Abstract
Background
Sex differences in pain sensitivity have been well documented, such that women often report greater sensitivity than men. However, clinical reports highlighting sex differences often equate gender and sex. This is a particularly critical oversight for those whose gender identity is different than their genetic sex.
Methods
This preliminary study sets to analyze differences in pain responses between cisgender and transgender individuals living with HIV and chronic pain. A total of 51 African-American participants (24 cisgender men, 20 cisgender women, 7 transgender women) with similar socioeconomic status were recruited. Genetic sex, gender identity, depression and anxiety, pain severity, pain interference and pain-related stigma were recorded. Participants also completed a quantitative sensory testing battery to assess pain in response to noxious heat and mechanical stimuli.
Results
Transgender women and cisgender women demonstrated a greater magnitude of temporal summation for heat pain stimuli or mechanical stimuli compared to cisgender men. Specifically, transgender women reported greater mechanical summation than either cisgender women or cisgender men. Transgender women and cisgender women similarly reported greater chronic pain severity compared to cisgender men.
Conclusion
These data support the notion that gender identity may play a more significant role in pain sensation than genetic sex. These results further maintain that not only gender identity and genetic sex are distinct variables but that treatment should be based on identity as opposed to genetic sex.
Introduction
Chronic pain is a major health concern with ever-increasing costs,Citation1,Citation2 but the burden is not equal. Sex differences exist in the prevalence of chronic pain conditions and pain sensitivityCitation3–Citation5 with females reporting more pain and greater sensitivity to stimuli.Citation6,Citation7 We Citation8,Citation9 and others Citation10,Citation12 have demonstrated sex differences in immune cell mediation of chronic pain in rodents to complement the reported sex differences in immune cell populations and responses in humans.Citation13–Citation16 As such, biological sex remains a significant factor related to pain experience.
The terms “sex” and “gender” have been used interchangeably in reports using human subjects, but they are not the same. Genetic/natal sex is defined by biological differences between males and females due to genetics and hormones. Gender identity refers to the social and psychologically constructed sense of oneself as a man or woman.Citation17 Research has shown that gender role expectations of pain explained more variability in pain sensitivity than genetic sex and was predictive of responses to nociceptive stimuli.Citation18 These results suggest sex differences in rodents, may be less directly translatable to human subjects with accompanying genders.
Transgender individuals, whose gender identities are not traditionally associated with their genetic sex,Citation19 have been disregarded and understudied in healthcare settings.Citation20 Whereas studies have examined sociodemographics,Citation21 healthcare access,Citation22 social supportCitation23 and other health-related behaviors,Citation24 little attention is given to specific health outcomes. This preliminary report describes the impact of gender identity on pain sensitivity in a sample of transgender women (TW) in comparison to cisgender men (CM) and women (CW). The first exploration of its kind, this study highlights the critical need for research in this area.
Methods
Participants for this study were a part of an on-going study examining the effects of HIV on chronic pain. As such, a complete methodology can be found elsewhere.Citation25 All participants provided written informed consent and study procedures were approved by the University of Alabama at Birmingham Institutional Review Board and conducted in compliance with the Declaration of Helsinki.
Medical Screening
Medical record reviews were completed to determine whether participants were prescribed antidepressants or opioids. For TW, estradiol prescription and dose were noted. Those who met study inclusion criteria were deemed eligible for ongoing participation. Blood was collected from each participant for the quantification of CD4 helper T-cell count (cells/microliter) and viral load (viruses/microliter) to confirm immune health and analyze HIV-related effects. Participants with 50 viruses/microliter of blood or greater were considered to have “detectable” viral loads.
Participants
A total of 51 African-American adults (age ≥18 years) were selected for this preliminary report. Chronic pain was defined as bodily pain that had persisted for at least three consecutive months and that was present on at least half the days in the past 6 monthsCitation26 and confirmed by medical records. No one chronic pain condition was targeted or excluded. Of the 51 participants that completed the study, 24 were CM, 20 were CW and 7 were TW. Following identification of our TW population, we identified comparable CM and CW participants in the study population based on race, SES and age – variables known to contribute to pain sensitivity.Citation27–Citation30 The post hoc examination reported herein and the primary focus of the data collection effort resulted in unequal groups and no representation of transgender men, factors that future studies will seek to remedy.
