Abstract
Purpose
To examine if a concomitant relationship exists between cognition and pain in an elderly population residing in long-term care.
Background/significance
Prior research has found that cognitive load mediates interpretation of a stimulus. In the presence of decreased cognitive capacity as with dementia, the relationship between cognition and increasing pain is unknown in the elderly.
Patients and methods
Longitudinal cohort design. Data collected from the Minimum Data Set-Resident Assessment Instrument (MDS-RAI) from the 2001–2003 annual assessments of nursing home residents. A covariance model was used to evaluate the relationship between cognition and pain at three intervals.
Results
The sample included 56,494 subjects from nursing homes across the United States, with an average age of 83 ± 8.2 years. Analysis of variance scores (ANOVAs) indicated a significant effect (P < 0.01) for pain and cognition, with protected t test revealing scores decreasing significantly with these two measures. Relative stability was found for pain and cognition over time. Greater stability was found in the cognitive measure than the pain measure. Cross-legged effects observed between cognition and pain measures were inconsistent. A concomitant relationship was not found between cognition and pain. Even though the relationship was significant at the 0.01 level, the correlations were low (r ≤ 0.08), indicating a weak association between cognition and pain.
Conclusion
Understanding the concomitance of pain and cognition aids in defining additional frameworks to extend models to include secondary needs, contextual factors, and resident outcomes. Cognitive decline, as with organic brain diseases, is progressive. Pain is a symptom that can be treated and reduced to improve resident quality of life. However, cognition can be used to determine the most appropriate method to assess pain in the elderly, thereby improving accuracy of pain detection in this population.
Introduction
Pain control is a primary concern across all care settings. Though a universal care concern, pain is frequently viewed in the elderly as a normal process of aging.Citation1 It is estimated that 49%–83% of the 1.8 million residents in long-term care have acute or chronic pain, yet the recognition and treatment of pain still presents a challenge.Citation2–Citation6 Recognizing a spectrum of pain behaviors beyond the traditional self-reporting methods, and increasing this knowledge among clinicians and support staff, is still a significant challenge in the provision of care to the elderly.
Predominantly, pain and cognitive decline often coexist in the elderly, with approximately 47% of residents in nursing homes having a diagnosis of dementia.Citation2 Pain assessment and treatment is complex, because residents have varying degrees of cognitive function, complicating how their needs are communicated. When these symptoms do coexist, little is known about the interaction of pain and cognitive decline, beyond laboratory imaging of the brain from a pathophysiological perspective.Citation7,Citation8 Empirical studies both support and refute poor neurocognitive performance in conjunction with increased pain intensity.Citation9–Citation13 Evaluating longitudinal data to assess if a relationship exists between pain and cognitive decline may assist in addressing these ambiguous findings.
The aim of this research was to examine if concomitance exists between cognition and pain in the elderly residing in long-term care.
Research questions
In a sample of nursing home residents,
Is cognitive decline a predictor of increased pain?
Is increasing pain a predictor of cognitive decline?
Research evaluating the theoretical constructs of pain and its contributing factors is lacking. Theoretical modeling using clinical data is a method used to evaluate resident characteristics and symptoms for inter-relationships between variables. Modeling whether chronic pain leads to worsening or declining cognition, thereby contributing to worsened pain, would test the theoretical constructs of this relationship. The significance and correlation of these variables creates a foundation for building additional models, with secondary needs and resident outcomes. Long-term unresolved pain may lead to secondary symptoms and comorbidities. Information of the relationship between pain and cognition adds to an understanding of how resident outcomes occur, and how quality initiatives can be approached. These items are all fundamental to determine if resident care needs are being met.
