Abstract
Many attempts have been made to increase the duration of local anesthetic action. One avenue of investigation has focused on encapsulating local anesthetics within carrier molecules to increase their residence time at the site of action. This article aims to review the literature surrounding the recently approved formulation of bupivacaine, which consists of bupivacaine loaded in multivesicular liposomes. This preparation increases the duration of local anesthetic action by slow release from the liposome and delays the peak plasma concentration when compared to plain bupivacaine administration. Liposomal bupivacaine has been approved by the US Food and Drug Administration for local infiltration for pain relief after bunionectomy and hemorrhoidectomy. Studies have shown it to be an effective tool for postoperative pain relief with opioid sparing effects and it has also been found to have an acceptable adverse effect profile. Its kinetics are favorable even in patients with moderate hepatic impairment, and it has been found not to delay wound healing after orthopedic surgery. More studies are needed to establish its safety and efficacy for use via intrathecal, epidural, or perineural routes. In conclusion, liposomal bupivacaine is effective for treating postoperative pain when used via local infiltration when compared to placebo with a prolonged duration of action, predictable kinetics, and an acceptable side effect profile. However, more adequately powered trials are needed to establish its superiority over plain bupivacaine.
Introduction
Pain is a protective mechanism which has adaptive value, and the inability to experience pain has been linked to early mortality from accidental injuries or damage to joints.Citation1,Citation2 However, pain in the postoperative setting is an unwanted side effect of surgery directed to improve morbidity or mortality. The potential benefits of optimal postoperative pain control include: improved cardiac, respiratory, and gastrointestinal functions; fewer thromboembolic complications; improved arterial graft survival; fewer septic complications; reduced chronic post surgical pain; reduced mortality in high-risk patients; and reduced health care costs.Citation3 Opioids have been the cornerstone of relief for perioperative pain; however, opioids have numerous side effects including nausea, vomiting, respiratory depression, prolonged ileus, itching, tolerance, and development of opiate induced hyperalgesia.Citation4,Citation5
Increasingly, multimodal analgesia is used to reduce perioperative opiate requirements, thus potentially reducing opioid side effects and improving the quality of analgesia.Citation6,Citation7 Local anesthetics are increasingly used perioperatively via different routes as part of a multimodal regimen.Citation8 The use of bolus injection of local anesthetics is limited by duration of post operative pain relief with the average duration of block via interscalene injection being 8 to 12 hours with either bupivacaine 0.5% or ropivacaine 0.5% or 0.75%.Citation9 Local anesthetic infusions via catheters are used to increase the duration of postoperative analgesia;Citation10 however, placement and maintenance of perineural catheters involves additional trainingCitation11 in addition to the added cost of pumps.Citation12
Complications due to perineural catheters are infrequent but can be life threatening, and these complications can include infection, septicemia, intravascular placement, or intravascular catheter migration.Citation13 The development of new, long acting local anesthetics, like liposomal bupivacaine is potentially important in the management of perioperative pain. This article will review liposomal bupivacaine as a potential addition to the clinician’s analgesic armamentarium.