Quantitative Sensory Testing (QST)
Our QST methodology has been described elsewhere.Citation25 In short, heat pain threshold (HPTh), heat pain tolerance (HPTo), and temporal summation (TS) of heat pain were assessed prior to TS of mechanical pain. Following TS procedure, conditioned pain modulation (CPM) was assessed.
Psychosocial Measures
Participants completed self-report questionnaires that included: the HIV Stigma Mechanism Measure,Citation31 the Center for Epidemiological Studies-Depression Scale (CES-D),Citation32 the State-Trait Anxiety Index (STAI),Citation33 the Everyday Discrimination Scale,Citation34 the 36-Item Short Form Health Survey (SF-36),Citation35 the Pain Sensitivity Questionnaire (PSQ),Citation36 the Pain Catastrophizing Scale (PCS),Citation37 Internalized Stigma of Chronic Pain Scale,Citation38 and the Brief Pain Inventory (BPI)-Short Form.Citation39,Citation40
Statistical Analyses
Differences in participant characteristics were examined using analysis of variance (ANOVA). Analyses included analgesics and antidepressants as covariates. For continuously measured variables, a repeated-measures ANOVA was used to analyze group differences. Differences in pain sensitivity were analyzed by ANOVA with post hoc analyses for pairwise comparisons across the three study groups. Finally, a correlation analysis was completed to assess the association of psychosocial variables with mechanical and heat pain sensitivity. There were no missing data for any of the study variables. All analyses were carried out using SPSS, version 24.
Results
Demographics
All participants (aged 46 1 years of age) were HIV-positive and living below the poverty line according to government standards. Sixteen had a detectable viral load (CM = 11; CW = 4; TW = 1). The average number of CD4+ cells was 662 (CM = 541; CW = 798; TW = 693). Thirty-four had current prescriptions for antidepressants and 35 had current prescriptions for analgesics. Only 5 of 7 TW participants had prescriptions for estradiol in varying doses in their medical records. Estradiol prescription did not predict pain scores, nor did estradiol dose in any test. Neither antidepressants nor analgesics had any effects on acute pain sensitivity measures differentially between groups.
Heat Pain Sensitivity
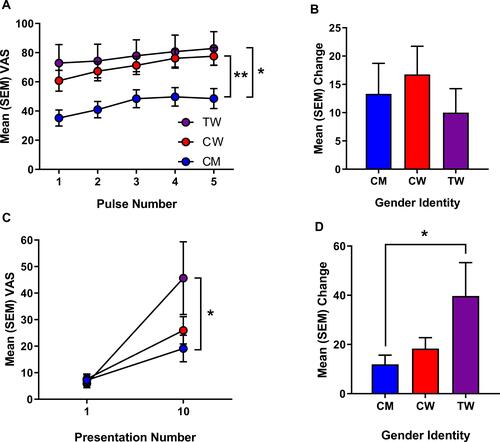
There were no significant differences between gender groups for HPTh or HPTo ( and ). There was a significant main effect of antidepressant prescription for HPTh (F(1,51) = 4.410, p = 0.041; ηp2 = 0.086, 1-β = 0.539 and HPTo (F(1,51) = 4.131, p = 0.048; ηp2 = 0.081, 1-β = 0.512), but no interaction with gender groups. TS of heat pain was determined by the change in the pain intensity rating elicited by the first heat pulse compared to the rating elicited by the fifth heat pulse for the 46°C stimulus. Data representing TS of heat pain and ratings over time are presented in and . Results of a repeated-measures ANOVA revealed that the magnitude of TS (ie, slope) did not differ according to gender identity. There was a significant main effect of analgesic prescription on TS (F(1,51) = 5.239, p = 0.027; ηp2 = 0.0.005, 1-β = 0.068), but no interaction with gender groups. Absolute pain intensity ratings in response to the repeated 46°C heat stimuli significantly differed according to gender identity (F(1,51) = 7.347, p = 0.002; ηp2 = 0.234, 1-β = 0.924)). Specifically, TW (p = 0.014) and CW (p = 0.019) rated the intensity of pain elicited by each of the five heat pulses as significantly greater in comparison to CM. Pain intensity ratings to the 46°C heat pulses did not significantly differ between TW and CW.
