Abstract
Nerve growth factor (NGF) is a neurotrophic protein essential for the growth, differentiation, and survival of sympathetic and sensory afferent neurons during development. A substantial body of evidence, based on both animal and human studies, demonstrates that NGF plays a pivotal role in modulation of nociception in adulthood. This has spurred development of a variety of novel analgesics that target the NGF signaling pathway. Here, we present a narrative review designed to summarize how NGF receptor activation and downstream signaling alters nociception through direct sensitization of nociceptors at the site of injury and changes in gene expression in the dorsal root ganglion that collectively increase nociceptive signaling from the periphery to the central nervous system. This review illustrates that NGF has a well-known and multifunctional role in nociceptive processing, although the precise signaling pathways downstream of NGF receptor activation that mediate nociception are complex and not completely understood. Additionally, much of the existing knowledge derives from studies performed in animal models and may not accurately represent the human condition. However, available data establish a role for NGF in the modulation of nociception through effects on the release of inflammatory mediators, nociceptive ion channel/receptor activity, nociceptive gene expression, and local neuronal sprouting. The role of NGF in nociception and the generation and/or maintenance of chronic pain has led to it becoming a novel and attractive target of pain therapeutics for the treatment of chronic pain conditions.
Introduction
Nerve growth factor (NGF) is a neurotrophic protein essential for the growth, differentiation, and survival of sympathetic and sensory afferent neurons during development.Citation1 NGF contributes to neuronal phenotype by modulating axonal guidance, gene transcription, neurotransmitter release, and synaptic plasticity.Citation2–Citation4 In addition, NGF plays a pivotal role in the modulation of nociception in adulthood.Citation5,Citation6
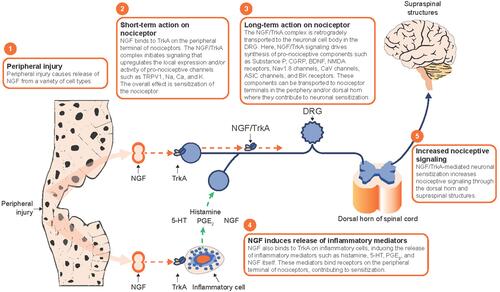
This review highlights how NGF receptor activation and subsequent downstream signaling alter nociception. Specifically, we discuss how NGF can (i) in a short time frame (typically within minutes) lead to direct sensitization of nociceptors via actions at the site of injury, and (ii) in a longer time frame (several hours to days) change gene expression and render nociceptors more responsive via actions in the dorsal root ganglion (DRG). These actions contribute to anatomic remodeling that results in a wider nociceptor input from injured tissue and increases the nociceptive signaling from the periphery to the central nervous system (CNS), providing a rationale for future study of novel analgesics that neutralize NGF or antagonizes its receptors.
Methods
This narrative review was intended to provide an overview of the effects of NGF on nociceptive signaling. Due to the broad scope of the review, and the substantial body of published literature, a narrative approach was utilized. The review was based on searches of PubMed and the authors’ familiarity with the published literature. Search terms included concepts related to NGF and pain or nociception. Results included both animal and human studies. Recent publications were prioritized, though older pivotal studies were also included.
Results
Overview of NGF and Its Receptors
NGF () is a member of the neurotrophin family, which in mammals also includes brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4/5.Citation7 NGF is initially translated as a precursor, proNGF, which can be (i) cleaved intracellularly into mature β-NGF by furin, (ii) cleaved extracellularly by plasmin or matrix metalloproteinases, or (iii) remain intact and signal in its proNGF precursor form.Citation8–Citation10 Inhibiting the processing of proNGF abolishes regulated secretion of the resulting mature NGF product.Citation11
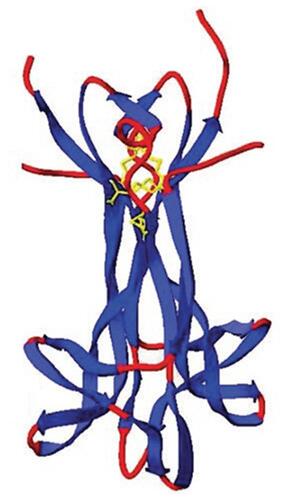
Figure 1 X-ray crystallographic structure of human NGF homodimer. NGF is a homodimer consisting of 2 strands of 120 amino acids each, which non-covalently dimerize to form a 26-kDa protein. Note the N-terminus of the monomers is not apparent (unresolved). Copyright© 2006. Portland Press. Reproduced with permission from Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond). 2006;110(2):175–191.Citation7
Abbreviation: NGF, nerve growth factor.
There are 2 receptors for NGF, p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase A (TrkA).Citation12 TrkA has a higher affinity for mature NGF than for proNGF and activates neurotrophic signaling.Citation9,Citation13 P75NTR has a higher affinity for proNGF and can activate both neurotrophic and apoptotic signaling, the later in the presence of sortilin.Citation8,Citation14 There is an intricate functional relationship between the 2 NGF receptors, and the signaling outputs of NGF and proNGF (survival versus apoptosis) depend on the cellular context and the ratio of TrkA to p75NTR.Citation13
TrkA is expressed in nociceptive sensory neurons and is thought to mediate most of the important effects of NGF on the nociceptive system.Citation6,Citation23 In rats, about 40% of DRG sensory neurons express TrkA, including peptidergic fibers that innervate bone, skin, muscle, and viscera.Citation6,Citation23 Following the release of NGF, which frequently occurs at sites of peripheral tissue injury, NGF can bind TrkA receptors located at peripheral nociceptor terminals. Upon binding of NGF to the extracellular region of TrkA, the receptor dimerizes, autophosphorylates, and initiates signaling events by docking and phosphorylating downstream targets.Citation24–Citation26 The NGF-TrkA complex is internalized into endosomes where it can be retrogradely transported, recycled, or degraded.Citation26 Immediate pro-nociceptive effects resulting from NGF/TrkA signaling (such as modulation of ion channel activity) occur in the peripheral nociceptor terminal, while longer-term effects (such as modification of gene expression) occur in the soma following retrograde axonal transport of the NGF/TrkA complex to the DRG.Citation5,Citation6 Three major signaling cascades initiated by TrkA activation include the phospholipase C-γ (PLCγ) pathway, the mitogen-activated protein kinase (MAPK)/Erk pathway, and the phosphoinositide 3-kinase (PI3K) pathway.Citation26
Role of NGF on the Nociceptive System During Development
A dominant effect of NGF during early development is its role as a survival factor for neurons, including sympathetic and sensory neurons.Citation1,Citation27 The density of the innervation of the target tissue is controlled by a spatially and temporally limited supply of NGF, and cells receiving insufficient support during this critical period of time succumb to cell death.Citation28
NGF null mice have a severe loss of sympathetic and sensory neurons, particularly in the population of peptidergic small- and medium-diameter DRG neurons.Citation30 Animals lacking TrkA receptors show a phenotype similar to NGF null mice, underscoring the importance of NGF-TrkA signaling for the development of the nociceptive system.Citation30,Citation31
In humans, Hereditary Sensory and Autonomic Neuropathy type V (HSAN V) is caused by mutations in the NGF gene.Citation32,Citation33 The first mutation identified, a cytosine to thymine point mutation at nucleotide 661, came from analysis of a northern Swedish multi-generational family.Citation32,Citation34 This particular NGF mutation results in a substitution of tryptophan (W) for arginine (R) at amino acid 221 in proNGF (R221W), which corresponds to amino acid 100 in mature NGF (R100W).Citation35 This mutation causes a substantial loss of unmyelinated nerve fibers and a moderate loss of thinly myelinated fibers.Citation32 Patients with this mutation present with impaired ability to sense deep pain (pain originating in the bones or joints) and temperature (thresholds for heat and cold sensing are increased), but most other neurological functions, including sweating, appear normal.Citation32 This mutation does not affect NGF binding to TrkA but does reduce PLC signaling downstream of TrkA.Citation35 This NGF mutation also inhibits processing of proNGF to mature NGF, which may lower systemic NGF levels, and abolishes NGF binding to p75NTR.Citation34,Citation35 Other mutations can alter the spectrum of HSAN V presentation. For example, a cytosine to adenosine mutation at nucleotide 680 (C680A) causes complete insensitivity to pain accompanied by anhidrosis, mild mental retardation, and immune deficiency.Citation33 Thus, different HSAN V NGF gene mutations may have a variety of effects on NGF-sensitive tissues.