Liposomal bupivacaine
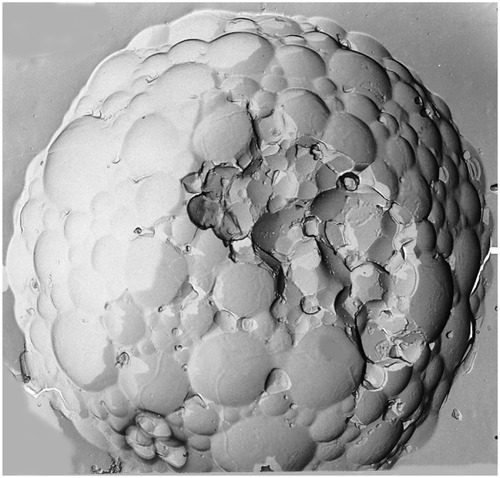
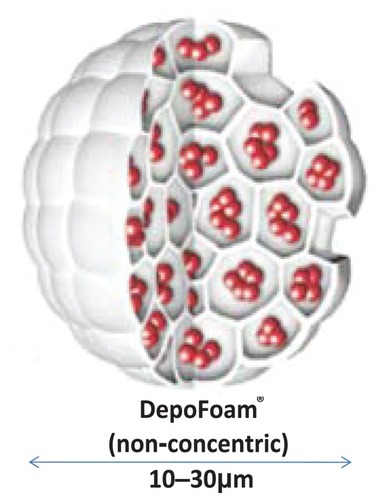
Liposomes are microscopic structures consisting of a phospholipid bilayer encapsulating an aqueous core. They may be unilamellar, multilamellar, or multivesicular. Unilamellar liposomes consist of a single lipid bilayer surrounding the aqueous core, whereas multilamellar liposomes consist of concentric lipid layers. Multivesicular liposomes (MVL), however, consist of nonconcentric lipid bilayers. The nonconcentric nature of MVL confers characteristic drug release patterns from the aqueous core that are different from the unilamellar and multilamellar liposomes, leading to increased stability and longer duration of drug release. The release of drug from the MVL requires only a breach in the external layer, and release of a drug from internal vesicles leads to redistribution of the drug within the particle without release. The multivesicular structure also ensures that the vesicles rearrange themselves without release of drug by internal fusion and division.Citation14,Citation15 These vesicles can encapsulate water soluble drugs in their core, and lipid soluble drugs within the membrane. They are used in the systemic delivery of antifungals, antineoplastics, and antibiotics.Citation16,Citation17 Currently available liposomal bupivacaine consists of vesicles of bupivacaine loaded in the aqueous chambers using DepoFoam® technology (Pacira Pharmaceuticals Inc, San Diego, CA). Each particle is composed of a honeycomb like structure of numerous internal aqueous chambers containing encapsulated bupivacaineCitation18,Citation19 ( and ).
Pharmacodynamics
Bupivacaine is an amide local anesthetic, which acts by inactivating voltage-dependent sodium channels. It has a pKa of 8.1 so only 15% is present in uncharged form at tissue pH. The uncharged fraction of bupivacaine travels across the cell membrane of the nerve, and once charged binds to the inner side of sodium channels, inactivating them.Citation20 The release of bupivacaine from its binding site is slow, which leads to a longer duration of action than lidocaine.Citation21
Pharmacokinetics
The pharmacokinetics of MVL bupivacaine have been studied in both animal and human models. Richard et alCitation18 compared MVL bupivacaine in doses of 9, 18, and 30 mg/kg with 9 mg/kg of plain bupivacaine injected by wound infiltration in rabbits. They found the Cmax to be dose dependent, being 107 ± 27.6, 222 ± 28.3, and 307 ± 148 ng/mL for the three doses of MVL bupivacaine, respectively. However, the Cmax was much lower than plain bupivacaine (620 ± 89.9 ng/mL). The plasma bupivacaine concentration in the group receiving plain bupivacaine peaked quickly compared to the MVL bupivacaine group: 1 ± 0 h compared to 12.5 ± 8.06, 7.0 ± 11.3, and 30.3 ± 22.5 hours for the three doses of MVL bupivacaine, respectively. Plasma bupivacaine concentrations were detectable in most animals (dogs) who received MVL bupivacaine 9 mg/kg over a 96-hour study period.