Figure 1 (A) Mean (SEM) temperature threshold (°C) when pain was detected. (B) Mean (SEM) temperature tolerance (°C). (C) Mean (SEM) change in pressure pain threshold (conditioned pain modulation) as a result of simultaneous exposure to the cold pressor and to pressure pain stimuli. CM = cisgender men (blue), CW = cisgender women (red), TW = transgender women (purple), individual circles represent participant values contributing to the overall means for each group.
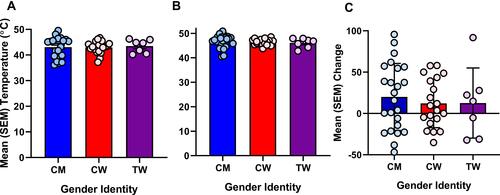
Figure 2 (A) Mean (SEM) visual analogue scale (VAS) pain ratings for the individual heat pulses. (B) Mean (SEM) change in VAS pain rating from pulse 1 to pulse 5. (C) Mean (SEM) VAS rating for the temporal summation of mechanical pain at 1 presentation and 10 presentations of 300 g monofilament. (D) Mean (SEM) change in VAS pain rating from 1 presentation to 10 presentations. CM = cisgender men (blue), CW = cisgender women (red), TW = transgender women (purple). *p < 0.05, **p<0.01.
Mechanical Pain Sensitivity
TS of mechanical pain was determined by calculating the change in the pain intensity rating elicited by the first contact with the nylon monofilament compared to the rating elicited following the series of 10 contacts, presented in and . Repeated-measures ANOVA revealed a significant effect of gender on TS of mechanical pain (F(1,51) = 4.647, p = 0.015; ηp2 = 0.168, 1-β = 0.756). Specifically, the magnitude (ie slope) of TS for mechanical pain demonstrated by TW was significantly greater than the TS demonstrated by CM (p = 0.013), but not CW (p = 0.078), though there was a trend. There were no significant effects of antidepressants or analgesics on mechanical sensitivity.
Conditioned Pain Modulation
For CPM, baseline pressure pain thresholds (PPTs) were compared to conditioned PPTs (ie, PPTs assessed during concurrent cold pressor application). There were no significant effects of gender on baseline PPTs or conditioned PPTs (). Further, there was no significant effect of gender on CPM.
Psychosocial Variables
There were no significant effects of gender identity on any of the psychosocial variables (data not shown) or evidence of significant correlations among the QST responses and psychosocial variables.
Discussion
To our knowledge, this is the first report to explore whether transgender individuals respond to pain more similarly to their genetic sex or their gender identity. Our preliminary findings suggest that TW respond to noxious mechanical and heat stimuli more similarly to CW and not CM, though not always statistically significant. In the case of TS of heat and mechanical pain, both TW and CW showed greater pain sensitivity compared to CM, but did not differ from one another. These findings could not be accounted for by any psychosocial variables, despite their relevance elsewhere. We assert that selection of comparator groups reduced the impact of these variables. In our preliminary study, gender identity was the major contributing factor to the summation of noxious stimuli, not genetic sex.