Significance
Evaluating cognition in conjunction with pain helps clarify if treating either symptom lessens the severity of the other, or if the symptoms are independent. Organic brain disorders cause a progressive process of cognitive decline.Citation14 If it is not possible for patients to regain a normal level of cognitive function, then the process is degenerative. Pain may add to and/or potentiate symptoms of cognitive decline. Understanding the relationship between cognition and pain establishes how these two variables could be included in a theoretical framework. This enables resident outcomes to be more accurately measured through symptoms and treatments, determining the most effective and cost-conscious actions. If pain and cognition were parallel to and not antecedent of each other, a symptom model would be inaccurate, making it difficult to determine where and what symptoms could be treated. Neglecting to include variables as a predictor of the other yields an incomplete clinical picture and theoretical model, making it difficult to find and measure care solutions because the root causes were not fully described.
Understanding the clinical pathways and interrelationships of resident symptoms is essential to the strategic planning and prioritizing of resident care needs. Resource allocation in a struggling Medicare-funded system is a difficult process to navigate. A new National Institutes of Health (NIH) nursing home rating system incorporates pain as a quality measure, previously neglected in long-term resident care assessments.Citation15,Citation16 Staff assessments, resident nonverbal cues, verbal complaints, facial expressions, and protective body movements were added as additional assessment items to more fully convey pain in this population. The use of federally-mandated resident assessment surveys is a cost-effective, time-efficient tool to gain insight into resident care needs, and an opportunity to increase our understanding of resident symptom pathways and the effectiveness of interventions used. Using existing clinical data to test theoretical constructs adds valuable information to the validity of the models posited against real world, resident care data.
Background
Pain is an intricate sensory experience involving physiological, pathological, social, cognitive, and emotional factors.Citation17,Citation18 Sensory process is modulated by cognitive load.Citation19–Citation22 Cognitive load helps to describe how hard it is for the individual to make sense of a stimulus. Cognitive decline is progressive and may manifest as symptoms of aphasia (language), apraxia (perform directed acts), agnosia (recognize objects), and/or disturbances in global functioning (planning, organizing, sequencing, and abstract thoughts). Considerable issues exist in the detection of pain in residents with moderate-to-severe cognitive impairment. A lower incidence of pain is reported as cognition declines, largely due to measurement and communication issues.Citation23,Citation24 The detection of pain behavioral cues by both formal and informal caregivers have marked differences depending on the resident’s cognitive status, especially with the interpretation of body movements.Citation25
A case report presented by Ashpole and KatzCitation17 described a patient with a lifelong history of pain (somatoform pain disorder). The patient’s refractory pain was unresolved, causing daily verbal complaints of discomfort. After the onset of dementia, the patient’s self-reports of pain sharply declined. The pain symptoms were posited to be presenting as an altered mood (eg, depression or irritability) and cognitive decline. Chronic pain is attributed to increased risk of depression in the elderly.Citation26–Citation29 Depressive symptoms are linked to decreased processing and motor function, but are not directly attributable to memory impairment.Citation30 Chronic pain results in changes to the resident’s personality, social interactions, lifestyle, and functional status, impacting their quality of life.Citation27 Unresolved pain may result in a decline of the resident’s quality of life causing delirium, depression, weight loss, social isolation, decreased activities of daily living, impaired gait, increased incidence of falls and comorbidities. Quality of life declines with chronic untreated pain, especially as the intensity of pain increases.Citation27 To date, the relationship between cognition and pain has been evaluated in case reports and pathophysiological studies, but not as a large-scale analysis of concomitance.
Theoretical framework
The concept of need-driven behaviorsCitation31 and the framework extending this model to include the consequences of need-driven, dementia-compromised behaviorsCitation32 serves as the theoretical platform for this research study. The need-driven behavior, ie, pain, is a coexisting symptom to cognitive state, a background factor. Proximal issues, eg, a decline in physical state and social and environmental causes, precipitate improvement or exacerbation of the original need: resolving the resident’s pain.