In the pharmacokinetic study of human volunteers, Davidson et alCitation22 compared subcutaneous injection of 20 mL of 2% liposomal bupivacaine versus 20 mL of 0.5% plain bupivacaine. They found no difference in the Cmax between the two groups (0.87 ± 0.45 versus 0.83 ± 0.34 in plain and liposomal groups, respectively) despite a 4-fold increase in bupivacaine dose and a 9.8-fold increase in the terminal half-life displayed by the liposomal bupivacaine group (131 ± 58 versus 1294 ± 860 min in plain and liposomal groups, respectively). The Tmax increased seven-fold in the liposomal bupivacaine group compared to the group administered plain bupivacaine, which was attributable to the slow release of liposomal bupivacaine. The attributes of slow release leading to prolonged Tmax and long T ½ leading to prolonged detectable plasma concentration of liposomal bupivacaine have been confirmed in a subsequent Phase II, multicenter clinical trial conducted by Langford et al.Citation23
Bupivacaine is metabolized mainly in the liver by glucuronide conjugation and hepatic N-dealkylation into pipecolylxylidine. Pipecolylxylidine is then hydroxylated and forms glucuronide conjugates. A small amount of bupivacaine is excreted unchanged in urine.Citation24 In a pharmacokinetic study of liposomal bupivacaine in patients with moderate hepatic impairment, Onel et alCitation25 found that although bupivacaine and pipecolylxylidine concentrations were higher in patients with moderate hepatic impairment than in patients with normal hepatic function, the concentration time plots were similar in both groups, and the differences were small enough not to warrant dose adjustments as per Food and Drug Administration (FDA) guidelines.
Efficacy in postoperative pain
Liposomal bupivacaine has been FDA approved for single dose wound infiltration in postoperative pain relief among patients undergoing hemorrhoidectomy and bunionectomy.Citation26 Gorfine et alCitation27 conducted a multicenter, randomized, double blind, placebo-controlled trial in patients undergoing hemorrhoidectomy. At the end of surgery, patients were randomized to receive either 300 mg (30 mL) extended release MVL bupivacaine or placebo (30 mL of 0.9% sodium chloride) in 5 mL increments via wound infiltration. Intraoperative use of all analgesics or local anesthetics, except fentanyl, was prohibited unless needed for the treatment of adverse effects. Patients remained at the study center for 72 hours, and were administered postsurgical analgesia in response to breakthrough pain consisting of morphine sulfate as needed.
The primary outcome measure consisted of a cumulative pain score in the first 72 hours as reflected in the AUC0–72 (area under the curve) numerical rating score (NRS) of pain intensity. Secondary efficacy measures consisted of assessing the proportion of patients who received no opioid rescue medications, total amount of opioid rescue medications consumed, time to first postsurgical use of rescue medications, and the patient’s rating of satisfaction with postsurgical analgesia. The researchers found the pain scores to be markedly lower in the bupivacaine extended release group compared to those receiving the placebo with a least mean square (SE) AUC ranging from 0 to 72 hours of 141.8 (10.7) in the MVL bupivacaine group versus 202.5 (10.7) in the placebo group (P < 0.0001). In the bupivacaine extended release group, 59% of patients were opioid free at 12 hours, and 28% were opioid free at 72 hours when compared to 14% and 10% in the placebo group, respectively (P < 0.0008 through 72 hours). In addition, the mean total amount of opioid consumed was lower in the MVL bupivacaine group (22.3 mg vs 29.1 mg, P ≤ 0.0006), and the median time to first opioid use was longer (14.3 hours vs 1.2 hours with P < 0.0001) and was associated with greater patient satisfaction with postoperative analgesia (95% vs 73%, P = 0.0007) when compared to placebo.