Mutations in the TrkA gene cause a related disorder, HSAN IV, which produces a phenotype similar to HSAN V.Citation36 These TrkA gene mutations result in defective binding of NGF to TrkA and, as a result, the inhibition of NGF-induced TrkA phosphorylation and downstream signaling cascades.Citation37
As development proceeds, the role of NGF in neuronal growth/survival during development diminishes and its role in modulating nociception becomes more relevant.Citation6 It is likely that the developmental role of NGF and the nociceptive role of NGF overlap temporally. The ability of NGF to modulate nociceptive signaling has been observed during early perinatal stages, with repeated postnatal (P0-14) exposure to exogenous NGF in rodents producing mechanical hyperalgesia that persists into adulthood.Citation38 Further, the ability of NGF to sensitize sensory neurons to capsaicin or heat stimuli begins between postnatal days 4 to 10.Citation39
Evidence for a Role of NGF Signaling in Nociception in Adulthood
NGF Levels are Increased During Pain Conditions
Though adult sensory and sympathetic neurons can survive in the absence of NGF, NGF remains capable of promoting neuronal growth and sprouting in adulthood.Citation40–Citation43 Basal NGF levels are lower in the adult than in development.Citation42,Citation44 In humans, serum NGF levels start to decrease at approximately 8 years of age, presumably reflecting increasing maturity of the nervous system.Citation45 Levels of NGF increase in adult rodents in several inflammatory conditions and in several models of pain.Citation46–Citation49 Further, blockage of NGF signaling can attenuate pain-related behavior in a variety of animal models including immune arthritis, fracture, bone cancer pain, osteoarthritis, and neuropathic pain.Citation50–Citation60 Increased levels of NGF are also found in chronic pain conditions in humans, such as osteoarthritis, low back pain, and interstitial cystitis ().Citation61–Citation77 However, an elevated level of NGF is not a hallmark of all chronic pain conditions and low levels of NGF have been found in the plasma of patients with fibromyalgia.Citation78 Thus, care should be taken when generalizing findings from one condition to another. It should also be noted that the physiologically relevant level of NGF required for neuronal sensitization at local sites of peripheral tissue injury is not known. It is also unclear how NGF levels at local sites of peripheral injury are correlated to overall levels measured in serum or other fluids.
Table 1 Summary of Disease States or Conditions in Humans in Which Increased Levels of NGF Were Detected Compared with Controls
NGF Administration Induces Hyperalgesia
In addition to the observation of increased NGF levels in chronic pain conditions and animal models of pain/inflammation, it has been demonstrated that exogenous administration or overexpression of NGF results in hyperalgesia and/or allodynia.Citation38,Citation79-Citation81
Interestingly, striking hyperalgesic effects of NGF administration have also been observed in humans. In healthy adults, for example, a single subcutaneous injection of recombinant NGF has been shown to elicit local injection-site hyperalgesia that persists for up to 7 weeks, depending on the dosage.Citation82 Likewise, intradermal injection of NGF produces long-lasting local thermal (early onset) and mechanical (delayed) hyperalgesia.Citation83–Citation87 Localized priming of nociceptors following intradermal injection of NGF has also been demonstrated through an enhancement of hyperalgesia in response to irradiation with ultraviolet-B.Citation88,Citation89
Intramuscular injection of NGF has been shown to cause lasting mechanical hyperalgesia in a variety of muscles.Citation90–Citation101 Notably, injection of NGF into the tibialis anterior muscle induces local mechanical hyperalgesia within 3 hours of injection that spreads to distant areas on days 1 to 4, suggesting involvement of central pain mechanisms.Citation93 Repeated injections result in both temporal summation and spreading of mechanical pain, again implicating both peripheral and central mechanisms.Citation102 Spreading of NGF-induced hyperalgesia has also been observed following injection into the supraspinatus muscles.Citation94 A single injection of NGF into the facia of the musculus erector spinae muscle produces both mechanical and chemical (proton) hyperalgesia.Citation103 Chemical hyperalgesia has also been demonstrated following the injection of NGF into the tibialis anterior.Citation101
NGF Treatment Lowers Nociceptor Activation Threshold
Intradermal injection of NGF increases the conduction velocity and decreases activity-dependent slowing of conduction velocity in unmyelinated porcine (pig) mechano-insensitive nociceptors.Citation104–Citation106 The activation threshold of mechano-sensitive nociceptors at the injection site decreases following NGF treatment and the proportion of mechano-sensitive nociceptors increases.Citation104 While the receptive field of these nociceptors increased, there was no increase in intraepidermal nerve fiber density, suggesting that previously silent nociceptors may be recruited in this circumstance.Citation104 These changes were measured 3 weeks after NGF administration and, therefore, likely represent long-term effects of NGF signaling. Sensitization of skin nociceptors has been confirmed in humans using microneurography techniques which demonstrate that axonal branches exhibit reduced activation thresholds within the NGF injection zone but not outside of the injection zone.Citation107
Nociceptive Actions of NGF Signaling
Effects of NGF on Inflammatory Cells and Mediators
There is evidence that NGF modulates nociception, in part, by influencing the actions of inflammatory cells and mediators. It has been shown that rodent mast cells produce and store NGF in granules until degranulation and NGF mRNA has been detected in a human mast cell line.Citation108,Citation109 Moreover, cultured media from this mast cell line is able to induce neurite outgrowth in cultured chick embryonic sensory neurons, suggesting that NGF is secreted from these cells.Citation109 NGF has also been found to be present in, and released from, human CD14+ T cell clones and human monocytes.Citation110,Citation111
NGF has been shown to increase the release of mediators from inflammatory cells (). These mediators, such as bradykinin, histamine, ATP, serotonin, and protons, are released during inflammation or injury from ruptured cells or from infiltrating inflammatory cells and are capable of activating receptors and ion channels found on the peripheral nociceptor terminal, leading to neuronal depolarization and sensitization that manifests as pain hypersensitivity.Citation112 For example, exogenous IL-1β causes mechanical and thermal hyperalgesia (measured as an increased nociceptive reflex) in rodents, and histamine has been shown to mediate pain-related behaviors in a rodent model of interstitial cystitis.Citation113,Citation114 Further, serotonin administered to healthy human volunteers causes mechanical hyperalgesia and stimulates calcium influx into cultured rat sensory neurons, an indication of cell excitability.Citation115,Citation116 Finally, bradykinin treatment causes mechanical hyperalgesia in rats and Protein Kinase C (PKC) signaling-dependent sensitization of the transient receptor potential cation channel subfamily V member 1 (TRPV1), when isolated via patch-clamp, which has a known role in nociception and noxious heat sensation.Citation117,Citation118
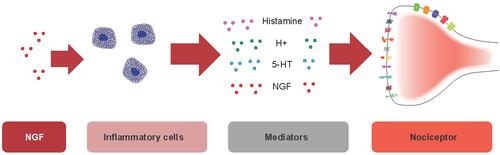
Figure 2 Nociceptive effects of NGF on inflammatory cells. NGF binds TrkA receptors on inflammatory cells. The resulting NGF/TrkA signaling increases the release of a variety of inflammatory mediators such as serotonin, histamine, and NGF itself, which are known to cause sensitization of nociceptors via modulation of receptor or ion channel activity at the peripheral terminal.
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); NGF, nerve growth factor; TrkA, tropomyosin receptor kinase A.
NGF can trigger the release of histamine and leukotriene from human basophils, serotonin and histamine from rodent mast cells, and histamine and tryptase from a human mast cell line.Citation119–Citation123 However, NGF administration did not activate mast cells in a separate rodent study, and there is some evidence that rodent mast cells do not express NGF receptors.Citation109,Citation124 Though the contribution of mast cells to NGF signaling in humans is not clear, human mast cells express TrkA receptors and, thus, species differences must be considered when discussing the influence of NGF on inflammatory cells.Citation109 Similar to effects seen in mast cells, isolated murine peritoneal macrophages exposed to NGF increase the production of interleukin 1β (IL-1β).Citation125 This may occur through TrkA activation as TrkA expression, but not p75NTR expression, was observed in these cells.Citation125 The effects that NGF-mediated release of inflammatory mediators have will depend on the tissue. For example, histamine evokes the sensation of itch when released in isolation in superficial skin and mucous membranes, but causes burning pain when applied to deep somatic tissues.Citation126,Citation127
In addition to affecting cytokine release, NGF can also affect the actions of inflammatory mediators. For example, NGF can potentiate the sensitivity of rat DRG neurons to bradykinin.Citation128 On the other hand, inflammatory mediators can influence the levels and effects of NGF. Evidence suggests that IL-1β contributes to increased NGF levels in cultured sciatic nerve explants, and inhibiting bradykinin-1 receptor activity blocks NGF-induced thermal hyperalgesia in rodents.Citation114,Citation129,Citation130 Thus, there may be instances of positive feedback loops in vivo in which NGF stimulates the release and actions of inflammatory mediators that in turn stimulate increased synthesis and/or release of NGF. However, the role, if any, such a feedback loop plays in the generation or maintenance of chronic pain is not known.
