Abstract
Background
The purpose of this study was to assess select medication utilization prior to duloxetine initiation among patients with major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, and musculoskeletal pain associated with osteoarthritis or low back pain.
Methods
Commercially insured duloxetine initiators between January 1, 2007 and March 31, 2010 were identified from a large US administrative claims database. Disease subgroups were constructed based on diagnosis from medical claims during the 12 months prior to duloxetine initiation. Prior use of antidepressants, anticonvulsants, opioids, nonsteroidal anti-inflammatory drugs, and muscle relaxants was assessed during the 12-month preinitiation period.
Results
This study identified 56,845 (2007), 44,838 (2008), and 65,840 (January 2009 to March 2010) duloxetine initiators. Among the 2009 initiators, utilization patterns were similar for patients with major depressive disorder and generalized anxiety disorder, with antidepressants being the most used (84% and 80%, respectively), followed by opioids (58% and 55%, respectively). Patients across pain-related conditions also had similar utilization patterns, with opioid use being the highest (76%–82%), followed by antidepressants (65%–72%). Use of other medication classes was common (29%–63%) but less frequent, and over 50% of the patients used any antidepressants, 70% used any antidepressants or anticonvulsants, and 90% used any antidepressants, anticonvulsants, or opioids. Trends in the use of these select medications were similar between 2007 and 2009.
Conclusion
Patients used several types of medications over the 12 months prior to initiating duloxetine across disease states, with antidepressants and opioids being the most frequently used medications. Trends of select medication use were similar over time.
Introduction
Duloxetine, a serotonin-norepinephrine reuptake inhibitor, received approval from the US Food and Drug Administration for the treatment of major depressive disorder (2004), generalized anxiety disorder (2007), and several chronic pain conditions, ie, diabetic peripheral neuropathic pain (2004), fibromyalgia (2008), and chronic musculoskeletal pain associated with osteoarthritis and chronic low back pain (2010).Citation1 Many studies have examined the efficacy and safety of duloxetine in a clinical trial setting over the past 10 years.Citation2–Citation11 More recently, the use of duloxetine in real-world practice for specific medical conditions has been assessed.Citation12–Citation21 However, no known published study has reported the pattern of medication use prior to duloxetine initiation for patients across the above-mentioned conditions.
Several types of medications, including antidepressants, anticonvulsants, opioids, muscle relaxants, and nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended by the clinical guidelines for use by individuals with major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, or low back pain.Citation22–Citation28 The purpose of this study was to describe the utilization of these common medications during the 12 months prior to duloxetine initiation among commercially insured patients with major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, or low back pain using the most recent claims data, and to evaluate the change in medication use patterns over the past three years. Using a large commercial administrative claims database from the US, this study describes the demographic characteristics of patients who initiated duloxetine across indications and examines the use of select medications during the 12 months prior to duloxetine initiation over time.
Materials and methods
Data source
This is a retrospective study using administrative medical and pharmacy claims data between January 1, 2006, and March 31, 2010, from the Thomson Medstat MarketScan commercial claims database. The database captures patient enrolment, prescription medication, and inpatient and outpatient resource utilization information gathered from around 100 US payers; it also links paid claims and encounter data to detailed patient information over time. Enrolment records provide health plan enrollees’ demographics and health insurance plan characteristics, including age, gender, region of residence, plan type, and plan enrolment status. The medical service claims collect detailed information during inpatient and outpatient health care encounters, including date and place of service, provider type, provider payments, and International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes. Finally, the pharmacy claims file contains information on medications dispensed, including National Drug Code, dispense date, quantity, days supplied, and plan-paid and patient-paid amounts.