Golf et alCitation28 conducted a multicenter, parallel group, placebo controlled, randomized, double blind study in which they compared extended release MVL bupivacaine to placebo in patients undergoing bunionectomy. The patients underwent primary first metatarsal bunionectomy under midazolam and/or propofol sedation with Mayo block with up to 25 mL of 2% lidocaine with epinephrine. Within 30 minutes after injection of lidocaine, the patients received either a single dose of 120 mg (8 mL) extended release bupivacaine or placebo (8 mL 0.9% sodium chloride) by local infiltration. Patients were observed for 24 hours at the study center. Rescue analgesia consisted of 5 mg oxycodone/325 mg acetaminophen tablets up to a maximum of 12 tablets per day with a single dose of intravenous ketorolac 15–30 mg as a second rescue. The primary outcome measure was the AUC of NRS pain scores through 24 hours. Secondary outcome measures consisted of: the proportion of patients who received no rescue pain medications; AUC of NRS pain scores through 36, 48, 60, and 72 hours; the proportion of patients who were pain free during the observation period; the time to first rescue medication use; and total oxycodone/acetaminophen consumption through 24, 36, 48, 60, and 72 hours. The researchers found markedly reduced pain intensity scores at 24 and 36 hours post injection in the MVL bupivacaine group compared to placebo (P = 0.0005 and P = 0.0229 at 24 and 36 hours) with no difference at 48 hours (P = 0.1316). The percentage of patients who were pain free showed a statistically significant difference at 2, 4, 8, and 48 hours only in the MVL bupivacaine group (P < 0.05), with more patients in the MVL bupivacaine group not receiving any rescue pain medication through 24 hours only (P < 0.05). The time to first opioid use was longer (7.2 hours vs 4.3 hours, P < 0.0001), and fewer mean total number of oxycodone/acetaminophen tablets were used through 24 hours (3.8 vs 4.7 tablets, P = 0.0077) in the MVL bupivacaine group compared to the placebo group.
Smoot et alCitation29 conducted a randomized, multicenter, double blind, parallel group, active control study comparing MVL bupivacaine 300 mg to bupivacaine HCl 100 mg (bupivacaine 0.5% with epinephrine 1:200,000) in patients undergoing bilateral cosmetic submuscular breast augmentation. At the end of the surgical procedure, the patients received either 300 mg of MVL bupivacaine or 100 mg of bupivacaine HCl (with epinephrine) on each side, injected locally at the breast implant pockets at the end of surgery. Postoperatively, the patients received 1000 mg of acetaminophen three times daily with rescue analgesia (oxycodone) for breakthrough pain through 96 hours. The primary outcome measure was the AUC of NRS pain scores through 72 hours. Secondary outcomes consisted of cumulative pain scores at time points other than 72 hours, proportion of patients not requiring rescue analgesia, total amount of rescue opioid medication consumed, and integrated rank assessment through multiple time points.
The mean cumulative pain score (numeric rating score with activity through 72 hours) was not significantly different in the two groups (441.5 in the MVL bupivacaine group vs 468.2 in the bupivacaine HCl group, P = 0.3999). The lack of a difference was attributed to a lack of statistical power. The NRS pain score with activity mean (SE) was markedly lower in the MVL bupivacaine group at 8 and 12 hours [4.9 (0.41) and 5.6 (0.40)] compared with the bupivacaine HCl group [6.7 (0.40) and 6.9 (0.37), P = 0.0016 and 0.0143, respectively]. The difference in mean (SE) pain scores at rest was also lower in the MVL bupivacaine group at 8 hours only compared to the bupivacaine HCl group [3.5 (0.35) vs 5.0 (0.34) respectively (P = 0.027)]. The total amount of postsurgical rescue opioid medication used at 24 and 48 hours was also lower in the MVL bupivacaine group compared to the bupivacaine HCl group (P = 0.0211 and 0.0459, respectively).
Bramlett et alCitation30 performed a randomized, double blind study comparing wound infiltration of MVL bupivacaine to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. They compared 150 mg of bupivacaine HCl (with 1:200,000 epinephrine) to MVL bupivacaine in doses of 133 mg, 266 mg, 399 mg, and 532 mg. The patients were between 18–75 years old and were classified as American Society of Anesthesiologists physical status 1–3 patients undergoing unilateral knee replacement under general anesthesia. For 24 hours prior to surgery, all patients received 1000 mg of acetaminophen three times daily. Intraoperatively, only intravenous fentanyl use was permitted. The study medications were diluted in 60 mL of 0.9% saline and were injected via local infiltration in the deep tissues, the capsulotomy incision, and the subcutaneous tissues intraoperatively. Postoperatively, patients received a single dose of a nonsteroidal anti-inflammatory drug parentally with oral acetaminophen. For rescue analgesia, patient-controlled intravenous morphine was used until patients could be switched to oral oxycodone 5–10 mg every 4–6 hours once oral intake was established. The primary outcome measure was AUC of NRS pain scores with activity (NRS-A) through day 4. Secondary outcome measures consisted of: AUC of NRS-A through time points other than day 4; AUC of NRS pain scores at rest (NRS-R); NRS-R and NRS-A scores at each assessed time point; total consumption of opioid rescue medications; total consumption of opioid medications; time to resumption of daily activities; and provider’s satisfaction with postoperative analgesia on day 8.