NGF Effects on Nociceptive Ion Channels, Receptors, and Peptides
In addition to enhancing the release of inflammatory mediators that alter sensory neuron excitability, NGF signaling itself also has effects on the activity of nociceptive ion channels and receptors that contribute to nociceptor sensitization (). The changes may be due either to direct, immediate effects on ion channel/receptor activity at the cell membrane and/or through longer-term effects such as enhanced gene transcription that leads to increased numbers of ion channels/receptors at the cell surface ().
Table 2 Summary of Short- and Intermediate/Long-Term Effects of NGF Signaling on Ion Channels, Receptors, and Peptides
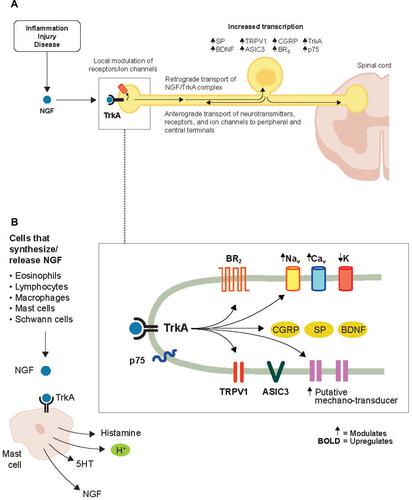
Figure 3 Effects of NGF on nociceptive ion channels, receptors, and peptides. (A) NGF signaling increases the activity of a variety of ion channels and receptors at the nociceptor peripheral terminal, which promotes depolarization and sensitization in a relatively short time frame. In a longer time frame, the NGF/TrkA complex is retrograde transported to the soma where NGF/TrkA signaling within the DRG promotes gene expression and leads to an upregulation of nociceptive ion channels, receptors, and peptides in the peripheral and central terminals. (B) NGF is released from a variety of cells following inflammatory injury. Reproduced with permission from Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology (Official Journal of the American Society of Anesthesiologists). 2011;115(1):189–20Citation6; https://anesthesiology.pubs.asahq.org/article.aspx?articleid=1933906.
Abbreviations: ASIC3, acid-sensing ion channel 3; BDNF, brain-derived neurotrophic factor; BR, bradykinin receptor; Ca, calcium; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; K, potassium; Na, sodium; NGF, nerve growth factor; SP, substance P; TrkA, tropomyosin receptor kinase A; TRPV1, transient receptor potential cation channel subfamily V member 1.
NGF Effects in Ion Channel Activity
The cation channel TRPV1, known to play a key role in nociception, is modulated by NGF activity. Cell culture studies have implicated each of the major signaling pathways downstream of TrkA activation in NGF-induced sensitization of TRPV1, though data particularly support a role for PI3K as a mediator of TRPV1 sensitization.Citation131–Citation135 Another non-selective cation channel predominantly expressed in sensory neurons, the ATP-gated P2X3 receptor, is also modulated by NGF.Citation136–Citation138 Cultured rodent trigeminal sensory neurons exposed to NGF exhibit potentiated P2X3 currents, while blocking NGF activity reduces such currents.Citation137,Citation138 NGF-induced enhancement of P2X3 activity may occur downstream of TrkA activation, as PKC-mediated phosphorylation of P2X3 threonine subunits has been shown to increase P2X3 currents in these cultured neurons.Citation137,Citation139
In isolated rat primary DRG sensory neurons, NGF enhances tetrodotoxin-resistant sodium currents and suppresses delayed rectifier potassium currents, which together lead to increased cell excitability.Citation140 Signaling molecules downstream of TrkA activation have been shown to potentiate sodium channel activation. In cultured rodent DRG neurons, for example, Nav1.7 activation is increased via Erk1/2 signaling, and activation of p38 MAPK can directly phosphorylate Nav1.8 leading to an increase in Nav1.8 current density in DRG neurons.Citation141,Citation142 However, whether these changes to sodium channel activation properties occur downstream of NGF-TrkA signaling, or as part of other signaling pathways, was not explored in these studies.
While numerous studies have demonstrated a role for NGF-TrkA signaling in the modulation of nociceptive ion channel activity, there is also evidence that NGF-p75NTR signaling can contribute to sensory neuron excitability.Citation6,Citation143-Citation145 For example, NGF-mediated activation of p75NTR has been shown to increase ceramide levels in a TrkA-independent manner in cell culture, and studies in rodents have shown that ceramide likely mediates NGF-induced sensitization of isolated sensory neurons in vitro and possibly NGF-induced pain-related behaviors in vivo.Citation140,Citation146,Citation147
NGF Effects on Gene Expression
In addition to enhancing the activity of nociceptive ion channels to promote depolarization and sensitization in a short time frame, NGF also mediates longer-term changes in gene expression and/or membrane localization, both of which contribute to increased sensory neuron excitability. For example, intramuscular injection of NGF into the masseter of rats causes an increase in the number of trigeminal ganglion neurons expressing the N-methyl-D-aspartate (NMDA) receptor subtype 2B, an increase that peaks after 3 days and is associated with mechanical sensitization.Citation148 NGF has also been shown to promote TRPV1 transcription in PC12 cells and increase translocation of TRPV1 protein to the cell surface of cultured rodent DRG neurons, the latter possibly mediated through PI3K and/or PKC signaling events downstream of TrkA.Citation134,Citation149–Citation151 Increased expression of sodium channels is evident in DRG neurons, accompanied by behaviors associated with thermal and mechanical allodynia, after subcutaneous administration of NGF in rats.Citation152 Intrathecal administration of NGF in rats causes novel P2X3 expression in axons projecting to lamina I and outer lamina II of the spinal cord.Citation153 In freshly isolated mouse DRG, NGF exposure increases bradykinin B2 receptor mRNA and membrane expression.Citation154 Likewise, a separate study found that NGF treatment increases the number of bradykinin binding sites in these cells, which is dependent on the presence of p75NTR.Citation155
Proton-gated acid-sensing ion channels (ASIC) levels may also be modulated by NGF. In cultured rodent DRG neurons, a mixture of inflammatory mediators including NGF, serotonin, interleukin-1, and bradykinin significantly increase ASIC3 currents, and NGF is known to increase ASIC3 expression.Citation156,Citation157 In humans, local NGF-induced hyperalgesia in the tibialis anterior muscle is enhanced by subsequent treatment with acid, an activator of ASIC channels.Citation101 In this study, however, acute acid-induced pain was not enhanced by previous intramuscular injection of NGF.Citation101 This contrasts with a separate human study in which injection of NGF into the fascia of the Musculus erector spinae muscle enhanced painful responses to acidic saline treatment compared with control saline.Citation103 This difference may be due to the time required for retrograde transport of the NGF signaling complex to the DRG, since acid treatment occurred 7 and 14 days after NGF administration in the former study (enhanced acid response) and only 1 day after NGF administration in the latter study (no enhancement of acid response).Citation101,Citation103 NGF signaling increases ASIC3 expression through a p75NTR-dependent transcriptional switch in primary cultured rat DRG neurons.Citation157 NGF controls a basal-level of ASIC3 transcription through constitutive activation of TrkA/PLC/PKC signaling, while increased levels of NGF promote ASIC overexpression via combined PLC/PKC and JNK/p38 MAPK signaling that depends on the presence of p75NTR.Citation157 ASIC1a protein expression has also been shown to increase following NGF treatment of cultured rat DRGs.Citation158
Overall, the cellular processes mediating NGF-induced upregulation of ion channel membrane expression are not completely delineated and may involve a combination of effects on transcription, translation, and exocytosis.
NGF Effects on Peptides
NGF has also been shown to increase levels of peptides expressed by nociceptors including substance P and calcitonin gene-related peptide (CGRP), both of which are increased during inflammation.Citation47,Citation159,Citation160 NGF-mediated increases in substance P protein levels occur downstream of both TrkA and p75NTR activation in cultured rat sensory neurons.Citation160 While NGF’s effects on nociceptive ion channels and cell surface receptors sensitize the nociceptor (more action potentials over time), NGF’s ability to enhance neurotransmitter release (substance P and CGRP) potentially increases neurotransmission independent of increases in the number of action potentials. This synergistic effect makes NGF a novel therapeutic target relative to other known neuronal mediators such as bradykinin and serotonin.