Sample selection
Patients who initiated duloxetine in 2007, 2008, and 2009 to March 2010 were identified. Initiation was defined as no duloxetine pill coverage in the previous 90 days. The corresponding dispense date for the initial duloxetine claim was denoted as the “index date”. Patients included were further required to have had at least one health care encounter for the following conditions in the 12 months prior to the index date, which was determined by the service date and associated ICD-9-CM diagnosis codes of the corresponding medical claims for major depressive disorder (ICD-9-CM: 296.2x, 296.3x), generalized anxiety disorder (ICD-9-CM: 300.02), diabetic peripheral neuropathic pain (ICD-9-CM: 250.6x, 357.2x), fibromyalgia (ICD-9-CM: 729.1x), osteoarthritis (ICD-9-CM: 715.xx), or low back pain (ICD-9-CM: 720.1x, 721.3x, 724.2x, 724.5x, 724.8x, 722.83, 724.02, 721.90, 722.10). Selected patients were also required to have at least 12 months of continuous enrollment prior to the index date and had to be 18–64 years old as of the index date.
Cohort construction
Annual cohorts of duloxetine initiators were created for 2007, 2008, and 2009 to March 2010 (referred to as the 2009 cohort). A patient could be included in multiple annual cohorts if s/he met the inclusion criteria in multiple years. All individuals were further assigned to six disease subcohorts (ie, major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, and low back pain) based on whether they had a medical claim for any of these conditions in the 12 months prior to the index date. If a patient had medical claims for multiple conditions, this individual was assigned to multiple disease subcohorts.
Study measures
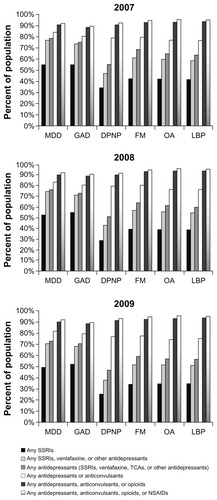
Medication utilization during the 12 months prior to duloxetine initiation was examined for the following therapeutic classes of medications individually: antidepressants, anticonvulsants, opioids, muscle relaxants, and NSAIDs (). In addition, use of medications from any of the following six selected lists of drug classes was also examined: any selective serotonin reuptake inhibitors; any selective serotonin reuptake inhibitors, venlafaxine, or other antidepressants; any antidepressants (selective serotonin reuptake inhibitors, venlafaxine, tricyclic antidepressants, or other antidepressants); any antidepressants or anticonvulsants; any antidepressants, anticonvulsants, or opioids; and any antidepressants, anticonvulsants, opioids, or NSAIDs.
Analysis
Demographic characteristics including age, gender, geographic region, and health plan type were assessed for all duloxetine initiators in each study year. Comparisons of duloxetine initiators for demographic characteristics between study years were examined using the Chi-square test for categorical variables and the t-test for continuous variables. For the 2009 initiators, demographic characteristics were also assessed for each disease subcohort. Proportions of duloxetine initiators with select medication utilization in the 12 months prior to duloxetine initiation were reported for each disease subcohort and the study periods (2007, 2008, and 2009). Utilization was examined for the six selected lists of medication classes.
Results
Demographic characteristics
The study identified 56,845 duloxetine initiators in 2007, 44,838 in 2008, and 65,840 in 2009 (). The mean patient age ranged between 46.6 years (2009) and 47.1 years (2008), and about 75% of the patients were female. Close to half of the patients lived in the southern region, and approximately two-thirds were enrolled in a preferred provider organization health plan. Among the 2009 duloxetine initiators, the low back pain cohort (n = 27,781; 42.2%) had the most patients, followed by major depressive disorder (n = 19,546; 29.7%), while the diabetic peripheral neuropathic pain cohort was the smallest (n = 2,334; 3.5%). On average, patients with generalized anxiety disorder or major depressive disorder were the youngest (mean age 42.3 years and 45.2 years, respectively) while those with diabetic peripheral neuropathic pain or osteoarthritis were the oldest (mean age 53.9 years and 53.1 years, respectively). Over 70% of the patients in each of the disease subcohorts were female except for those with diabetic peripheral neuropathic pain (58.1%). The distribution by region or health plan type was similar across cohorts. Similar trends across disease subcohorts were found in 2007 and 2008 (data not shown).