There was no difference between the groups for the primary outcome measure of the mean AUC of NRS pain scores with activity. The mean (SD) scores were 20.4 (3.9) in the bupivacaine HCl group versus 19.1 (4.4), 18.8 (5.3), 19.5 (5.3), and 20.7 (5.4), in the MVL bupivacaine 532 mg, 399 mg, 266 mg, and 133 mg groups, respectively. There was no detectable difference in the groups with regard to mean numeric rating scale pain scores, total consumption of rescue opioids, or the time to resumption of work or normal daily activities ().
Table 1 Studies comparing the efficacy of MVL bupivacaine versus placebo or bupivacaine HCl
Boogaerts et alCitation31 compared 0.5% bupivacaine (with 1:200,000 epinephrine) with 0.5% liposomal bupivacaine (a multilamellar formulation different from the clinically available multivesicular DepoFoam) administered epidurally for the management of postsurgical pain. The patients were classified as American Society of Anesthesiologists physical status 2 and 3 undergoing major abdominal surgery. The epidural catheter was inserted with a test dose of bupivacaine (0.5% 3 mL) with epinephrine 1:200,000 given at the time of insertion. Postoperatively, when patients experienced pain after complete recovery of motor function, they received a 10 mL bolus of either liposomal bupivacaine 0.5% or 10 mL of plain bupivacaine 0.5% (with 1:200,000 epinephrine). The researchers found no detectable difference in the time of onset of analgesia (13.75 ±1.25 min in the plain bupivacaine group versus 13.92 ± 1.58 min in the liposomal bupivacaine group), though the duration of analgesia increased significantly in the liposomal bupivacaine group (6.25 ± 1.13 hours in the liposomal bupivacaine group versus 3.2 ± 0.4 hours in the plain bupivacaine group, P < 0.05). In a subset of patients who underwent abdominal aortic surgery, the duration of analgesia was 10.6 ± 1.4 hours in the liposomal bupivacaine group versus 2.42 ± 0.35 hours in the plain bupivacaine group (P < 0.001). There was no motor block in the liposomal bupivacaine group though intraoperative surgical anesthesia was not observed with the liposomal bupivacaine group. The lack of surgical block was thought to be due to alterations in the pharmacodynamics of the drug preventing the necessary amount of free bupivacaine available at the site of action, thus producing only postsurgical analgesia. There are no studies evaluating the epidural use of DepoFoam bupivacaine to assess whether the lack of surgical analgesia is seen with the DepoFoam formulation as well.