In preclinical studies, genetic sex is a significant biological variable. Our early work demonstrated sex differences in the role of immune cells in the mediation of chronic pain.Citation8,Citation9 Others have replicated our work across speciesCitation11 and various chronic pain models Citation10,Citation12. In each case, the utilization of specific immune cells was found to hormone-dependent.Citation9,Citation11,Citation41 In fact, there is evidence in humans of similar differences in immune cell responses,Citation14 populationsCitation13,Citation15,Citation16 and hormone-dependenceCitation42 that encourages the translatability of the preclinical work. However, emerging evidence suggests that psychosocial factors can modulate pain in rodents in ways that mirror human interactions.Citation43,Citation44
Social and cultural factors can alter pain experience. Women with chronic pain report greater hostility and dismissalCitation45 and are more likely to have their pain attributed to psychological issues.Citation46 These disparities appear to be reinforced through gender stereotypes that attribute distinct pain resilience patterns for men and women. The Gender Role Expectations of Pain Questionnaire (GREP) was developed to examine the impact of these beliefs.Citation18 Researchers using the GREP have demonstrated that gender identity predicted more of the variability in responses than genetic sex.Citation47 These data support the notion that beliefs about pain sensitivity may play a greater role than other biological variables, in alignment with the striking similarity in summation responses between CW and TW in our study. However, there are biologically based reasons for expecting that TW and CW would show similar responses.
Central sensitization is a phenomenon whereby nociceptor stimulation can increase the excitability of neurons in central pain pathways manifesting as pain hypersensitivityCitation48–Citation50 and may underlie chronic pain. In support of clinical epidemiological data, there is evidence to suggest that central sensitization and resulting secondary hyperalgesia are indeed greater in women,Citation51 possibly accounting for the greater prevalence of chronic pain in women. To that end, temporal summation may be predictive of the development of chronic painCitation52 and we have reported putative sex differences in temporal summation of heat,Citation53 but we are the first to expand these investigations to TW. Additionally, in pain-relevant brain regions such as the thalamus, hypothalamus, putamen, INAH3 subnucleus and the bed nucleus of the stria terminalis, there are documented differences in neuron number, connectivity and cortical thickness that are dependent on gender identity, as opposed to genetic sex.Citation54–Citation59 There is debate as to whether these similarities are innateCitation60 or due to social factors,Citation61,Citation62 but gender identity appears to be a critical component. These data suggest that TW may be at increased risk for central sensitization, hypersensitivity and subsequent chronic pain. Therefore, we assert that gender identity be recognized as a critical factor in treatment recommendations and future studies.
Our study had a number of limitations due to the preliminary nature of the work. First, the sample size for the TW group was small, based on the conditions surrounding the collection of the data and the focus of the parent project. Second, the lack of transgender men in the study is an aspect that future studies should address. Third, to provide comparator groups to the TW in the study, we selected participants that were similar in race, SES and health status. This choice increased internal validity but may result in data that is not generalizable to the population as a whole. Fourth, as mentioned, we did not test hormone status and there is ample data to suggest that hormones affect the mediation of chronic pain.Citation8,Citation9 We feel that the limitations of our study do not reduce the potential importance of our work and believe that future large-scale studies can overcome these obstacles to provide a clearer understanding of the role that gender identity plays on pain sensitivity in humans.
The transgender community is a vulnerable population dealing with stigmas that impact the quality of life.Citation21,Citation63 Despite the evidence of sex differences in pain that are dependent on hormone status and genetic sex, there are a host of reasons for caution when applying such findings. Our study demonstrated that TW and CW show very similar temporal summation of thermal stimuli and that TW may have an even greater summation of mechanical stimuli than CW. This latter finding argues that TW may be at greater risk for chronic pain than CW, supporting the need for further study and assistance. In our hands, gender identity appears to have a significant impact on pain sensitivity, supporting the recognition of gender identity over genetic sex.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge the efforts of the staff at the UAB CCTS Clinical Research Unit for their assistance with data collection, sample preparation and blood analysis, as well as Tammie Quinn for aiding with the conceptualization of the manuscript.
Disclosure
The authors declare no personal or financial conflicts of interest in this work.