Table 1 Characteristics of duloxetine initiators
Prior medication utilization
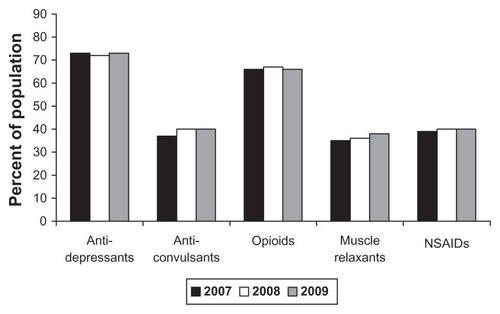
shows utilization of select medications over the 12 months prior to duloxetine initiation between 2007 and 2009. Among the five medication therapeutic classes examined, antidepressants were used most frequently (by approximately three-quarters of patients), followed by opioids (about two-thirds of patients). Between 35% and 40% of the patients used anticonvulsants, muscle relaxants, or NSAIDs during the previous 12 months. The medication utilization trend was consistent over time.
Figure 1 Use of select medications during the 12 months prior to duloxetine initiation across disease states.
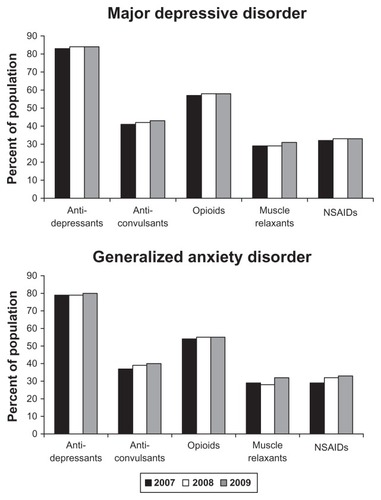
Patterns of prior medication use were similar among duloxetine initiators with major depressive disorder or generalized anxiety disorder (). Among the 2009 duloxetine initiators, utilization of antidepressants was the highest (around 80%) across the medications examined, followed by opioids (around 60%). Use of anticonvulsants, muscle relaxants, and NSAIDs was frequent but less common (between 30% and 40%). The trends across years were very similar.
Figure 2 Use of select medications during the 12 months prior to duloxetine initiation among patients with major depressive disorder or generalized anxiety disorder.
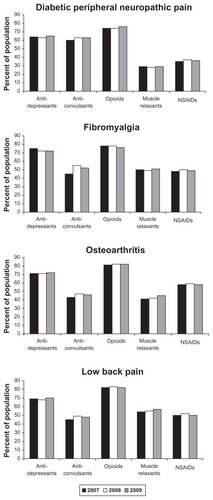
Prior medication use among duloxetine initiators across pain-related conditions is presented in . Across all cohorts in 2009, opioid use was the highest, followed by antidepressants. The use of anticonvulsants was different across cohorts, with the highest use in diabetic peripheral neuropathic pain but similar use among the other cohorts. Muscle relaxants and NSAIDs had the lowest use among patients with diabetic peripheral neuropathic pain. The highest utilization of muscle relaxants was among patients with low back pain, while the highest use of NSAIDS was among patients with osteoarthritis. Similar trends were found in 2007 and 2008.