Safety
Bupivacaine may produce many adverse effects. The most common life threatening side effects involve the cardiovascular and central nervous systems.Citation32,Citation33 Bupivacaine is more cardiotoxic than lidocaine, and it produces its toxicity by producing cardiac conduction block.Citation34 Animal studies have shown that bupivacaine uncouples oxidative phosphorylation, may induce apoptosis in muscle cells, and may cause Schwann cell damage. The damage to Schwann cells happens in both a time as well as a concentration dependent fashion.Citation35 The myotoxicity of bupivacaine is well described and may be related to Ca2+-induced apoptosis of muscle cells. The myotoxicity of bupivacaine is most pronounced after retrobulbar and peribulbar blocks with an overall incidence of anesthesia-related diplopia reported to be 0.25%.Citation36,Citation37 Although the diplopia may resolve spontaneously, it may require surgical correction.Citation38
The most common side effects of MVL bupivacaine in clinical trials included nausea, vomiting, constipation, pyrexia, dizziness, and headache.Citation28,Citation30 Bergese et alCitation39 compared the cardiac safety of MVL bupivacaine in four doses (150, 300, 450, or 600 mg) to bupivacaine HCl with epinephrine injected via wound infiltration intraoperatively in patients undergoing total knee arthroplasty. They found no significant differences in change from baseline in QRS or QTc duration in the two groups, nor did the two groups differ in mean change from baseline heart rate and PR interval. Naseem et alCitation40 examined the effect of four doses of MVL bupivacaine (300, 450, 600, and 750 mg) injected subcutaneously on the QTc interval in healthy volunteers. None of the participants receiving MVL bupivacaine had a maximum QTc interval greater than 500 ms, and there were no changes in QTc of greater than 60 ms at any measured time point.
In a 2-year follow up study assessing the effect of MVL bupivacaine on the integrity of breast implants after augmentation mammoplasty, Minowitz et alCitation41 found no negative impact of intraoperative use of MVL bupivacaine on the integrity of breast implants. Local anesthetics have inhibitory effects on platelet aggregation in response to different agonists. Pinto et alCitation42 studied the effect of multilamellar liposomal local anesthetics on the inhibition of platelet aggregation in response to adenosine diphosphate. They found that encapsulation of local anesthetics into liposomes increased the inhibitory effect of local anesthetics; however, the clinical impact (if any) of this finding remains to be seen in larger trials. In an animal studies by Richard et alCitation18,Citation19 evaluating the safety and efficacy of MVL bupivacaine compared to plain bupivacaine and saline, the authors did find granulomatous inflammation in the MVL bupivacaine group, which was considered to be a normal reaction to liposomes; however, there was no effect on wound healing. MVL bupivacaine did not alter wound healing or wound scarring when used for postsurgical analgesia after total knee arthroplasty in humans.Citation30
DepoFoam® should not be coadministered with any other local anesthetic as it may increase the release of bupivacaine from the liposomes. It should not be allowed to come in contact with antiseptics like chlorhexidine or povidine iodine as they may disrupt the lipid layers leading to uncontrolled release of bupivacaine.Citation43
Discussion
DepoFoam-encapsulated bupivacaine is a new formulation of bupivacaine that provides slow sustained release of bupivacaine from multivesicular liposomes. Compared to placebo, it has been shown to produce prolonged analgesia with an opioid sparing effect, although more adequately powered trials are needed to assess its efficacy and duration of analgesia compared to standard local anesthetic solutions. At present, it is approved by the FDA for use via local infiltration after bunionectomy and hemorrhoidectomy. It has not been shown to be more toxic compared to plain bupivacaine, and it does not have markedly different cardiac effects than plain bupivacaine. It appears safe for use in patients with moderate hepatic impairment and does not warrant dose adjustment in that group.Citation25 It has not been evaluated for use via intrathecal, epidural, or perineural administration or in pediatric and pregnant patients.Citation43 More multicenter trials are needed to evaluate its efficacy and safety in these populations. If its safety and efficacy are established for epidural, intrathecal, and perineural use, it holds a potentially valuable place in the analgesic arsenal for use against postoperative pain and may substantially reduce the cost and complications associated with catheter and local anesthetic infusion pumps. In addition, the opioid sparing effects of MVL bupivacaine are valuable in potentially reducing opioid-related side effects. This in turn may reduce unwanted hospital admissions related to postoperative pain or opioid side effects.
In summary, the current literature studying MVL bupivacaine has, in general, demonstrated prolonged analgesia and reduced opioid side effects compared to placebo. However, its increased analgesic efficacy (and cost effectiveness) compared to plain bupivacaine in various clinical settings needs to be evaluated in adequately powered clinical trials. At present, the literature supports only a limited role for MVL bupivacaine. This may change as larger studies are conducted.