The long-term consequence of unresolved, need-driven behaviors gives rise to additional behavioral symptoms and secondary unmet needs. The primary relationship between cognition and pain has been evaluated for this study. Future theoretical constructs, including the complete model, would further evaluate the relationship of secondary needs (ie, depression, weight loss, social isolation, higher risk of falls, decreased activities of daily living, impaired gait), and how appropriate interventions mitigate the occurrence of secondary needs. Appropriate interventions to primary needs could improve resident quality of life, use healthcare resources more efficaciously, and reduce staff burden. This theoretical framework enables the clinician to translate a complex system encompassing such factors as resident, caregiver, environment, and outcomes, into a measurable tool to improve care.
Methods
Design and sample
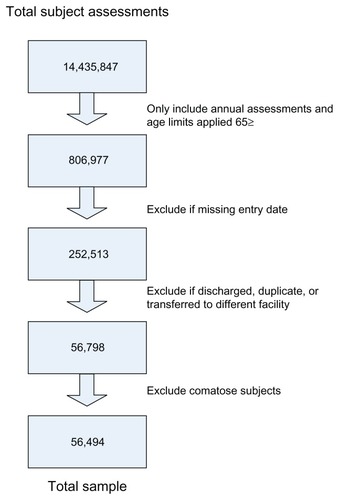
A longitudinal cohort design was used. Data were collected from 2001–2003 (inclusive) on residents residing in Medicare- receiving nursing homes across the United States. Minimum Data Set (MDS) 2.0Citation33 annual assessments were used as the data source, including all residents aged ≥65 years. Comatose residents were excluded from the sample, because key item sections (Sections B–F) could not be scored. These items are required for the pain index instrument used in this study. Noncompletion of the cognitive, communications/hearing, mood and behavior, and psychosocial well-being sections of MDS adheres to the instructions given to assessors completing the resident assessment forms.
Data were extracted from a deidentified resident database containing the MDS items. The sample yielded 56,494 subjects (see for sample methods). The University of Central Florida Institutional Review Board (IRB) assigned an exempt status to the study. Data collection was retrospective and no interventions were tested.
Instruments
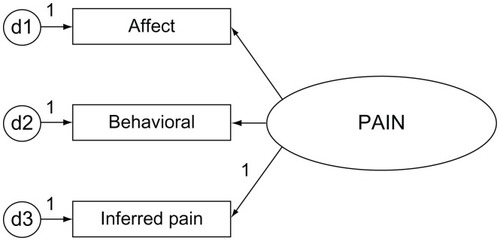
The MDS is a nationally required assessment providing information on the quality of care provided in nursing homes.Citation16 Core items from the MDS instrument are used for care planning to trigger events or symptoms requiring intervention (eg, pressure ulcers, delirium, cognitive loss, falls, and mood state). Pain is not a care-planning trigger from the Resident Assessment Protocol (RAP) however, it is a quality measure.Citation33 MDS items have demonstrated good-to-excellent validity and reliabilityCitation34–Citation36 with interrater and test–retest reliability from 0.40–0.80, depending on the item section.Citation34 A composite score was used to detect pain from core MDS items (pain items analyzed are detailed in ).
Table 1 Pain score items
The significance (P = 0.01) and validity of the measures used in the pain index have been established in a previous pilot study.Citation24 Pain scores could range from 0–34. Score weighting is determined by the ordinal scoring used in the MDS instrument. The pain index includes Fries’ pain scaleCitation37 (PS) items (eg, J2a for pain frequency and item J2b, pain intensity). The PS items highly correlated with a pain sites summary score.Citation24 Additional dimensions of affective and behavioral items are also included to aid in detecting pain across cognitive states ().
The Cognitive Performance Scale (CPS) was used to determine resident cognitive state. The CPS instrument uses key MDS items from sections B, C, and G of the resident assessment form.Citation38,Citation39 The CPS measure correlates highly (r ≥ 0.70) with the Folstein Mini-Mental Status Examination (MMSE).Citation40 The MDS-derived CPS scores were converted to MMSE average totals. The averaged scores could range from 0.04 (severe impairment) to 24.9, an intact cognitive state. A CPS score of 6 converts to an average MMSE of 0.4, a 3 to 15.4, and 0 to an MMSE of 24.9.Citation38 In validation testing of the CPS scores against the MMSE, a sensitivity of 0.94 and specificity of 0.94 were shown,Citation40 indicating that the utility of this instrument is viable in determining resident cognitive status from MDS-derived scores.