Figure 3 Use of select medications during the 12 months prior to duloxetine initiation among patients with pain indications.
shows the use of six different select medication classes during the 12 months prior to duloxetine initiation over time. Patterns of use in 2009 were similar between major depressive disorder and generalized anxiety disorder patients: about 50% of the patients used selective serotonin reuptake inhibitors; 70% used any antidepressants (selective serotonin reuptake inhibitors, venlafaxine, tricyclic antidepressants, and other antidepressants); 80% used any antidepressants or anticonvulsants; 90% of the patients used any antidepressants, anticonvulsants, or opioids; and the percentages did not change much after adding NSAIDs to the examined list of medications. The use of these medications in 2009 was also similar across the pain-related conditions: antidepressants had the lowest use in diabetic peripheral neuropathic pain, but similar use among the other cohorts and similar to the trends observed in major depressive disorder and generalized anxiety disorder; 70%–80% of the patients used any antidepressants or anticonvulsants; 90% used any antidepressants, anticonvulsants, or opioids; and adding NSAIDs had minimal impact. Overall, the patterns were consistent from 2007 through 2009.
Discussion
Using the Thomson Medstat MarketScan commercial claims database, this study is the first to assess select medication use during the 12 months prior to duloxetine initiation among patients with major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, fibromyalgia, or chronic musculoskeletal pain due to osteoarthritis or low back pain between 2007 and 2009. Many patients were found to have used other medications, including antidepressants, anticonvulsants, opioids, NSAIDs, and muscle relaxants, before their initiation of duloxetine. Use of select medications was similar among patients with major depressive disorder and generalized anxiety disorder, and a similar medication use pattern was also found for the pain-related medical conditions (fibromyalgia, diabetic peripheral neuropathic pain, osteoarthritis, low back pain). From 2007 to 2009, utilization of these medications was relatively stable over time.
Not surprisingly, antidepressants were the most commonly used medications prior to duloxetine initiation among patients with major depressive disorder, because some patients may have been required to use a generically available medication before some of the branded medications could be prescribed. Use of antidepressants was also high among duloxetine initiators with diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, or low back pain. This may be because many of these patients also have comorbid depression.Citation29,Citation30 In general, claims data do not capture the reason for which a medication is prescribed. With the multiple approved indications for duloxetine and the likelihood of comorbid medical conditions associated with the cohorts examined, we are unable to identify the reason why patients were prescribed duloxetine.
Opioid use was high among duloxetine initiators with pain-related medical conditions, and this is consistent with the treatment guidelines because opioids are recommended for treating diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, and low back pain.Citation22,Citation25,Citation31–Citation34 The widespread use of opioids by patients with major depressive disorder or generalized anxiety disorder might be because many of these patients also have comorbid pain.Citation29,Citation30 Long-term opioid treatment may result in significant side effects and lead to tolerance and dependence,Citation35 so further research is warranted to assess such use in these conditions.
Use of muscle relaxants and NSAIDs was similar across the disease subcohorts, with relatively lower use in major depressive disorder, generalized anxiety disorder, and diabetic peripheral neuropathic pain, and relatively higher use in fibromyalgia, osteoarthritis, and low back pain. Unlike in osteoarthritis and low back pain, where NSAIDs are recommended by the treatment guidelines,Citation24,Citation25,Citation32,Citation36 the high use of NSAIDs observed in the fibromyalgia cohort may be driven by other pain comorbidities,Citation18,Citation20,Citation37 given that no studies have confirmed the efficacy of NSAIDs in patients with fibromyalgia.Citation38 Because over-the-counter medications are not captured in the database examined here, the use of NSAIDs might be underestimated in this study.
In general, the pattern of select medication use prior to duloxetine initiation within each disease subcohort was consistent across the study years. However, a notable change was observed among fibromyalgia patients, ie, anticonvulsant use increased by 10 percentage points from 2007 to 2008 followed by a three percentage point decrease from 2008 to 2009. This might be related to the approval of pregabalin in 2007 and duloxetine in 2008 for the management of fibromyalgia.Citation1,Citation39 Additionally, this study examined data prior to the approval of duloxetine for chronic musculoskeletal pain associated with osteoarthritis and low back pain (end of 2010 which is after the end of our study period). Further research is needed to examine whether the trends observed in this study will persist after approval of the new indication.