Acknowledgements
and a copy of References Citation23, Citation25, Citation39, and Citation40 were provided by Pacira Pharmaceuticals, Inc, 5 Sylvan Way, Parsippany, NJ 07054. The company had no input in the preparation or editing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- AcerbiAParisiDThe evolution of painCostaFARochaLMCostaEHarveyICoutinhoAAdvances in Artificial LifeECAL 2007: Proceedings of the 9th Annual Conference on Advances in Artificial LifeSeptember 10–14, 2007Lisbon, PortugalBerlinSpringer2007816824
- LoeserJDMelzackRPain: an overviewLancet199935391641607106910334273
- BreivikHPain managementBaillieres Clin Anaesthesiol199484775795
- KoppertWSchmelzMThe impact of opioid-induced hyperalgesia for postoperative painBest Pract Res Clin Anaesthesiol2007211658317489220
- ChauDLWalkerVPaiLChoLMOpiates and elderly: use and side effectsClin Interv Aging20083227327818686750
- BuvanendranAKroinJSMultimodal analgesia for controlling acute postoperative painCurr Opin Anaesthesiol200922558859319606021
- DahlVRaederJCNon-opioid postoperative analgesiaActa Anaesthesiol Scand200044101191120311065198
- TuncerSAysolmazGReisliRErolAYalçinNYosunkayaAThe effects of the administration of subfacial levobupivacaine infusion with the ON-Q pain pump system on postoperative analgesia and tramadol consumption in cesarean operationsAgri2010222737820582749
- BorgeatAEkatodramisGAnaesthesia for shoulder surgeryBest Pract Res Clin Anaesthesiol200216221122512491553
- OsadaRZukawaMSekiEKimuraTContinuous peripheral nerve block in forearm for severe hand traumaHand Surg201116323924422072454
- ChellyJEDelaunayLWilliamsBBorghiBOutpatient lower extremity infusionsBest Pract Res Clin Anaesthesiol200216231132012491560
- RichmanJMLiuSSCourpasGDoes continuous peripheral nerve block provide superior pain control to opioids? A meta-analysisAnesth Analg2006102124825716368838
- ChellyJEGhisiDFanelliAContinuous peripheral nerve blocks in acute pain managementBr J Anaesth2010105Suppl 1i86i9621148658
- MantripragadaSA lipid based depot (DepoFoam technology) for sustained release drug deliveryProg Lipid Res200241539240612121719
- NewRRCInfluence of liposome characteristics on their properties and fatePhilippotJRSchuberFLiposomes as Tools in Basic Research and IndustryBoca RatonCRC Press1995320
- MowatJJMokMJMacLeodBAMaddenTDLiposomal bupivacaine. Extended duration nerve blockade using large unilamellar vesicles that exhibit a proton gradientAnesthesiology19968536356438853095
- OstroMJCullisPRUse of liposomes as injectable-drug delivery systemsAm J Hosp Pharm1989468157615872672806
- RichardBMOttLRHaanDThe safety and tolerability evaluation of DepoFoam bupivacaine (bupivacaine extended-release liposome injection) administered by incision wound infiltration in rabbits and dogsExpert Opin Investig Drugs2011201013271341
- RichardBMNewtonPOttLRThe safety of EXPAREL® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogsJ Drug Deliv [Serial on the Internet]12012 [cited July 2, 2012];962101:[10 p.]. http://www.hindawi.com/journals/jdd/2012/962101/Accessed July 2, 2012
- DullenkopfABorgeatALocal anesthetics. Differences and similarities in the “-cains”Anaesthesist200352432934012715136
- CovinoBGPharmacology of local anaesthetic agentsBr J Anaesth19865877017162425835
- DavidsonEMBarenholzYCohenRHaroutiunianSKaganLGinosarYHigh-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humansAnesth Analg201011041018102320357145