Statistical analysis
Descriptive statistics, correlations and repeated measures analyses of variance (ANOVAs) were completed using SPSS software (v 14.0; SPSS Inc, Chicago, IL). The SPSS statistical modeling program, AMOS (v 6.0; SPSS Inc), was used to build the covariance model of pain and cognitive state at three different time intervals for 2001, 2002, and 2003. Pain and cognition scores were hypothesized to be inversely related. Increasing pain score items indicated higher levels of pain. Cognitive decline was noted with a lower MMSE score. The analyses are one-tailed.
The covariance model was evaluated for integrity-of-fit statistics; however, the model is simplistic, with only six discrete measures and five residual terms, so fit statistics would indicate a recently identified model. Due to the required large sample size to run structural equation modeling, assessment of statistical power is complex.Citation41,Citation42 Sample size requirements generally are the number of free parameters (n = 17) times five to ten, to estimate sample size. The sample total (n = 56,494) far exceeds this rule.
Results
Select MDS items were collected on 56,494 subjects with a mean age of 83 years. In total, 80% of the sample was female, and 84% Caucasian. shows the study demographics.
Table 2 Demographic of resident characteristics
The most prevalent diagnosis was arthritis (33.7%) with 14.2% of the sample complaining of joint pain at the first data collection ().
Table 3 Diseases/events with potential pain symptoms
Over the 3-year period, the diagnoses of arthritis increased by 8%, and recorded joint pain dropped to 11.3%. Cognitive state did not fluctuate over the three measures observed. Cognition declined slightly over the 3-year period, as did pain ().
Table 4 Longitudinal chart of the cognitive and pain scores
The majority of the sample, 60%–67%, was moderately-to-severely cognitively impaired. A one-way repeated measure ANOVA was calculated for cognition and pain. Each variable compared subject scores at three different time intervals: 2001, 2002, and 2003. A significant effect was found for cognition (F(2,112986) = 5949.23; P < 0.01) and pain (F(2,112986) = 271.82; P < 0.01). Significant ANOVAs require a post-hoc analysis. Follow-up protected t-test with repeated measures was used, because of limitations of SPSS software to run a post-hoc analysis for within-subject factors.Citation43 A protected t-test between each measure inflates the risk of Type I errors, so a significance level of 0.017 was used (0.05/3 measures) instead of 0.05. The follow-up protected t-test revealed that scores decreased significantly (P < 0.017) for cognition1 (mean [M] = 14.5, standard deviation [SD] = 1.80), cognition2 (M = 13.7, SD = 8.1), and cognition3 (M = 12.8, SD = 8.3), and decreased significantly (P = 0.017) for pain1 (M = 2.4, SD = 2.9), pain2 (M = 2.34, SD = 2.8), and pain3 (M = 2.18, SD = 2.8).
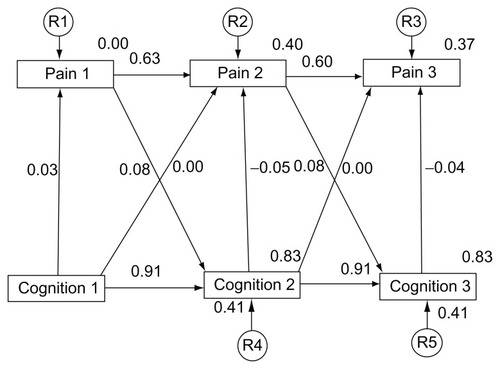
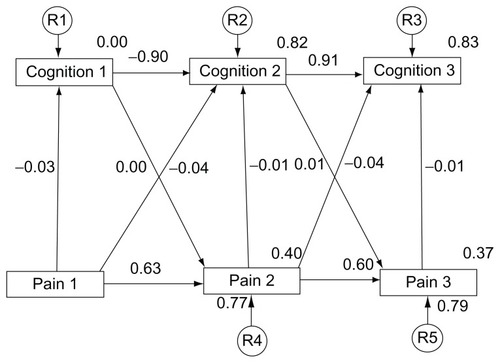
Regression weights of 1 were assigned to each residual variable. A residual term was not attached to Cognition1 (), because there was no predictor for these variables.