Additional information
Funding
References
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–294. doi:10.1046/j.1526-4637.2003.03034.x
- Green CR. Being present: the role of narrative medicine in reducing the unequal burden of pain. Pain. 2011;152(5):965–966. doi:10.1016/j.pain.2011.01.041
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–380; discussion 435–513. doi:10.1017/S0140525X97221485
- Fillingim RB, CD K, Ribeiro-Dasilva MC, Rahim-Williams B, JL R 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi:10.1016/j.jpain.2008.12.001
- Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4(1):24–30. doi:10.1007/s11916-000-0006-6
- Riley JL 3rd, Gilbert GH, Heft MW. Orofacial pain symptom prevalence: selective sex differences in the elderly? Pain. 1998;76(1–2):97–104. doi:10.1016/S0304-3959(98)00030-X
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. doi:10.1038/nrn3360
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31(43):15450–15454. doi:10.1523/JNEUROSCI.3859-11.2011
- Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083.
- Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull. 2017.
- Mapplebeck JCS, Dalgarno R, Tu Y, et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159(9):1752–1763. doi:10.1097/j.pain.0000000000001265
- Taves S, Berta T, Liu DL, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2015.
- Abdullah M, Chai PS, Chong MY, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272(2):214–219. doi:10.1016/j.cellimm.2011.10.009
- Wegner A, Elsenbruch S, Rebernik L, et al. Inflammation-induced pain sensitization in men and women: does sex matter in experimental endotoxemia? Pain. 2015;156(10):1954–1964. doi:10.1097/j.pain.0000000000000256
- Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1(12):1279–1283. doi:10.1038/nm1295-1279
- Sankaran-Walters S, Macal M, Grishina I, et al. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4(1):10. doi:10.1186/2042-6410-4-10
- Torgrimson BN, Minson CT. Sex and gender: what is the difference? J Appl Physiol. 2005;99(3):785–787. doi:10.1152/japplphysiol.00376.2005
- Robinson ME, Riley JL, Myers CD, et al. Gender role expectations of pain: relationship to sex differences in pain. J Pain. 2001;2(5):251–257. doi:10.1054/jpai.2001.24551
- Mayer KH, Bradford JB, Makadon HJ, Stall R, Goldhammer H, Landers S. Sexual and gender minority health: what we know and what needs to be done. Am J Public Health. 2008;98(6):989–995. doi:10.2105/AJPH.2007.127811
- Fredriksen-Goldsen KI, Cook-Daniels L, Kim H-J, et al. Physical and mental health of transgender older adults: an at-risk and underserved population. Gerontologist. 2013;54(3):488–500. doi:10.1093/geront/gnt021
- Motmans J, Meier P, Ponnet K, Female TG. Male transgender quality of life: socioeconomic and medical differences. J Sex Med. 2012;9(3):743–750. doi:10.1111/j.1743-6109.2011.02569.x
- Kenagy GP. Transgender health: findings from two needs assessment studies in Philadelphia. Health Soc Work. 2005;30(1):19–26. doi:10.1093/hsw/30.1.19
- Budge SL, Adelson JL, Howard KAS. Anxiety and depression in transgender individuals: the roles of transition status, loss, social support, and coping. J Consult Clin Psychol. 2013;81(3):545–557. doi:10.1037/a0031774
- Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84:22–29. doi:10.1016/j.socscimed.2013.02.019
- Owens MA, Parker R, Rainey RL, et al. Enhanced facilitation and diminished inhibition characterizes the pronociceptive endogenous pain modulatory balance of persons living with HIV and chronic pain. J Neurovirol. 2019;25(1):57–71. doi:10.1007/s13365-018-0686-5
- Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. doi:10.1097/j.pain.0000000000000160
- Cruz-Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014;66(7):1800–1810. doi:10.1002/art.38620
- Goodin BR, Pham QT, Glover TL, et al. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol. 2013;32(11):1117–1126. doi:10.1037/a0031592
- Herbert MS, Goodin BR, Bulls HW, et al. Ethnicity, cortisol, and experimental pain responses among persons with symptomatic knee osteoarthritis. Clin J Pain. 2017;33(9):820–826. doi:10.1097/AJP.0000000000000462
- Thompson KA, Terry EL, Sibille KT, et al. At the intersection of ethnicity/race and poverty: knee pain and physical function. J Racial Ethn Health Disparities. 2019;6:1131–1143.
- Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 2009;13(6):1160–1177. doi:10.1007/s10461-009-9593-3
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401.
- Speilberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
- Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi:10.1177/135910539700200305
- Ware JE Jr., Sherbourne CD, The MOS. 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002
- Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S. Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain. 2009;146(1):65–74. doi:10.1016/j.pain.2009.06.020
- Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
- Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. J Pain. 2014;15(5):550e551–510. doi:10.1016/j.jpain.2014.02.001
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
- Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manag. 2005;29(4):401–410. doi:10.1016/j.jpainsymman.2004.06.018
- Rosen SF, Ham B, Drouin S, et al. T-Cell mediation of pregnancy analgesia affecting chronic pain in mice. J Neurosci. 2017;37(41):9819–9827. doi:10.1523/JNEUROSCI.2053-17.2017
- Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi:10.1073/pnas.251140998
- Martin LJ, Acland EL, Cho C, et al. Male-specific conditioned pain hypersensitivity in mice and humans. Curr Biol. 2019;29(2):192–201. doi:10.1016/j.cub.2018.11.030
- Martin LJ, Hathaway G, Isbester K, et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol. 2015;25(3):326–332. doi:10.1016/j.cub.2014.11.028
- Igler EC, Defenderfer EK, Lang AC, Bauer K, Uihlein J, Davies WH. Gender differences in the experience of pain dismissal in adolescence. J Child Health Care. 2017;21(4):381–391. doi:10.1177/1367493517727132
- Miller MM, Allison A, Trost Z, et al. Differential effect of patient weight on pain-related judgements about male and female chronic low back pain patients. J Pain. 2017.
- Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5(2):77–82. doi:10.1016/j.jpain.2003.11.004
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3, Supplement):S2–S15. doi:10.1016/j.pain.2010.09.030
- Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8(11):893–901. doi:10.1016/j.jpain.2007.06.006
- Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2016;68(4):472–480. doi:10.1002/acr.22712
- Jensen MT, Petersen KL. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. J Pain. 2006;7(3):211–217. doi:10.1016/j.jpain.2005.10.013
- Goodin BR, Bulls HW, Herbert MS, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med. 2014;76(4):302–310. doi:10.1097/PSY.0000000000000058
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109(1):115–123. doi:10.1016/j.pain.2004.01.019
- Rametti G, Junque C, Zubiaurre-Elorza L, et al. Cortical thickness in untreated transsexuals. Cereb Cortex. 2012;23(12):2855–2862. doi:10.1093/cercor/bhs267
- Guillamon A, Junque C, Gomez-Gil E. A review of the status of brain structure research in transsexualism. Arch Sex Behav. 2016;45(7):1615–1648. doi:10.1007/s10508-016-0768-5
- Savic I, Arver S. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex. 2011;21(11):2525–2533. doi:10.1093/cercor/bhr032
- Pool CW, Swaab DF, Hofman MA, Kruijver FPM, Gooren LJG, Zhou J-N. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J Clin Endocrinol Metab. 2000;85(5):2034–2041. doi:10.1210/jcem.85.5.6564
- Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131(Pt 12):3132–3146. doi:10.1093/brain/awn276
- Hahn A, Kranz GS, Küblböck M, et al. Structural connectivity networks of transgender people. Cereb Cortex. 2014;25(10):3527–3534. doi:10.1093/cercor/bhu194
- Bao A-M, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32(2):214–226. doi:10.1016/j.yfrne.2011.02.007
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64(8):644–658. doi:10.1037/0003-066X.64.8.644
- Martin CL, Ruble D. Children’s search for gender cues: cognitive perspectives on gender development. Curr Dir Psychol Sci. 2004;13(2):67–70. doi:10.1111/j.0963-7214.2004.00276.x
- Nobili A, Glazebrook C, Arcelus J. Quality of life of treatment-seeking transgender adults: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2018;19(3):199–220. doi:10.1007/s11154-018-9459-y