This study had several limitations. First, it used a crosssectional design. Although three years of data were analyzed, we did not track patients longitudinally. Second, all patients included had 12 months of continuous enrollment prior to duloxetine initiation. Therefore, individuals who dropped out of the plan at any time during the previous 12 months were excluded from the analysis. Third, this study was conducted among commercially insured individuals who received their health insurance through employment. The findings from this study may not be generalizable to other populations with different characteristics, such as the Medicare or Medicaid populations. Fourth, identification of medical conditions was based on ICD-9-CM diagnosis codes recorded in administrative claims, thus was not validated by medical chart review. Therefore, medical problems encountered by patients but not captured in administrative claims were not recognized. Fifth, this study only focused on select medication utilization rather than comprehensive medication use. Finally, only outpatient prescription medication use was examined. Over-the-counter medication utilization was not captured in the database because its use was not assessed in this study.
Conclusion
Commercially insured patients used several types of medications prior to duloxetine initiation. Antidepressants were the most commonly prescribed among patients with major depressive disorder or generalized anxiety disorder, followed by opioids. Among duloxetine initiators with diabetic peripheral neuropathic pain, fibromyalgia, osteoarthritis, or low back pain, opioids were the most frequently used followed by antidepressants. Over the 12 months prior to duloxetine initiation, over 50% of the patients used any antidepressants, 70% used any antidepressants or anticonvulsants, and 90% used any antidepressants, anticonvulsants, or opioids. The use of select medications was stable between 2007 and 2009.
Disclosure
This study was funded by Eli Lilly and Company.
References
- Cymbalta® [package insert] http://pi.lilly.com/us/cymbalta-pi.pdf2010
- ArnoldLMLuYCroffordLJA double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorderArthritis Rheum20045092974298415457467
- ChappellASBradleyLAWiltseCDetkeMJD’SouzaDNSpaethMA six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgiaInt J Gen Med200919110220428412
- ChappellASDesaiahDLiu-SeifertHA double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the kneePain Pract2011111334120602715
- ChappellASOssannaMJLiu-SeifertHDuloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trialPain2009146325326019625125
- DetkeMJLuYGoldsteinDJMcNamaraRKDemitrackMADuloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depressionJ Psychiatr Res200236638339012393307
- GoldsteinDJLuYDetkeMJLeeTCIyengarSDuloxetine vs placebo in patients with painful diabetic neuropathyPain20051161–210911815927394
- RussellIJMeasePJSmithTREfficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trialPain2008136343244418395345
- RynnMRussellJEricksonJEfficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive- titration, placebo-controlled trialDepress Anxiety200825318218917311303
- SkljarevskiVOssannaMLiu-SeifertHA double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back painEur J Neurol20091691041104819469829
- WernickeJFPritchettYLD’SouzaDNA randomized controlled trial of duloxetine in diabetic peripheral neuropathic painNeurology20066781411142017060567
- ChenSYWuNBoulangerLFraserKAZhaoYThe relationship between average daily dose, medication adherence, and health-care costs among diabetic peripheral neuropathic pain patients initiated on duloxetine therapyPain Pract201010653053920412505
- ZhaoYSunPWatsonPMedication adherence and healthcare costs among patients with diabetic peripheral neuropathic pain initiating duloxetine versus pregabalinCurr Med Res Opin201127478579221303196
- RaySChogtuBPrescribing trends in depression: a drug utilization study done at a tertiary healthcare centreJ Clin Diag Res201153573577
- BauerMMonzBUMontejoALPrescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) studyEur Psychiatry2008231667318164600