NGF Effects on Nerve Sprouting
First-in-human studies using recombinant human NGF were designed to prevent or reverse peripheral neuropathy.Citation161 Phase 3 clinical trials not only failed to demonstrate a significant beneficial effect, but it was also observed that NGF injection produced generalized myalgia and localized hyperalgesia at the injection site.Citation161 This observation revealed that intradermal NGF injections could be used as an experimental model for hyperalgesia and opened the door into research on how NGF modulates pain signaling. One thought was that local peripheral neuronal sprouting of sensory nerves can increase nerve terminal density in peripheral tissues. Such anatomical remodeling at sites of injury or inflammation could, potentially, contribute to increased nociceptive input and increased pain perception. For example, pathological sensory and/or sympathetic nerve sprouting, sometimes resulting in the formation of painful neuroma-like structures, has been observed in disease models of bone cancer pain and arthritis pain.Citation49,Citation53,Citation54,Citation162 Evidence suggests that NGF can drive neuronal sprouting (). For example, administration of NGF antibody inhibits sprouting and neuroma formation in the aforementioned models of bone and arthritis pain.Citation53–Citation55,Citation163
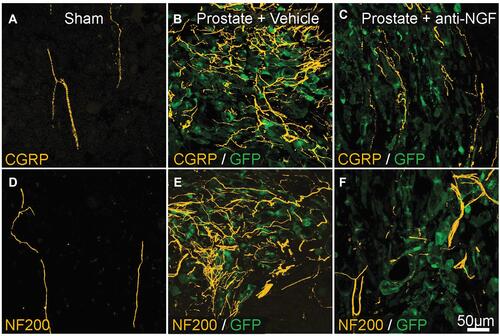
Figure 4 Preventative administration of anti-NGF antibody reduces metastatic prostate cancer-induced CGRP+ and NF200+ sensory nerve sprouting. (A and D) CGRP+ and NF200+ innervation of the bone marrow in sham-operated mice (yellow). (B and E) 26 days post-injection. Proliferation of prostate cancer cells (transfected with green fluorescent protein; green) and increased sprouting of CGRP+ and NF200+ fibers (yellow). (C and F) Effects of anti-NGF antibody (mAb911) administered at 10, 15, 20, and 25 days after cell injection. CGRP+ and NF200+ nerve sprouting has significantly reduced. Republished with permission from Pathological Sprouting of Adult Nociceptors in Chronic Prostate Cancer-Induced Bone Pain. Juan M. Jimenez-Andrade, Aaron P. Bloom, James I. Stake, William G. Mantyh, Reid N. Taylor, Katie T. Freeman, Joseph R. Ghilardi, Michael A. Kuskowski and Patrick W. Mantyh. J Neurosci. 2010;30 (44) :14649-14656.Citation163 https://doi.org/10.1523/JNEUROSCI.3300-10.2010.
Abbreviations: CGRP, calcitonin gene-related peptide; GFP, green fluorescent protein; NF200, 200-kDa neurofilament; NGF, nerve growth factor.
In addition to its effect at peripheral sites, NGF may also play a role in neuronal sprouting at sites such as the DRG and dorsal horn of the spinal cord.Citation164–Citation166 For example, axonal sprouting of peptidergic nociceptive neurons in the dorsal horn and into the ventral horn of the spinal cord can be induced by adenovirus-driven overexpression of NGF in rats.Citation165,Citation166 Such sprouting leads to chronic pain, characterized by thermal-mechanical and hyperalgesia, in these animals.Citation165,Citation166
Although aberrant nerve sprouting has been seen in animal models of pain and evidence suggests this is NGF-dependent, the exact signaling pathways downstream of NGF receptor activation are unknown. Under in vitro experimental conditions, chick DRG axonal sprouting towards NGF-coated beads is blocked both by treatment with a pan-Trk inhibitor and with PI3K inhibition, consistent with the hypothesis that pathological sprouting may be mediated by NGF-TrkA signaling pathways.Citation167
NGF also mediates sprouting of TrkA+ sympathetic nerve fibers.Citation168–Citation171 Exogenous administration of NGF in adult mice, for example, leads to increased adrenergic nerve sprouting in several peripheral organs and in the brain.Citation168 An increase in sympathetic drive may represent another mechanism through which NGF contributes to pain. For example, increased sympathetic signaling plays a role in the maintenance of pain associated with complex regional pain syndrome (CPRS) and elevated sympathetic activity increases the spatial distribution of hyperalgesia in these patients.Citation172,Citation173
NGF Effects Within the CNS
As discussed above, NGF signaling contributes to acute and long-term nociceptive hypersensitivity by increasing the activity and/or expression of nociceptive ion channels, receptors, and peptides in the periphery. However, NGF may also have sensitizing effects within the CNS.
NGF has been shown to affect levels of nociceptive peptides within the CNS. Repeated subcutaneous administration of NGF increases CGRP and substance P release at central afferent terminals of sensory neurons in rodents.Citation174 CGRP increases neuronal excitability of spinal neurons and substance P has been shown to increase dorsal horn neuron excitability by potentiating NMDA activity in these animals.Citation175–Citation177 NGF also affects BDNF levels, a neurotrophin that is expressed by some TrkA+ sensory neurons, and BDNF release in the spinal cord is thought to contribute to the central sensitization thought to underlie many chronic pain conditions.Citation178 In adult rats, BDNF mRNA levels are selectively increased in TrkA-expressing DRG cells in response to intrathecal administration of NGF.Citation179 Following NGF treatment, BDNF is retrogradely and anterogradely transported from the DRG to the peripheral and central sensory nerve terminals.Citation179,Citation180 BDNF is also released directly in the dorsal horn following electrical stimulation of dorsal roots in isolated rat dorsal horns, and this release is enhanced by systemic or intrathecal NGF administration.Citation181 BDNF increases sensory neuron excitability via binding to p75NTR and subsequent downstream sphingosine kinase signaling.Citation182 BDNF can also sensitize rodent spinal lamina II neurons via NMDA receptor activation and PLC/PKC signaling, though it is not known whether the PLC/PKC signaling pathway is initiated downstream of TrkA activation in this case.Citation183
NGF may also play a role in wind-up, the process by which central neuron excitability is increased following repeated low-frequency stimulation.Citation184 Isolated rat spinal cords treated with NGF exhibit a novel wind-up response with low-frequency stimulation of group I/II Aβ fibers that were found to be mediated through enhanced neurokinin-1 receptor activation.Citation185
Overall, NGF signaling initiated at distal peripheral locations can have long-lasting effects within the CNS that may contribute to chronic pain (). A single subcutaneous administration of NGF in the rat, for example, causes transient thermal and mechanical allodynia (up to 24 hours), but persistent (up to 3 months) increases in sodium channel levels within neurons of the DRG.Citation152
Figure 5 Summary of NGF effects on nociception. NGF/TrkA signaling has relatively short-term actions at the peripheral nociceptor terminal and on inflammatory cells, followed by longer-term actions within the nociceptor soma in the DRG. The overall effect is neuronal sensitization in the periphery and in the dorsal horn, leading to increased nociceptive signaling to higher-order pathways. Reproduced with permission from Schmelz et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain (Official Journal of the International Association for the Study of Pain). 2019 Oct;160(10):2210–2220; https://journals.lww.com/pain/Fulltext/2019/10000/Nerve_growth_factor_antibody_for_the_treatment_of.6.aspx.Citation207
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); ASIC, acid-sensing ion channels; BDNF, brain-derived neurotrophic factor; BK, bradykinin; Ca, calcium; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; K, potassium; Na, sodium; NGF, nerve growth factor; PGE2, prostaglandin E2; SubP, substance P; TrkA, tropomyosin receptor kinase A; TRPV1, transient receptor potential cation channel subfamily V member 1.
Future Perspectives
Given the role of NGF in the modulation of nociception, the analgesic benefits of drugs targeting the NGF pathway have been explored in pre-clinical pain models and in human studies. Monoclonal antibodies against NGF (eg, tanezumab and fasinumab) that bind and neutralize NGF activity are in late stages of clinical development, having demonstrated significant analgesic effects over placebo in Phase 2 or Phase 3 trials of osteoarthritis.Citation186–Citation196 Small molecule TrkA inhibitors (ASP7962 and GZ389988A) have advanced to Phase 2 clinical testing with mixed results. A single intra-articular injection of the TrkA inhibitor GZ389988A has been shown to modestly improve osteoarthritis knee pain at 8 weeks.Citation197 In contrast, treatment with the oral TrkA inhibitor ASP7962 at a dose of 100 mg BID failed to improve pain and function in patients with knee osteoarthritis after 4 weeks of treatment.Citation198 Finally, a Phase 1 trial of LEVI-04, an injectable p75NTR fusion protein designed to bind excess NGF, is currently recruiting healthy volunteers and patients with knee OA (NCT03227796).
