S151562
- Savva AD, Kassinopoulos M, Smyrnis N, Matsopoulos GK, Mitsis GD. Effects of motion related outliers in dynamic functional connectivity using the sliding window method. J Neurosci Methods. 2020;330:108519. doi:10.1016/j.jneumeth.2019.108519
- Worthen SF, Hobson AR, Hall SD, Aziz Q, Furlong PL. Primary and secondary somatosensory cortex responses to anticipation and pain: a magnetoencephalography study. Eur J Neurosci. 2011;33(5):946–959. doi:10.1111/j.1460-9568.2010.07575.x
- Zhang J, Su J, Wang M, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol. 2017;264(4):654–663. doi:10.1007/s00415-017-8404-4
- S K K, Nabekura J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J Neurosci. 2011;31(14):5477–5482. doi:10.1523/JNEUROSCI.0328-11.2011
- Nair DG. About being BOLD. Brain Res Rev. 2005;50(2):229–243. doi:10.1016/j.brainresrev.2005.07.001
- Helmchen C, Klinkenstein JC, Kruger A, et al. Structural brain changes following peripheral vestibulo-cochlear lesion may indicate multisensory compensation. J Neurol Neurosurg Psychiatry. 2011;82(3):309–316. doi:10.1136/jnnp.2010.204925