- LiuXGelwicksSFariesDEAbleSLInitial duloxetine prescription dose and treatment adherence and persistence in patients with major depressive disorderInt Clin Psychopharmacol201025631532220706125
- WuNChenSBoulangerLFraserKBledsoeSLZhaoYDuloxetine compliance and its association with healthcare costs among patients with diabetic peripheral neuropathic painJ Med Econ200912319220219705975
- WuNChenSBoulangerLRaoPZhaoYAverage daily dose, medication adherence, and healthcare costs among commercially-insured patients with fibromyalgia treated with duloxetineCurr Med Res Opin20112761131113921456939
- YeWZhaoYRobinsonRLSwindleRWTreatment patterns associated with duloxetine and venlafaxine use for major depressive disorderBMC Psychiatry2011111921281479
- ZhaoYChenSYWuNFraserKABoulangerLMedication adherence and healthcare costs among fibromyalgia patients treated with duloxetinePain Pract201111438139121199311
- ZhaoYSunPWatsonPMitchellBSwindleRComparison of medication adherence and healthcare costs between duloxetine and pregabalin initiators among patients with fibromyalgiaPain Pract201111320421620807351
- American College of RheumatologyRecommendations for the medical management of osteoarthritis of the hip and knee, 2000 Available from: http://www.rheumatology.org/practice/clinical/guidelines/oa-mgmt.aspAccessed November 8, 2011
- [No authors listed]American Pain Society releases new clinical practice guideline for fibromyalgia pain Available from: http://www.ampainsoc.org/press/2005/101005.htmAccessed July 12, 2012
- [No authors listed]American Academy of Orthopaedic Surgeons guideline on the treatment of osteoarthritis of the knee (non-arthroplasty)2008 Available from: http://www.aaos.org/Research/guidelines/OAKguideline.pdfAccessed July 13, 2012
- Institute for Clinical Systems improvement health care guideline: adult low back pain2010 Available from: http://www.icsi.org/low_back_pain/adult_low_back_pain__8.htmlAccessed November 8, 2011
- ArgoffCEBackonjaMMBelgradeMJConsensus guidelines: treatment planning and options. Diabetic peripheral neuropathic painMayo Clin Proc200681Suppl 4S12S2516608049
- American Psychiatric AssociationPractice guideline for the treatment of patients with major depressive disorder, third edition2010 Available from: http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485#654001Accessed November 30, 2011
- National Institute for Health and Clinical ExcellenceAnxiety: management of anxiety (panic disorder, with or without agoraphobia, and generalised anxiety disorder) in adults in primary, secondary and community care2004 Accessed from: http://www.nice.org.uk/nicemedia/live/13314/52667/52667.pdfAccessed November 30, 2011
- BairMJRobinsonRLKatonWKroenkeKDepression and pain comorbidity: a literature reviewArch Intern Med2003163202433244514609780
- LepineJPBrileyMThe epidemiology of pain in depressionHum Psychopharmacol200419Suppl 1S3S715378670
- ArgoffCEBackonjaMMBelgradeMJConsensus guidelines: treatment planning and options. Diabetic peripheral neuropathic painMayo Clin Proc200681S12S2516608049
- RichmondJHunterDIrrgangJAmerican Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the kneeJ Bone Joint Surg Am201092499099320360527
- [No authors listed]American Pain Society releases new clinical practice guideline for fibromyalgia pain2005 Accessed from: http://www.ampainsoc.org/press/2005/101005.htmAccessed November 8, 2011
- [No authors listed]American Academy of Orthopaedic Surgeons guideline on the treatment of osteoarthritis of the knee (non-arthroplasty)2008 Available from: http://www.aaos.org/Research/guidelines/OAKguideline.pdfAccessed November 8, 2011
- BenyaminRTrescotAMDattaSOpioid complications and side effectsPain Physician200811Suppl 2S105S12018443635
- ChouRQaseemASnowVDiagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain SocietyAnn Intern Med2007147747849117909209
- BoulangerLWuNChenSYPredictors of pain medication selection among patients diagnosed with fibromyalgiaPain Pract201212426627521899718
- MeasePFibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatmentJ Rheumatol Suppl20057562116078356
- Lyrica® [package insert] http://labeling.pfizer.com/ShowLabeling.aspx?id=5612008