Other novel pain therapeutics targeting the NGF pathway are in the early stages of discovery or pre-clinical development. These include monoclonal antibodies that bind and neutralize TrkA and small molecule NGF/pro-NGF inhibitors that disrupt NGF/proNGF binding to TrkA and p75NTR.Citation199–Citation201 While still in early developmental stages, these small molecule-based inhibitors may be of therapeutic interest in attenuating NGF-induced sensitization of nociceptive signaling pathways.
The nociceptive signaling pathways mediated by NGF have been studied primarily in vitro in cell culture studies or in vivo using animal models. However, signaling pathways may differ in human cells. With advances in human induced pluripotent stem cells, it may be possible in the future to study NGF-induced nociceptive signaling pathways in sensory neuron-like cells derived from human pluripotent stem cells, allowing for a better understanding of the cellular role of NGF in human nociception.Citation171
Conclusions
NGF has a well-known and multifunctional role in nociceptive processing; however, the precise signaling pathways downstream of NGF receptor activation that mediate nociception are complex and not completely understood. Additionally, much of the existing knowledge derives from studies performed in animal models, and this may not accurately represent the human condition. However, available data establish a role for NGF in the modulation of nociception through effects on the release of inflammatory mediators, nociceptive ion channel/receptor activity, nociceptive gene expression, and local neuronal sprouting. The role of NGF in nociception and the generation and/or maintenance of chronic pain have led it to become a novel and attractive target of pain therapeutics for the treatment of chronic pain conditions.
Acknowledgments
The study was sponsored by Pfizer and Eli Lilly and Company. Medical writing support was provided by Matt Soulsby, PhD, CMPP, of Engage Scientific Solutions and was funded by Pfizer Inc and Eli Lilly & Co.
Disclosure
P Barker has served as a scientific consultant to Pfizer. L. Arendt-Nielsen is part of Center for Neuroplasticity and Pain (CNAP) which is supported by the Danish National Research Foundation (DNRF121). P. Mantyh has served as a consultant to Pfizer and has received anti-NGF antibody from Rinat (Pfizer) for research use. L. Viktrup is a full-time employee of Eli Lilly & Co. L. Tive is a full-time employee of, and owns stock/options in, Pfizer Inc. The authors report no other conflicts of interest in this work.
References
- Levi-Montalcini R, Angeletti PU. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963;6:653–659. doi:10.1016/0012-1606(63)90149-0
- Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17(14):5445–5454. doi:10.1523/JNEUROSCI.17-14-05445.1997
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337(6205):362–364. doi:10.1038/337362a0
- Berry A, Bindocci E, Alleva E. NGF, brain and behavioral plasticity. Neural Plast. 2012;2012:784040. doi:10.1155/2012/784040
- Chang DS, Hsu E, Hottinger DG, Cohen SP. Anti-nerve growth factor in pain management: current evidence. J Pain Res. 2016;9:373–383. doi:10.2147/JPR.S89061
- Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115(1):189–204. doi:10.1097/ALN.0b013e31821b1ac5
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond). 2006;110(2):175–191. doi:10.1042/CS20050161
- Clewes O, Fahey MS, Tyler SJ, et al. Human ProNGF: biological effects and binding profiles at TrkA, P75NTR and sortilin. J Neurochem. 2008;107(4):1124–1135. doi:10.1111/j.1471-4159.2008.05698.x
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. doi:10.1126/science.1065057
- Seidah NG, Benjannet S, Pareek S, et al. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314(Pt 3):951–960. doi:10.1042/bj3140951
- Lim KC, Tyler CM, Lim ST, Giuliano R, Federoff HJ. Proteolytic processing of proNGF is necessary for mature NGF regulated secretion from neurons. Biochem Biophys Res Commun. 2007;361(3):599–604. doi:10.1016/j.bbrc.2007.07.039
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67(3):203–233. doi:10.1016/S0301-0082(02)00016-3
- Ioannou MS, Fahnestock M. ProNGF, but not NGF, switches from neurotrophic to apoptotic activity in response to reductions in TrkA receptor levels. Int J Mol Sci. 2017;18(3). doi:10.1016/0306-4522(92)90237-V
- Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. doi:10.1038/nature02319
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350(6320):678–683. doi:10.1038/350678a0
- Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron. 1992;9(6):1067–1079. doi:10.1016/0896-6273(92)90066-M
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13(1):203–215. doi:10.1016/0896-6273(94)90470-7
- Berg MM, Sternberg DW, Hempstead BL, Chao MV. The low-affinity p75 nerve growth factor (NGF) receptor mediates NGF-induced tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1991;88(16):7106–7110. doi:10.1073/pnas.88.16.7106
- Hantzopoulos PA, Suri C, Glass DJ, Goldfarb MP, Yancopoulos GD. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron. 1994;13(1):187–201. doi:10.1016/0896-6273(94)90469-3
- Sharma N, Deppmann CD, Harrington AW, et al. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67(3):422–434. doi:10.1016/j.neuron.2010.07.018
- Ceni C, Kommaddi RP, Thomas R, et al. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J Cell Sci. 2010;123(Pt 13):2299–2307. doi:10.1242/jcs.062612
- Bilderback TR, Gazula V-R, Dobrowsky RT. Phosphoinositide 3-kinase regulates crosstalk between Trk A tyrosine kinase and p75NTR-dependent sphingolipid signaling pathways. J Neurochem. 2001;76(5):1540–1551. doi:10.1046/j.1471-4159.2001.00171.x
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7(7):1484–1494.
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53(1):25–38. doi:10.1016/j.neuron.2006.09.034
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350(6314):158–160. doi:10.1038/350158a0
- Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257.
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130(1):149–156. doi:10.1083/jcb.130.1.149
- Davies AM. The neurotrophic hypothesis: where does it stand? Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):389–394.
- Fitzgerald M. Developmental biology of inflammatory pain. Br J Anaesth. 1995;75(2):177–185. doi:10.1093/bja/75.2.177
- Crowley C, Spencer SD, Nishimura MC, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–1011. doi:10.1016/0092-8674(94)90378-6
- Smeyne RJ, Klein R, Schnapp A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368(6468):246–249. doi:10.1038/368246a0
- Einarsdottir E, Carlsson A, Minde J, et al. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13(8):799–805. doi:10.1093/hmg/ddh096
- Carvalho OP, Thornton GK, Hertecant J, et al. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet. 2011;48(2):131–135. doi:10.1136/jmg.2010.081455
- Larsson E, Kuma R, Norberg A, Minde J, Holmberg M. Nerve growth factor R221W responsible for insensitivity to pain is defectively processed and accumulates as proNGF. Neurobiol Dis. 2009;33(2):221–228. doi:10.1016/j.nbd.2008.10.012
- Capsoni S, Covaceuszach S, Marinelli S, et al. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One. 2011;6(2):e17321. doi:10.1371/journal.pone.0017321
- Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13(4):485–488. doi:10.1038/ng0896-485
- Indo Y. Molecular basis of congenital insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum Mutat. 2001;18(6):462–471. doi:10.1002/humu.1224
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13(5):2136–2148. doi:10.1523/JNEUROSCI.13-05-02136.1993
- Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92(5):3148–3152. doi:10.1152/jn.00356.2004
- Kemp SWP, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229(2):460–470. doi:10.1016/j.expneurol.2011.03.017
- Hui L, Yuan J, Ren Z, Jiang X. Nerve growth factor reduces apoptotic cell death in rat facial motor neurons after facial nerve injury. Neurosciences (Riyadh). 2015;20(1):65–68.
- Orike N, Thrasivoulou C, Wrigley A, Cowen T. Differential regulation of survival and growth in adult sympathetic neurons: an in vitro study of neurotrophin responsiveness. J Neurobiol. 2001;47(4):295–305. doi:10.1002/neu.1036
- Jones MG, Munson JB, Thompson SWN. A role for nerve growth factor in sympathetic sprouting in rat dorsal root ganglia. Pain. 1999;79(1):21–29. doi:10.1016/S0304-3959(98)00142-0
- Heumann R, Lindholm D, Bandtlow C, et al. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987;84(23):8735–8739. doi:10.1073/pnas.84.23.8735
- Calamandrei G, Alleva E, Cirulli F, et al. Serum NGF levels in children and adolescents with either Williams syndrome or Down syndrome. Dev Med Child Neurol. 2000;42(11):746–750. doi:10.1017/S0012162200001389
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62(2):327–331. doi:10.1016/0306-4522(94)90366-2
- Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49(3):693–698.