The covariance models indicate pain (1–3) and cognition (1–3) measurements were stable over time with previous measures being a good predictor of subsequent measures. Higher stability was observed with the cognitive measure than with the measure of pain. The cross-legged effect of both cognitive and pain measures was inconsistent. Very little association was found between cognition and pain variables, regardless of the time interval. A concomitant relationship was significant (P < 0.01), but the associations were weak and ranged from absolute values of 0.03 to 0.08 ().
Table 5 Correlations
Model 1 depicts cognitive decline as a predictor of increased pain, and Model 2 represents the inverse model, increasing pain as a predictor of cognitive decline. The root-mean-square residual (RMR) is the averaged squared amount by which the sample variances and covariances differ in their estimates.Citation42 A smaller RMR is preferred with a value of 0 indicating a perfect fit (see ).
Table 6 Goodness-of-fit statistics of the covariance model
The goodness-of-fit index (GFI), as it approaches 1 indicates a perfect fit. The optimal values yielded by the model for the GFI, Tucker–Lewis Index, and adjusted GFI could be attributed to the simplicity of the model, even though all three were approaching 1.0.
Discussion
The sample data do not confirm the presence of concomitance between pain and cognition in this long-term care population. The theoretical construct does not support either measure as a predictor of the other. These findings support Kovach’s model of consequences of need-driven, dementia- compromised behaviors (C-NDB).Citation32 Cognition (background factor) and pain (proximal factor) exist as contributing aspects of how need-driven behaviors are manifested and communicated. Kovach’s C-NDB model serves as the template for understanding how symptoms and environmental factors interact. This system contains environmental and contextual factors, affecting both resident and care outcomes. Failing to identify resident care needs is not in isolation of the resident, but is a complex system involving clinician, support staff, environmental factors, and the resident.
MDS can be used as a reliable tool to track resident characteristics and outcomes over time. Reporting was consistent for cognition and pain over the 3-year period, and considerable fluctuations in recorded values of cognition and pain did not occur. Because pain assessments were recorded annually, differences in pain would be anticipated. The findings showed a gradual decline in recorded pain over the 3-year period, as cognition also declined. This raises concern, because these findings may support previous research, indicating pain is underreported and undertreated in residents with cognitive decline.Citation44–Citation46
Reductions in pain scores at the third interval may also be attributable to residents having less pain, or residents having received appropriate interventions for their pain. Differences in pain would be expected with recent events like fracture, surgery, or falls. Partitioning this group of residents into a separate cohort could evaluate the consistency of pain reporting, and pain measures specific to these acute events. Until clinicians and support staff increase their awareness of affective, cognitive, and behavioral indicators of pain, the reliability of MDS for pain measures will continue to be a concern.
Results suggest the importance of assessing memory function when managing physiologically distressed residents, because this information aids in determining the best methods to assess resident pain.Citation26,Citation47,Citation48 Over the 3-year period, declines in cognitive status occurred which were consistent with the progression of organic brain disease. Acute declines in cognition may be indicative of a change in mental status not attributable to the progression of a pre-existing disease, but the onset of infection (ie, urinary tract infection, pneumonia, or sepsis), or psychiatric illness.