- LangfordRMChappellGMKarraschJAA single administration of DepoBupivacaine (TM) intraoperatively results in prolonged detectable plasma bupivacaine and analgesia in patients undergoing inguinal hernia repairPoster presented at: 62nd Annual Postgraduate Assembly of the New York State Society of AnesthesiologistsDecember 12–16, 2008New York, NY
- GantenbeinMAttoliniLBruguerolleBOxidative metabolism of bupivacaine into pipecolylxylidine in humans is mainly catalyzed by CYP3ADrug Metab Dispos200028438338510725304
- OnelEWarnottKLambertWPatouGPharmacokinetics of depobupivacaine (EXPAREL™), a novel bupivacaine extended release liposomal injection, in volunteers with moderate hepatic impairmentPoster presented at: 112th Annual Meeting of the American Society of Clinical Pharmacology and TherapeuticsMarch 3–6, 2011Dallas, TX
- US Food and Drug AdministrationFDA Label Approved on 10/28/2011 (PDF) for EXPARELUS Silver Spring, MDUS Food and Drug Administration Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022496s000lbl.pdfAccessed May 01, 2012
- GorfineSROnelEPatouGKrivokapicZVBupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trialDis Colon Rectum201154121552155922067185
- GolfMDanielsSEOnelEA phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomyAdv Ther201128977678821842428
- SmootJDBergeseSDOnelEWilliamsHTHeddenWThe efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammoplasty: a randomized, double-blind, active-control studyAesthet Surg J2012321697622179931
- BramlettKOnelEViscusiERJonesKA randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplastyKneeIn press1272012
- BoogaertsJGLafontNDDeclercqAGEpidural administration of liposome-associated bupivacaine for the management of postsurgical pain: a first studyJ Clin Anesth1994643153207946368
- AstraZeneca CanadaBupivacaine Prescribing InformationMississauga, ONAstraZeneca Canada Inc Available from: http://www.astrazeneca.ca/documents/ProductPortfolio/SENSORCAINE_PM_en.pdfAccessed April 28, 2012
- TuckerGTMatherLEClinical pharmacokinetics of local anaestheticsClin Pharmacokinet197944241278385208
- WolfeJWButterworthJFLocal anesthetic systemic toxicity: update on mechanisms and treatmentCurr Opin Anaesthesiol201124556156621841477
- YangSAbrahamsMSHurnPDGrafeMRKirschJRLocal anesthetic Schwann cell toxicity is time and concentration dependentReg Anesth Pain Med201136544445121857272
- ZinkWGrafBMLocal anesthetic myotoxicityReg Anesth Pain Med200429433334015305253
- Gómez-ArnauJIYangüelaJGonzálezAAnaesthesia-related diplopia after cataract surgeryBr J Anaesth200390218919312538376
- VlăduţiuCSevanSCiuicăMDiplopia through toxic myopathy after cataract surgeryOftalmologia20085247782
- BergeseSDOnelEMorrenMMorganrothJBupivacaine extended-release liposome injection exhibits a favorable cardiac safety profileReg Anesth Pain Med201237214515122266897
- NaseemAHaradaTWangDBupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteersJ Clin Pharmacol Epub November 4, 2011
- MinkowitzHSOnelEPatronellaCKSmootJDA two-year observational study assessing the safety of DepoFoam bupivacaine after augmentation mammaplastyAesthet Surg J201232218619322238339
- PintoLMPereiraRde PaulaEde NucciGSantanaMHDonatoJLInfluence of liposomal local anesthetics on platelet aggregation in vitroJ Liposome Res2004141–2515915461932
- Pacira Pharmaceuticals Inc.Exparel (Bupivacaine Liposome Injectable Suspension) Product MonographParsippany, NJPacira Pharmaceuticals, Inc Available from: http://www.exparel.com/pdf/Exparel_Monograph.pdfAccessed May 01, 2012