- Grills BL, Schuijers JA. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop Scand. 1998;69(4):415–419. doi:10.3109/17453679808999059
- Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J Neurosci. 2013;33(24):10066–10074. doi:10.1523/JNEUROSCI.5784-12.2013
- Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116(1–2):8–16. doi:10.1016/j.pain.2005.03.039
- Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133(1–3):183–196. doi:10.1016/j.pain.2007.06.016
- Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79(2–3):265–274. doi:10.1016/S0304-3959(98)00164-X
- Bloom AP, Jimenez-Andrade JM, Taylor RN, et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12(6):698–711. doi:10.1016/j.jpain.2010.12.016
- Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum. 2012;64(7):2223–2232.
- Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–2574. doi:10.1016/j.pain.2011.07.020
- Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2015;23(6):925–932. doi:10.1016/j.joca.2015.02.002
- Koewler NJ, Freeman KT, Buus RJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. 2007;22(11):1732–1742. doi:10.1359/jbmr.070711
- LaBranche TP, Bendele AM, Omura BC, et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis. 2017;76(1):295–302. doi:10.1136/annrheumdis-2015-208913
- Miyagi M, Ishikawa T, Kamoda H, et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord. 2017;18(1):428. doi:10.1186/s12891-017-1792-x
- Sevcik MA, Ghilardi JR, Peters CM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1–2):128–141. doi:10.1016/j.pain.2005.02.022
- Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992;35(3):351–355. doi:10.1002/art.1780350315
- Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem Res. 1998;23(6):919–922. doi:10.1023/A:1022475432077
- Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852–1861. doi:10.1093/rheumatology/keq188
- Iannone F, De Bari C, Dell’Accio F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford). 2002;41(12):1413–1418. doi:10.1093/rheumatology/41.12.1413
- Jiang YH, Peng CH, Liu HT, Kuo HC. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One. 2013;8(10):e76779. doi:10.1371/journal.pone.0076779
- Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161(2):438–441; discussion 441–432. doi:10.1016/S0022-5347(01)61915-3
- Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104(10):1476–1481. doi:10.1111/j.1464-410X.2009.08675.x
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79(4):572–577. doi:10.1046/j.1464-410X.1997.00097.x
- Watanabe T, Inoue M, Sasaki K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108(2):248–251. doi:10.1111/j.1464-410X.2010.09716.x
- Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26(7):1564–1569.
- Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001;57(1):132–134. doi:10.1212/WNL.57.1.132
- Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T. Expression of mRNAs for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75NGFR, trk, trkB, and trkC) in human peripheral neuropathies. Neurochem Res. 1998;23(6):821–829. doi:10.1023/A:1022434209787
- Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–292. doi:10.1002/path.1108
- Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11(4):R126. doi:10.1186/ar2793
- Aoki Y, Nakajima A, Ohtori S, et al. Increase of nerve growth factor levels in the human herniated intervertebral disc: can annular rupture trigger discogenic back pain? Arthritis Res Ther. 2014;16(4):R159. doi:10.1186/ar4674
- Zhu Z, Friess H, diMola FF, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17(8):2419–2428. doi:10.1200/JCO.1999.17.8.2419
- Paterson S, Schmelz M, McGlone F, Turner G, Rukwied R. Facilitated neurotrophin release in sensitized human skin. Eur J Pain. 2009;13(4):399–405. doi:10.1016/j.ejpain.2008.05.005
- Jablochkova A, Backryd E, Kosek E, et al. Unaltered low nerve growth factor and high brain-derived neurotrophic factor levels in plasma from patients with fibromyalgia after a 15-week progressive resistance exercise. J Rehabil Med. 2019;51(10):779–787. doi:10.2340/16501977-2593
- Andreev N, Dimitrieva N, Koltzenburg M, McMahon SB. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995;63(1):109–115. doi:10.1016/0304-3959(95)00024-M
- Davis BM, Lewin GR, Mendell LM, Jones ME, Albers KM. Altered expression of nerve growth factor in the skin of transgenic mice leads to changes in response to mechanical stimuli. Neuroscience. 1993;56(4):789–792. doi:10.1016/0306-4522(93)90127-2
- Schnegelsberg B, Sun TT, Cain G, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R534–R547. doi:10.1152/ajpregu.00367.2009
- Petty BG, Cornblath DR, Adornato BT, et al. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36(2):244–246. doi:10.1002/ana.410360221
- Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148(3):407–413. doi:10.1016/j.pain.2009.11.022
- Weinkauf B, Obreja O, Schmelz M, Rukwied R. Differential time course of NGF-induced hyperalgesia to heat versus mechanical and electrical stimulation in human skin. Eur J Pain. 2015;19(6):789–796. doi:10.1002/ejp.603
- Andersen HH, Lo Vecchio S, Elberling J, Yosipovitch G, Arendt-Nielsen L. UVB- and NGF-induced cutaneous sensitization in humans selectively augments cowhage- and histamine-induced pain and evokes mechanical hyperknesis. Exp Dermatol. 2018;27(3):258–267. doi:10.1111/exd.13508
- Andresen T, Nilsson M, Nielsen AK, Lassen D, Arendt-Nielsen L, Drewes AM. Intradermal injection with nerve growth factor: a reproducible model to induce experimental allodynia and hyperalgesia. Pain Pract. 2016;16(1):12–23. doi:10.1111/papr.12267
- Weinkauf B, Obreja O, Schmelz M, Rukwied R. Differential effects of lidocaine on nerve growth factor (NGF)-evoked heat- and mechanical hyperalgesia in humans. Eur J Pain. 2012;16(4):543–549. doi:10.1016/j.ejpain.2011.08.004
- Rukwied B, Weinkauf B, Main M, Obreja O, Schmelz M. Axonal hyperexcitability after combined NGF sensitization and UV-B inflammation in humans. Eur J Pain. 2014;18(6):785–793. doi:10.1002/j.1532-2149.2013.00423.x
- Rukwied R, Weinkauf B, Main M, Obreja O, Schmelz M. Inflammation meets sensitization–an explanation for spontaneous nociceptor activity? Pain. 2013;154(12):2707–2714. doi:10.1016/j.pain.2013.07.054
- Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104(1–2):241–247. doi:10.1016/S0304-3959(03)00012-5
- Svensson P, Wang K, Arendt-Nielsen L, Cairns BE. Effects of NGF-induced muscle sensitization on proprioception and nociception. Exp Brain Res. 2008;189(1):1–10. doi:10.1007/s00221-008-1399-4
- Svensson P, Castrillon E, Cairns BE. Nerve growth factor-evoked masseter muscle sensitization and perturbation of jaw motor function in healthy women. J Orofac Pain. 2008;22(4):340–348.
- Andersen H, Arendt-Nielsen L, Svensson P, Danneskiold-Samsoe B, Graven-Nielsen T. Spatial and temporal aspects of muscle hyperalgesia induced by nerve growth factor in humans. Exp Brain Res. 2008;191(3):371–382. doi:10.1007/s00221-008-1531-5
- Gerber RK, Nie H, Arendt-Nielsen L, Curatolo M, Graven-Nielsen T. Local pain and spreading hyperalgesia induced by intramuscular injection of nerve growth factor are not reduced by local anesthesia of the muscle. Clin J Pain. 2011;27(3):240–247. doi:10.1097/AJP.0b013e3182048481
- Lo Vecchio S, Petersen LJ, Finocchietti S, et al. Interaction between ultraviolet B-induced cutaneous hyperalgesia and nerve growth factor-induced muscle hyperalgesia. Eur J Pain. 2016;20(7):1058–1069. doi:10.1002/ejp.828
- Vecchio SL, Finocchietti S, Gazerani P, Petersen LJ, Arendt-Nielsen L, Graven-Nielsen T. Heat-rekindling in UVB-irradiated skin above NGF-sensitized muscle: experimental models of prolonged mechanical hypersensitivity. Int J Physiol Pathophysiol Pharmacol. 2014;6(3):143–152.