Further research could look at specific diagnoses and the consistency of cognitive decline and pain measures over time. Additional variables like the use of multiple medications (eg, polypharmacy), or certain classes of medications, ie, antipsychotics or hypnotics, may yield valuable information about the contributing factors to resident decline, and create an index of outcomes for pharmacoeconomic and clinical data supporting resident care guidelines and health policy reform. Supplemental theoretical modeling could evaluate latent growth models, with predictors combining pain, cognition, age, sex, and facility characteristics, enabling a greater understanding of pain and cognition in the elderly beyond this concomitance study. Additionally, research examining a growth curve model, plotting parallel points in time, would provide valuable information to trends in data distribution and would clarify if the model were polynomial.
One limitation of this method – that of using scores derived only from annual assessments of cognition and pain – is that it measures only a single point in time, and does not fully capture the day-to-day variation in resident scores. A linear relationship was statistically analyzed between pain and cognition. Additional research could examine the non-linear relationship of these two variables, to determine if a nonlinear relationship exists. Additionally, composite pain scores were used to increase the ease of score totaling from individual score items, to improve the use of the pain indicators in the long-term care setting. Further research is required to examine the effect of medication use by class, and how other comorbidities influence pain measures.
A further limitation of this research was the data distribution. Normality and population distribution were unequal. The majority of the population assessed was not experiencing pain, and cognitive groups were also unequal. While the population demographics are representative of nursing homes, very distinct population demographics (ie, sex, race, educational background, socioeconomic factors) limit generalizability beyond this setting. Variations in the interpretation of reliability measures between those rating the MDS sections for mood and behavior have been reported.Citation49,Citation50 The research was limited to the available items in MDS, and these items might not capture, define or describe all pain symptoms. Even with the further criteria added to measure pain across cognitive states, there remain dimensions of pain still to be discovered and defined.
Table 7 Goodness-of-Fit statistical terms
Conclusion
This research sought to ascertain whether in fact a relationship exists between pain and cognition, and if so, to gain preliminary insight into the relationship. Investigating whether cognition is a predictor of pain in a concomitant relationship helped to define how secondary patient outcomes might be mediated. Further research should be used to link cognition, resident ability to communicate, and levels of pain for significance with quality of life measures like depression, disturbances in gait, weight loss, decreased activity, declines in functional status, or social isolation. In the case of most organic brain diseases, instead of returning to a normal level of cognitive functioning, a progressive decline occurs. Pain is a cycle that can be intervened upon, and symptoms can be lessened through medicinal and non-medicinal treatments, improving resident comfort. With an understanding of the role of cognition in identifying how pain is communicated, we can improve pain detection and uniformity of measures to ameliorate symptoms. The significance of confirming, theoretical frameworks with advanced multivariate analysis is an opportunity to evaluate interactions of key variables. A global assessment of concomitance between pain and cognition offers a unique insight towards a better understanding of the relationship of pain and cognition in a general nursing home population.
Acknowledgments
This research could not have been possible without the assistance of Seung Chun Paek, MS, PhD, Research Associate, Korean Health Insurance Corporation, Seoul, Korea, for running the dataset parameters and queries. Thank you to Drs Steven Talbert and Diane Andrews at the University of Central Florida, College of Nursing for their reviews and edits of this manuscript. This research was completed at the University of Central Florida, College of Nursing in partnership with the College of Health and Public Affairs, Orlando, Florida and funded by the UCF Provost Fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
- CannPPain in older adults should not be seen as part of ageingBr J Community Nurs2008131257457619060835
- American Health Care AssociationOSCAR Data Reports: Patient CharacteristicsAHCA Available at: http://www.ahca.org/research/oscar_patient.htmAccessed April 15, 2006
- EppsCDRecognizing pain in the institutionalized elder with dementiaGeriatr Nurs2001222717911326213
- LoweSCensus Bureau releases new data on residents of adult correctional facilities, nursing homes and other group quarters annual data also pain diverse portrait of nation’s race, ethnic and ancestry groupU.S. Census Bureau Available at: http://www.census.gov/Press-Release/www/releases/archives/american_community_survey_acs/010709.htmlAccessed November 27, 2007
- SawyerPLillisJPBodnerEVAllmanRMSubstantial daily pain among nursing home residentsJ Am Med Dir Assoc20078315816517349944
- ParmeleePASmithBKatzIRPain complaints and cognitive status among elderly institution residentsJ Am Geriatr Soc19934155175228486885
- SeminowiczDAMikulisDJDavisKDCognitive modulation of pain-related brain responses depends on behavioral strategyPain20041121/2485815494184
- BrommBBrain images of painNews in Physiological Sciences20011624424911572930
- LandrøNIStilesTCSletvoldHMemory functioning in patients with primary fibromyalgia and major depression and healthy controlsJ Psychosom Res19974232973069130186
- GraceGMNeilsonWRHopkinsMBergMAConcentration and memory deficits in patients with fibromyalgia syndromeJ Clin Exp Neuropsychol199921447748710550807
- IezziTDuckworthMPVuongLNArchibaldYMKlinckAPredictors of neurocognitive performance in chronic pain patientsInt J Behav Med2004111566115194520
- KewmanDGVaishampayanNZaldDHanBCognitive impairment in musculoskeletal pain patientsInt J Psychiatry Med19912132532621955277
- DuftonBDCognitive failure and chronic painInt J Psychiatry Med19891932912972807747
- PsychnetOrganic brain syndromePsychnet-UK Available at: http://www.psychnet-uk.com/dsm_iv/organic_brain_syndrome.htmAccessed February 18, 2009
- CMS to create nursing home quality new five-star system to be added to nursing home compare sitePress ReleasesCMS Office of Public Affairs Available at: http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=3163&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=dateAccessed February 9, 2012
- Centers for Medicare and Medicaid ServicesAction plan for the further improve of nursing home qualityCMS Available at: http://www.cms.hhs.gov/CertificationandComplianc/Downloads/2008NHActionPlan.pdfAccessed February 9, 2012
- AshpoleEKatzBEffect of dementia on pain: A case reportAustralas J Ageing19971627172
- ChapmanCRThe affective dimension of pain: a modelBrommBDesmedtJEPain and the Brain: From Nociception to CognitionNew York, NYRaven Press1995283301
- KastnerSPinskMADe WeerdPDesimoneRUngerleiderLGIncreased activity in human visual cortex during directed attention in the absence of visual stimulationNeuron199922475176110230795
- KastnerSDe WeerdPDesimoneRUngerleiderLGMechanisms of directed attention in the human extrastriate cortex as revealed by functional MRIScience199828253861081119756472
- PessoaLKastnerSUngerleiderLGNeuro-imaging studies of attention: from modulation of sensory processing to top-down controlJ Neurosci200323103990399812764083
- SeminowiczDADavisKDPain enhances functional connectivity of a brain network evoked by performance of a cognitive taskJ Neurophysiol20079753651365917314240
- SengstakenEAKingSAThe problems of pain and its detection among geriatric nursing home residentsJ Am Geriatr Soc19934155415448486889