- De Martino E, Zandalasini M, Schabrun S, Petrini L, Graven-Nielsen T. Experimental muscle hyperalgesia modulates sensorimotor cortical excitability, which is partially altered by unaccustomed exercise. Pain. 2018;159(12):2493–2502. doi:10.1097/j.pain.0000000000001351
- Sorensen LB, Boudreau SA, Gazerani P, Graven-Nielsen T. Enlarged areas of pain and pressure hypersensitivity by spatially distributed intramuscular injections of low-dose nerve growth factor. J Pain. 2019;20(5):566–576. doi:10.1016/j.jpain.2018.11.005
- Mista CA, Bergin MJG, Hirata RP, et al. Effects of prolonged and acute muscle pain on the force control strategy during isometric contractions. J Pain. 2016;17(10):1116–1125. doi:10.1016/j.jpain.2016.06.013
- Nie H, Madeleine P, Arendt-Nielsen L, Graven-Nielsen T. Temporal summation of pressure pain during muscle hyperalgesia evoked by nerve growth factor and eccentric contractions. Eur J Pain. 2009;13(7):704–710. doi:10.1016/j.ejpain.2008.06.015
- Munkholm TK, Arendt-Nielsen L. The interaction between NGF-induced hyperalgesia and acid-provoked pain in the infrapatellar fat pad and tibialis anterior muscle of healthy volunteers. Eur J Pain. 2017;21(3):474–485. doi:10.1002/ejp.941
- Hayashi K, Shiozawa S, Ozaki N, Mizumura K, Graven-Nielsen T. Repeated intramuscular injections of nerve growth factor induced progressive muscle hyperalgesia, facilitated temporal summation, and expanded pain areas. Pain. 2013;154(11):2344–2352. doi:10.1016/j.pain.2013.07.007
- Deising S, Weinkauf B, Blunk J, Obreja O, Schmelz M, Rukwied R. NGF-evoked sensitization of muscle fascia nociceptors in humans. Pain. 2012;153(8):1673–1679. doi:10.1016/j.pain.2012.04.033
- Hirth M, Rukwied R, Gromann A, et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain. 2013;154(11):2500–2511. doi:10.1016/j.pain.2013.07.036
- Obreja O, Ringkamp M, Turnquist B, et al. Nerve growth factor selectively decreases activity-dependent conduction slowing in mechano-insensitive C-nociceptors. Pain. 2011;152(9):2138–2146. doi:10.1016/j.pain.2011.05.021
- Obreja O, Kluschina O, Mayer A, et al. NGF enhances electrically induced pain, but not axon reflex sweating. Pain. 2011;152(8):1856–1863. doi:10.1016/j.pain.2011.04.002
- Obreja O, Rukwied R, Nagler L, Schmidt M, Schmelz M, Namer B. Nerve growth factor locally sensitizes nociceptors in human skin. Pain. 2018;159(3):416–426. doi:10.1097/j.pain.0000000000001108
- Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91(9):3739–3743. doi:10.1073/pnas.91.9.3739
- Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27(9):2295–2301. doi:10.1002/eji.1830270925
- Lambiase A, Bracci-Laudiero L, Bonini S, et al. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100(3):408–414. doi:10.1016/S0091-6749(97)70256-2
- Rost B, Hanf G, Ohnemus U, et al. Monocytes of allergics and non-allergics produce, store and release the neurotrophins NGF, BDNF and NT-3. Regul Pept. 2005;124(1–3):19–25. doi:10.1016/j.regpep.2004.06.024
- Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–131. doi:10.1093/bja/75.2.125
- Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ, Zimmer J. Mast cell-derived histamine mediates cystitis pain. PLoS One. 2008;3(5):e2096. doi:10.1371/journal.pone.0002096
- Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. doi:10.1016/j.brainresrev.2008.12.020
- Babenko V, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain and muscular hyperalgesia induced by combinations of serotonin and bradykinin. Pain. 1999;82(1):1–8. doi:10.1016/S0304-3959(99)00026-3
- Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118(1):69–74. doi:10.1016/S0306-4522(02)00960-0
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88(1):544–548. doi:10.1152/jn.2002.88.1.544
- Khasar SG, Miao FJ-P, Janig W, Levine JD. Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur J Neurosci. 1998;10(2):435–444. doi:10.1046/j.1460-9568.1998.00030.x
- Bischoff SC, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79(10):2662–2669. doi:10.1182/blood.V79.10.2662.bloodjournal79102662
- Tal M, Liberman R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neurosci Lett. 1997;221(2–3):129–132. doi:10.1016/S0304-3940(96)13318-8
- Horigome K, Pryor JC, Bullock ED, Johnson EM Jr. Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J Biol Chem. 1993;268(20):14881–14887.
- Mazurek N, Weskamp G, Erne P, Otten U. Nerve growth factor induces mast cell degranulation without changing intracellular calcium levels. FEBS Lett. 1986;198(2):315–320. doi:10.1016/0014-5793(86)80428-8
- Groneberg DA, Serowka F, Peckenschneider N, et al. Gene expression and regulation of nerve growth factor in atopic dermatitis mast cells and the human mast cell line-1. J Neuroimmunol. 2005;161(1–2):87–92. doi:10.1016/j.jneuroim.2004.12.019
- Lopes DM, Denk F, Chisholm KI, et al. Peripheral inflammatory pain sensitisation is independent of mast cell activation in male mice. Pain. 2017;158(7):1314–1322. doi:10.1097/j.pain.0000000000000917
- Susaki Y, Shimizu S, Katakura K, et al. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 1996;88(12):4630–4637. doi:10.1182/blood.V88.12.4630.bloodjournal88124630
- Shim W-S, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi:10.1186/1744-8069-4-29
- Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170(1):38–45. doi:10.1111/bph.12266
- Kasai M, Kumazawa T, Mizumura K. Nerve growth factor increases sensitivity to bradykinin, mediated through B2 receptors, in capsaicin-sensitive small neurons cultured from rat dorsal root ganglia. Neurosci Res. 1998;32(3):231–239. doi:10.1016/S0168-0102(98)00092-3
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330(6149):658–659. doi:10.1038/330658a0
- Rueff A, Dawson AJ, Mendell LM. Characteristics of nerve growth factor induced hyperalgesia in adult rats: dependence on enhanced bradykinin-1 receptor activity but not neurokinin-1 receptor activation. Pain. 1996;66(2–3):359–372. doi:10.1016/0304-3959(96)03060-6
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(Pt 2):433–446. doi:10.1113/jphysiol.2003.039990
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34(4):689–700. doi:10.1016/j.mcn.2007.01.005
- Chuang HH, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi:10.1038/35082088
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi:10.1038/sj.emboj.7600893
- Bernstein CD, Diaz JH, Gould HJ 3rd. A possible role for zonisamide in treating neuropathic pain: a case of idiopathic polyneuropathy. Pain Pract. 2002;2(2):134–136. doi:10.1046/j.1533-2500.2002.02015.x
- Vulchanova L, Riedl MS, Shuster SJ, et al. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10(11):3470–3478. doi:10.1046/j.1460-9568.1998.00355.x
- D’Arco M, Giniatullin R, Simonetti M, et al. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. 2007;27(31):8190–8201. doi:10.1523/JNEUROSCI.0713-07.2007
- Simonetti M, Fabbro A, D’Arco M, et al. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain. 2006;2:11. doi:10.1186/1744-8069-2-11
- Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. 2008;37(1):83–90. doi:10.1007/s12035-008-8020-5
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544(2):385–402. doi:10.1113/jphysiol.2002.024265
- Stamboulian S, Choi JS, Ahn HS, et al. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30(5):1637–1647. doi:10.1523/JNEUROSCI.4872-09.2010
- Hudmon A, Choi JS, Tyrrell L, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28(12):3190–3201. doi:10.1523/JNEUROSCI.4403-07.2008
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21(12):3387–3394. doi:10.1111/j.1460-9568.2005.04173.x
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24(38):8300–8309. doi:10.1523/JNEUROSCI.2893-04.2004
- Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366(2):187–192. doi:10.1016/j.neulet.2004.05.042
- Khodorova A, Nicol GD, Strichartz G. The p75NTR signaling cascade mediates mechanical hyperalgesia induced by nerve growth factor injected into the rat hind paw. Neuroscience. 2013;254:312–323. doi:10.1016/j.neuroscience.2013.09.046
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265(5178):1596–1599. doi:10.1126/science.8079174
- Wong H, Kang I, Dong XD, et al. NGF-induced mechanical sensitization of the masseter muscle is mediated through peripheral NMDA receptors. Neuroscience. 2014;269:232–244. doi:10.1016/j.neuroscience.2014.03.054
- Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101(1):212–222. doi:10.1111/j.1471-4159.2006.04363.x
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128(5):509–522. doi:10.1085/jgp.200609576
- Camprubi-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009;23(11):3722–3733. doi:10.1096/fj.09-134346
- Gould HJ 3rd, Gould TN, England JD, Paul D, Liu ZP, Levinson SR. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000;854(1–2):19–29. doi:10.1016/S0006-8993(99)02216-7
- Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem. 2001;77(3):864–875. doi:10.1046/j.1471-4159.2001.00288.x
- Lee YJ, Zachrisson O, Tonge DA, McNaughton PA. Upregulation of bradykinin B2 receptor expression by neurotrophic factors and nerve injury in mouse sensory neurons. Mol Cell Neurosci. 2002;19(2):186–200. doi:10.1006/mcne.2001.1073
- Petersen M, Segond von Banchet G, Heppelmann B, Koltzenburg M. Nerve growth factor regulates the expression of bradykinin binding sites on adult sensory neurons via the neurotrophin receptor p75. Neuroscience. 1998;83(1):161–168. doi:10.1016/S0306-4522(97)00374-6
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–10670. doi:10.1523/JNEUROSCI.22-24-10662.2002
- Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278(49):48907–48913. doi:10.1074/jbc.M309468200
- Matricon J, Muller E, Accarie A, et al. Peripheral contribution of NGF and ASIC1a to colonic hypersensitivity in a rat model of irritable bowel syndrome. Neurogastroenterol Motil. 2013;25(11):e740–e754. doi:10.1111/nmo.12199
- Amann R, Schuligoi R, Herzeg G, Donnerer J. Intraplantar injection of nerve growth factor into the rat hind paw: local edema and effects on thermal nociceptive threshold. Pain. 1996;64(2):323–329. doi:10.1016/0304-3959(95)00120-4
- Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197(2):430–436. doi:10.1016/j.expneurol.2005.10.006
- Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413.
- Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14(3):R101. doi:10.1186/ar3826
- Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30(44):14649–14656. doi:10.1523/JNEUROSCI.3300-10.2010
- Cheng CF, Cheng JK, Chen CY, Rau RH, Chang YC, Tsaur ML. Nerve growth factor-induced synapse-like structures in contralateral sensory ganglia contribute to chronic mirror-image pain. Pain. 2015;156(11):2295–2309. doi:10.1097/j.pain.0000000000000280
- Lin CL, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience. 2014;272:76–87. doi:10.1016/j.neuroscience.2014.04.053
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20(12):4435–4445. doi:10.1523/JNEUROSCI.20-12-04435.2000
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18(14):5403–5414. doi:10.1523/JNEUROSCI.18-14-05403.1998
- Bjerre B, Bjorklund A, Mobley W, Rosengren E. Short- and long-term effects of nerve growth factor on the sympathetic nervous system in the adult mouse. Brain Res. 1975;94(2):263–277. doi:10.1016/0006-8993(75)90061-X
- Mantyh WG, Jimenez-Andrade JM, Stake JI, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171(2):588–598. doi:10.1016/j.neuroscience.2010.08.056
- Deng YS, Zhong JH, Zhou XF. Effects of endogenous neurotrophins on sympathetic sprouting in the dorsal root ganglia and allodynia following spinal nerve injury. Exp Neurol. 2000;164(2):344–350. doi:10.1006/exnr.2000.7432
- Campenot RB. Local control of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Brain Res. 1987;465(1–2):293–301. doi:10.1016/0165-3806(87)90250-1
- Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi:10.1186/s41038-016-0066-4
- Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002;359(9318):1655–1660. doi:10.1016/S0140-6736(02)08589-6
- Malcangio M, Garrett NE, Tomlinson DR. Nerve growth factor treatment increases stimulus-evoked release of sensory neuropeptides in the rat spinal cord. Eur J Neurosci. 1997;9(5):1101–1104. doi:10.1111/j.1460-9568.1997.tb01462.x
- Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V. Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP. Mol Pain. 2006;2:31. doi:10.1186/1744-8069-2-31
- Murase K, Nedeljkov V, Randic M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 1982;234(1):170–176. doi:10.1016/0006-8993(82)90483-8
- Randic M, Hecimovic H, Ryu PD. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurones. Neurosci Lett. 1990;117(1–2):74–80. doi:10.1016/0304-3940(90)90122-P
- Nijs J, Meeus M, Versijpt J, et al. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets. 2015;19(4):565–576. doi:10.1517/14728222.2014.994506
- Michael GJ, Averill S, Nitkunan A, et al. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–8490. doi:10.1523/JNEUROSCI.17-21-08476.1997
- Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74(4):945–953. doi:10.1016/0306-4522(96)00237-0
- Lever IJ, Bradbury EJ, Cunningham JR, et al. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21(12):4469–4477. doi:10.1523/JNEUROSCI.21-12-04469.2001
- Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol. 2008;586(13):3113–3127. doi:10.1113/jphysiol.2008.152439
- Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18(9):2467–2476. doi:10.1046/j.1460-9568.2003.02982.x
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66(2–3):105–108. doi:10.1097/00006396-199608000-00001
- Thompson SW, Dray A, McCarson KE, Krause JE, Urban L. Nerve growth factor induces mechanical allodynia associated with novel A fibre-evoked spinal reflex activity and enhanced neurokinin-1 receptor activation in the rat. Pain. 1995;62(2):219–231. doi:10.1016/0304-3959(94)00271-F
- Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre Phase III randomised clinical trial. Ann Rheum Dis. 2014;73(9):1665–1672. doi:10.1136/annrheumdis-2012-203164
- Birbara C, Dabezies EJ Jr, Burr AM, et al. Safety and efficacy of subcutaneous tanezumab in patients with knee or hip osteoarthritis. J Pain Res. 2018;11:151–164. doi:10.2147/JPR.S135257
- Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13(8):790–798. doi:10.1016/j.jpain.2012.05.006
- Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 2013;65(7):1795–1803. doi:10.1002/art.37950
- Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009–1021. doi:10.1016/j.pain.2013.03.006
- Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2015;74(6):1202–1211. doi:10.1136/annrheumdis-2013-204905
- Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154(9):1603–1612. doi:10.1016/j.pain.2013.04.035
- Nickel JC, Mills IW, Crook TJ, et al. Tanezumab reduces pain in women with interstitial cystitis/bladder pain syndrome and patients with nonurological associated somatic syndromes. J Urol. 2016;195(4 Pt 1):942–948. doi:10.1016/j.juro.2015.10.178
- Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155(7):1245–1252. doi:10.1016/j.pain.2014.03.018
- Tiseo PJ, Ren H, Mellis S. Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: results of a proof-of-concept study. J Pain Res. 2014;7:523–530. doi:10.2147/JPR.S65974
- Schnitzer TJ, Easton R, Pang S, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. 2019;322(1):37–48. doi:10.1001/jama.2019.8044
- Krupka E, Jiang GL, Jan C. Efficacy and safety of intra-articular injection of tropomyosin receptor kinase A inhibitor in painful knee osteoarthritis: a randomized, double-blind and placebo-controlled study. Osteoarthritis Cartilage. 2019;27(11):1599–1607. doi:10.1016/j.joca.2019.05.028
- Watt FE, Blauwet MB, Fakhoury A, Jacobs H, Smulders R, Lane NE. Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: results from a randomized controlled phase 2a trial. Osteoarthritis Cartilage. 2019;27(11):1590–1598. doi:10.1016/j.joca.2019.05.029
- Miller RE, Block JA, Malfait AM. What is new in pain modification in osteoarthritis? Rheumatology (Oxford). 2018;57(suppl_4):iv99–iv107. doi:10.1093/rheumatology/kex522
- Sheffield KS, Kennedy AE, Scott JA, Ross GM. Characterizing nerve growth factor-p75(NTR) interactions and small molecule inhibition using surface plasmon resonance spectroscopy. Anal Biochem. 2016;493:21–26. doi:10.1016/j.ab.2015.09.019
- Sheffield KS, Vohra R, Scott JA, Ross GM. Using surface plasmon resonance spectroscopy to characterize the inhibition of NGF-p75(NTR) and proNGF-p75(NTR) interactions by small molecule inhibitors. Pharmacol Res. 2016;103:292–299. doi:10.1016/j.phrs.2015.12.005
- Jiang H, Takeda K, Lazarovici P, et al. Nerve growth factor (NGF)-induced calcium influx and intracellular calcium mobilization in 3T3 cells expressing NGF receptors. J Biol Chem. 1999;274(37):26209–26216.
- Jarvis CR, Xiong ZG, Plant JR, et al. Neurotrophin modulation of NMDA receptors in cultured murine and isolated rat neurons. J Neurophysiol. 1997;78(5):2363–2371. doi:10.1152/jn.1997.78.5.2363
- Okuse K, Chaplan SR, McMahon SB, et al. Regulation of EXPRESSION of the Sensory Neuron-Specific Sodium Channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci. 1997;10(3):196–207. doi:10.1006/mcne.1997.0657
- Lewis DL, De Aizpurua HJ, Rausch DM. Enhanced expression of Ca2+ channels by nerve growth factor and the v-src oncogene in rat phaeochromocytoma cells. J Physiol. 1993;465:325–342. doi:10.1113/jphysiol.1993.sp019679
- Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7(2):134–142. doi:10.1006/mcne.1996.0010
- Schmelz M, Manthy P, Malfait AM, et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain. 2019;160(10):2210–2220. doi:10.1097/j.pain.0000000000001625