- BurfieldAHCohort study of pain behaviors in the elderly residing in skilled nursing careDiss. University of Central Florida, 2009Orlando, FLProQuest-CSA, LLC2010
- ClossSJCashKBarrBBriggsMCues for the identification of pain in nursing home residentsInt J Nurs Stud200542131215582634
- HadjistavropoulosTVoyerPSharpeDVerreaultRAubinMAssessing pain in dementia patients with co-morbid delirium and/or depressionPain Manag Nurs200892485418513661
- ZanocchiMMaeroBNicolaEChronic pain in a sample of nursing home residents: prevalence, characteristics, influence on quality of life (QOL)Arch Gerontol Geriatr200847112112818006088
- LeongIYNuoTHPrevalence of pain in nursing home residents with different cognitive and communicative abilitiesClin J Pain200723211912717237660
- BarsevickAMThe elusive concept of the symptom clusterOncol Nurs Forum200734597198017878126
- BauneBTSuslowTEngelienAAroltVBergerKThe association between depressive mood and cognitive performance in an elderly general population – the MEMO studyDement Geriatr Cogn Disord200622214214916741362
- AlgaseDLBeckCKolanowskiANeed-driven dementia-compromised behavior: An alternative view of disruptive behaviorAm J Alzheimer’s Dis Other Demen19961161219
- KovachCRNoonanPESchlidtAMWellsTA model of consequences of need-driven, dementia-compromised behaviorJ Nurs Scholarsh200537213414015960057
- Centers for Medicare and Medicaid ServicesMDS 2.0 for Nursing HomesCMS Available at: http://www.cms.hhs.gov/NursingHomeQualityInits/20_NHQIMDS20.aspAccessed June 23, 2008
- WonAMorrisJNNonemakerSLipsitzLAA foundation for excellence in long-term care: the Minimum Data SetAnn Long-Term Care1999739297
- SnowdenMMcCormickWRussoJValidity and responsiveness of the Minimum Data SetJ Am Geriatr Soc19994781000100410443863
- LawtonMPCastenRParmeleePAVan HaitsmaKCornJKlebanMHPsychometric characteristics of the Minimum Data Set II: validityJ Am Geriatr Soc19984667367449625190
- FriesBESimonSEMorrisJNFlodstromCBooksteinFLPain in U.S. nursing homes: validating a pain scale for the Minimum Data SetGerontologist200141217317911327482
- MorrisJNFriesBEMehrDRMDS cognitive performance scaleJ Gerontol1994494M174M1828014392
- FriesBENew opportunities for research in cognitive aging: the National Nursing Home Resident Assessment Instrument. Division 20American Psychological AssociationInstitute of Gerontology, University of Michigan Available at: http://apadiv20.phhp.ufl.edu/fries.htmAccessed February 9, 2012
- Gruber-BaldiniALZimmermanSIMortimoreEMagazinerJThe validity of the Minimum Data Set in measuring the cognitive impairment of persons admitted to nursing homesJ Am Geriatr Soc200048121601160611129749
- KaplanDStatistical power in structural equation modelsDepartment of Educational Studies, University of Delaware Available at: http://www2.gsu.edu/~mkteer/power.htmlAccessed February 9, 2012
- WanTTHEvidence-Based Health Care ManagementBoston, MAKluwer Academic Publishers2002
- CronkBCHow to Use SPSS3rd edGlendale, CAPyrcazk Publishing2004
- HuttEBuffumMDFinkRJonesKRPepperGAOptimizing pain management in long-term care residentsGeriatrics Aging [serial online]200710523527 Available at: http://www.medscape.com/viewarticle/564630_2Accessed November 7, 2007
- TaitRCChibnallJTUnder-treatment of pain in dementia: assessment is keyJ Am Med Dir Assoc20089637237418585637
- HorgasALYoonSLPain managementCapezutiEZwickerDFulmerTTGray-MiceliDEvidence-Based Geriatric Nursing Protocols for Best Practice3rd edNew York, NYSpringer Publishing Co2007199222
- ShegaJWRudyTKeefeFJPerriLCMenginOTWeinerDKValidity of pain behaviors in persons with mild to moderate cognitive impairmentJ Am Geriatr Soc20085691631163718662203
- HorgasALNicholsALSchapsonCAVietesKAssessing pain in persons with dementia: relationships among the non-communicative patient’s pain assessment instrument, self-report, and behavioral observationsPain Manag Nurs200782778517544127
- HawesCMorrisJNPhillipsCDMorVFriesBENonemakerSReliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS)Gerontologist19953521721787750773
- CastenRLawtonMPParmeleePAKlebanMHPsychometric characteristics of the Minimum Data Set I: confirmatory factor analysisJ Am Geriatr Soc19984667267359625189